Assisted Reproduction Associated With Monozygotic Twinning
Infertility treatments are the only known factor to increase the rate of monozygotic twinning (MZT) in humans. As the main means of infertility treatments, assisted reproductive technology (ART) has gradually been widely used all over the world over the past several decades, which may have at least doubled the incidence of MZT (Ferm, Reference Ferm1969).
Artificial induction of ovulation (AIO), intracytoplasmic sperm injection (ICSI), assisted hatching (AH), frozen embryos and embryo transfer stages (Table 1) are all considered as possible factors that affect the incidence of MZT (Aston et al., Reference Aston, Peterson and Carrell2008; Knopman et al., Reference Knopman, Krey, Lee, Fino, Novetsky and Noyes2010). Ovulation induction and superovulation are both methods used to treat female infertility. Ovulation induction treatment aims to induce the maturity of at least one follicle and usually applies to an ovulatory women without other reproductive disorders, while superovulation is usually used to obtain a higher number of follicles for in vitro operation (Kafy & Tulandi, Reference Kafy and Tulandi2007). Ovulation induction treatments increase the incidence of monozygotic multiples pregnancies; the first report about it from the East Flanders Prospective Twin Study (EFPTS) showed that the incidence of MZT following ovulation induction (1.2%) was significantly higher than that of natural conception (0.45%). Furthermore, AIO seems to be the first identified means of increasing the MZT rate (Elizur et al., Reference Elizur, Levron, Shrim, Sivan, Dor and Shulman2004; Steinman, Reference Steinman2001).
Table 1. Review of ART-associated MZT publications

Note: ART, assisted reproductive technology; MZT, monozygotic twinning; AH, assisted hatching; ICSI, intracytoplasmic sperm injection.
Both ICSI and AH are primary zona manipulation techniques in assisted reproduction. ICSI involves injecting a single sperm cell into the oocyte cytoplasm directly, which is one of the major means to treat fertilization failure due to dysfunction of sperm (Palermo et al., Reference Palermo, O’Neill, Chow, Cheung, Parrella, Pereira and Rosenwaks2017). AH involves artificially disrupting the zona pellucida via chemical, mechanical or laser manipulation before embryo transfer to improve implantation rate (Hammadeh et al., Reference Hammadeh, Fischer-Hammadeh and Ali2011).
The manipulation of the zona pellucida is considered to be the crucial trigger for the raised incidence of MZT in assisted reproduction (Abusheika et al., Reference Abusheika, Salha, Sharma and Brinsden2000); the zona pellucida hardens after fertilization and if the hatching opening of the zona pellucida is too narrow it may bisect the blastocyst during blastocyst expansion. If each part possesses sufficient ICM to develop an independent embryo it will probably result in MZT formation (Aston et al., Reference Aston, Peterson and Carrell2008; Hershlag et al., Reference Hershlag, Paine, Cooper, Scholl and Kvapil1999; Ménézo & Sakkas, Reference Ménézo and Sakkas2002; Wehbe et al., Reference Wehbe, Tucker, Palermo and Scott Sills2003). However, other studies have demonstrated that manipulation of the zona pellucida does not affect the incidence of MZT in pregnancy outcomes (Elizur et al., Reference Elizur, Levron, Shrim, Sivan, Dor and Shulman2004; H. Liu et al., Reference Liu, Liu, Chen, Kang, Du and Li2018; Nakasuji et al., Reference Nakasuji, Saito, Araki, Nakaza, Nakashima, Kuwahara, Ishihara, Irahara, Kubota, Yoshimura and Sakumoto2014; Sills et al., Reference Sills, Moomjy, Zaninovic, Veeck, McGee, Palermo and Rosenwaks2000; Wu et al., Reference Wu, Huang, Wu, Chen, Soong and Huang2014). Frankfurter et al. (Reference Frankfurter, Hackett, Meng and Keefe2001) used pronase to remove the entire zona pellucida before embryo transfer to avoid MZT occurrence; however, this manipulation did not result in a statistical difference in the incidence of MZT in pregnancy outcomes compared to those with integral zona pellucida. Moreover, surprisingly, Shi et al. (Reference Shi, Jin, Liu, Zhang, Mi, Shi, Wang and Liang2021) found that zona pellucida manipulation instead reduced the incidence of MZT in a study of 26,254 assisted reproduction cases. A study by Wang et al. (Reference Wang, Liu, Chen, Sun and Li2018) also reached the same conclusion.
Although numerous studies have proved that the increased frequency of MZT is not associated with AH and ICSI, the zona pellucida manipulation is still considered to be the main possible cause for increasing the occurrence of MZT.
Embryo transfer stage is associated with implantation rate, and the transfer performed at blastocyst stage is significantly higher than that at cleavage stage, suggesting that delayed embryo transfer can improve the conception rate (Glujovsky et al., Reference Glujovsky, Retamar, Sedo, Ciapponi, Cornelisse and Blake2022). The effect of delayed embryo transfer on the incidence of MZT appears to have the same trend. Milki et al. (Reference Milki, Jun, Hinckley, Behr, Giudice and Westphal2003) found that blastocyst stage (5.6%) transfers have a higher risk than cleavage stage (2%) transfer of producing MZT, and other studies have reached the same conclusion (Mateizel et al., Reference Mateizel, Santos-Ribeiro, Done, Van Landuyt, Van de Velde, Tournaye and Verheyen2016; Shi et al., Reference Shi, Jin, Liu, Zhang, Mi, Shi, Wang and Liang2021). However, a study of 9969 cases of assisted reproductive pregnancy by Franasiak et al. (Reference Franasiak, Dondik, Molinaro, Hong, Forman, Werner, Upham and Scott2015) proved that the incidence of MZT was not associated with the embryo transfer stage (Papanikolaou et al., Reference Papanikolaou, Fatemi, Venetis, Donoso, Kolibianakis, Tournaye, Tarlatzis and Devroey2010). Interestingly, Moayeri et al. (Reference Moayeri, Behr, Lathi, Westphal and Milki2007) also demonstrated that the embryo transfer stage is not related to the incidence of MZT, but in overturning their previous conclusion from 2003, the explanation they gave was that it was probably due to the optimization of experimental conditions and techniques. Liu et al. (Reference Liu, Liu, Chen, Kang, Du and Li2018) found that delaying embryo transfer within a certain time frame significantly increased the rates of MZT, with the highest rate occurring on day 5.
Whether pregnancy outcomes are related to maternal age in assisted reproduction is also controversial (Aston et al., Reference Aston, Peterson and Carrell2008). A study of 8459 cases on the effect of maternal age on pregnancy outcomes in assisted reproduction pointed out that advanced maternal age was associated with a lower rate of MZT and the threshold age was 36 years (X. Liu & Shi, Reference Liu and Shi2021) but other studies have shown that there is no relationship between maternal age and MZT frequency (H. Liu et al., Reference Liu, Liu, Chen, Kang, Du and Li2018; Nakasuji et al., Reference Nakasuji, Saito, Araki, Nakaza, Nakashima, Kuwahara, Ishihara, Irahara, Kubota, Yoshimura and Sakumoto2014; Wu et al., Reference Wu, Huang, Wu, Chen, Soong and Huang2014). Shi et al.’s (Reference Shi, Jin, Liu, Zhang, Mi, Shi, Wang and Liang2021) conclusion from 26,254 assisted reproduction cases showed that the incidence of MZT in fresh embryos was significantly lower than that in thawed embryos, and this conclusion was also obtained by Liu and Shi (Reference Liu and Shi2021) and Mateizel et al. (Reference Mateizel, Santos-Ribeiro, Done, Van Landuyt, Van de Velde, Tournaye and Verheyen2016); some of this data is shown in Table 1 and I will not repeat the description here.
The components of the culture media and their concentrations are considered to play a role in a MZT event, especially the ambient calcium concentration (Steinman, Reference Steinman2001). However, an ART study of 1876 cases from Japan indicated that type of cultivation media was not associated with MZT incidence (Sobek et al., Reference Sobek, Zbořilová, Procházka, Šilhánová, Koutná, Klásková, Tkadlec and Sobek2015); the same conclusion was also reached in some studies from other countries (Skiadas et al., Reference Skiadas, Missmer, Benson, Gee and Racowsky2008). Moreover, the blastocyst morphology before transfer was proved to be related to MZT incidence, when the embryo with looser inner cell mass (ICM) has a higher rate of forming MZT following implantation (Shi et al., Reference Shi, Jin, Liu, Zhang, Mi, Shi, Wang and Liang2021).
Ovulation induction is the only uncontroversial factor that can heighten the incidence of MZT without any in vitro manipulation, suggesting that the MZT events can be unilaterally determined by the follicles before zygotes are formed. No matter how the experimental conditions and technologies are optimized, in vitro fertilization and culture cannot completely mimic the natural uterine environment (Ménézo & Sakkas, Reference Ménézo and Sakkas2002) and it could be the interference factor in MZT studies. In addition, various studies have reached different and even opposite conclusions; no one has observed embryo splitting before the blastocyst transfer in ART, which indicates the requirement for more convincing biological research than just statistical analysis of clinical data.
MZT Fetus Papyraceous in Triplets
Fetus papyraceous (FP) happens when one of a pair of monozygotic twins has died in utero, and while the other survives, the dead one has not been completely reabsorbed. If one of the twins dies during the first trimester it is usually completely reabsorbed, whereas if it dies in the second or third trimester, the incompletely absorbed remains are compressed and lie between the amniotic sac of its co-twin and the uterine wall, becoming a mummified, parchment-like shape, known as a paper fetus. The incidence of FP is reported to be 1:184 twin births (≈1:12000 live births; Ikpeze & Nwosu, Reference Ikpeze and Nwosu1998; Lau & Rogers, Reference Lau and Rogers1999; Tayade & Kumar, Reference Tayade and Kumar2012) and it is higher in triplets pregnancies.
Surviving fetuses are usually born prematurely and are smaller than normal singleton fetuses. Clinical cases suggest that surviving fetuses are at risk of renal failure and central nervous system (CNS) developmental abnormalities, including polymicrogyria, multicystic encephalopathy or porencephaly, ventriculomegaly or hydranencephaly, cortical atrophy, and cerebral infarction (Bukar et al., Reference Bukar, Chama, Bako and Bi2013; Ikpeze & Nwosu, Reference Ikpeze and Nwosu1998; Pharoah, Reference Pharoah2006). Disseminated intravascular coagulopathy, preeclampsia, polyhydramnios, antepartum hemorrhage, preterm birth, and anemia may occur in pregnant mothers (Børlum, Reference Børlum1991).
Bukar et al. (Reference Bukar, Chama, Bako and Bi2013) reported a rare case of MZT FP in a triplet pregnancy when a 39-year-old Nigerian multiparous pregnant woman showed signs of premature labor at 36 weeks. She gave birth to a 2.3 kg baby girl and two monochorionic MZT male papyraceous fetuses weighing 150 g and 130 g respectively. The difference in weight suggested that the second male fetus did not survive for long after the first died (Figure 1).

Figure 1. Monochorionic female MZT fetus papyraceous in triplet pregnancy (Bukar et al., Reference Bukar, Chama, Bako and Bi2013).
Conjoined Triplets
In early human development, the chorion begins to form at about day 3, and the amnion begins to form about day 7. So the timing of embryo splitting can be estimated according to the type of chorion and amnion (Hall, Reference Hall2003), but this method of estimation is not completely accurate because of a rare kind of monochorionic dizygotic (MCDZ) twinning that is developed from two zygotes (Peters et al., Reference Peters, König, Verhoeven, Schats, Mijatovic, Ket and Lambalk2017; see Table 2). Dichorionic diamniotic (DCDA), monochorionic diamniotic (MCDA) and monochorionic monoamniotic (MCMA) are the three types MZT placental membranes.
Table 2. Different twin types based on placenta and membranes

Note: DC, dichorionic; DA, diamniotic; MC, monochorionic; MA, monoamniotic; DZ, dizygotic.
However, conjoined twins can occur when embryonic division occurs at the primitive streak stage. Two hypotheses — ‘fission’ versus ‘fusion’ — have been proposed for the mechanism of conjoined twinning; incomplete splitting of the embryonic axis is the widely accepted one and early reattachment of the split fetuses has also been suggested as a mechanism in conjoined twins (Hall, Reference Hall2003; Machin, Reference Machin1993). Herranz (Reference Herranz2015) put forward a hypothesis that the MZT event occurs only in the first cleavage division of the zygote (two-cell stage), and the diversity of chorionicity and amnionicity depends on the degree of fusion of the two embryonic membranes within the zona pellucida (Figure 2).
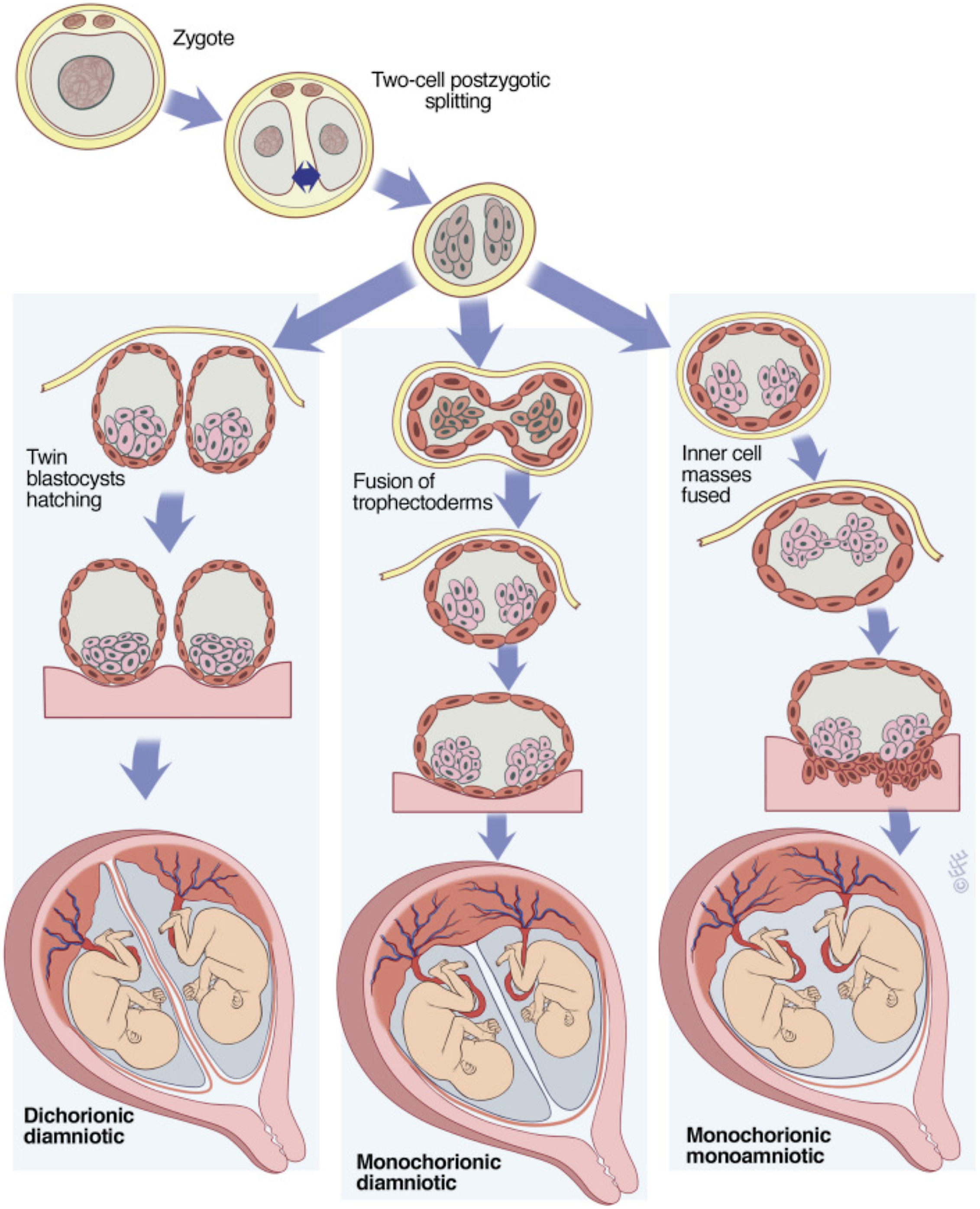
Figure 2. Model of three forms of MZT from Herranz, the placenta and membranes types result from the degree of fusion of the two embryonic membranes (Herranz, Reference Herranz2015).
Note: Reprinted with permission from Elsevier from McNamara H. C. et al. (Reference McNamara, Kane, Craig, Short and Umstad2016). A review of the mechanisms and evidence for typical and atypical twinning, American Journal of Obstetrics and Gynecology, 214, 172–191.
The spontaneous incidence of conjoined twinning is approximately 15 per million deliveries, and the most common varieties encountered are thoraco-omphalopagus, thoracopagus, omphalopagus, parasitic twins and craniopagus (Villarreal et al., Reference Villarreal, Yoeli, Masand, Galvan, Olutoye and Goss2020). Conjoined twins show significant differences in gender in that about 75% of conjoined twins are female; whether this is because male conjoined fetuses have less survivability or conjoined events are more likely to arise in female embryos remains unknown (Kaufman, Reference Kaufman2004). Some conjoined cases can be separated by surgery but this does not always result in survival of both individuals, and it is evidently impossible for the conjoined fetuses with a high degree of crucial organ sharing such as the heart to survive a surgical separation. So, when severe conjoined malformation is diagnosed early, that pregnancy termination is an option that should be considered.
Certain teratogenic or toxic drugs are capable of performing as an embryo splitting trigger, and probably act by interfering with the normal progress of mitosis. After treatment of a pregnant golden hamster with dimethyl sulfoxide (DMSO) and urethan, three cases of conjoined twinning and other embryo malformation were observed (Ferm, Reference Ferm1969). Vincristine is an alkaloid commonly used for treating acute leukemias in children and has been found to be extremely teratogenic. In a study on the embryonic teratogenicity of vincristine, eight normal monoamniotic twins together with one pair of conjoined twins were found in 228 embryos after injection of pregnant mice with vincristine (Kaufman, Reference Kaufman2004; Kaufman & O’Shea, Reference Kaufman and O’Shea1978). Roux reported one pair of conjoined twins induced by in utero early exposure to prochlorperazine (Brambati et al., Reference Brambati, Lanzani, Sanchioni and Tului1990). Furthermore, over-ripe follicles are thought to be an extremely severe event for animal reproduction and embryonic development (Bomsel-Helmreich, Reference Bomsel-Helmreich1976; Steinman, Reference Steinman2001), and is one of the major causes of human birth defects. Hormonal modifications just prior to ovulation could initiate biochemical processes in the oocyte which proceed to an abnormal sequence of events when ovulation or fertilization does not take place at the optimum time (Butcher, Reference Butcher1976; Witschi & Laguens, Reference Witschi and Laguens1963), Over-ripeness of follicles can lead to abnormal mitosis and meiosis, with a higher risk of malformed embryos with chromosomal abnormalities after fertilization (Witschi, Reference Witschi1952) and which can cause spontaneous abortion in women (Mikamo, Reference Mikamo1970). However, over-ripe follicles are considered one of the possible reasons for the occurrence of MZT (Smits et al., Reference Smits, Jongbloet and Zielhuis1995; Witschi, Reference Witschi1952). Delayed fertilization and over-ripeness can enhance the incidence of MZT in rabbits, but there are is also high embryonic mortality and chromosomal anomalies such as trisomies, triploidies, and chimaeras (Bomsel-Helmreich & Papiernik-Berkhauer, Reference Bomsel-Helmreich and Papiernik-Berkhauer1976).
Athanasiadis et al. (Reference Athanasiadis, Tzannatos, Mikos, Zafrakas and Bontis2005) reported an extremely rare conjoined triplet case from spontaneous pregnancy. The conjoined monster fetuses appeared to have three distinct heads, four arms and four legs; two of the fetuses showed the characteristics of dicephalus conjoined twinning with two heads, two arms and two legs (Figure 3), sharing a common stomach, a common spleen and a pair of kidneys, while the third fetus formed a conjoined fusion with them at the thorax and the upper abdomen level. The three fetuses, which were all female, shared one single heart, one single liver, and a common umbilical cord. They were removed by hysterotomy at 22 weeks (Athanasiadis et al., Reference Athanasiadis, Tzannatos, Mikos, Zafrakas and Bontis2005). According to the widely accepted fission hypothesis, in this case, the embryo splitting occurred after 14 days following fertilization. After the first incomplete splitting to form thoracopagus conjoined embryos, a second division arose in one of the fetuses, which resulted in the formation of dicephalus conjoined fetuses. However, if the fusion hypothesis is correct, it indicated that reattachment occurred twice in this case. Monozygotic triplets were formed initially at an early stage, then the first reattachment arose between two of the embryos to form the dicephalus conjoined fetuses, and the second reattachment arose between the dicephalus conjoined fetuses and the third fetus, which resulted in thoracopagus conjoined fetuses.

Figure 3. Conjoined triplet monster with three heads, four arms and four legs (Athanasiadis et al., Reference Athanasiadis, Tzannatos, Mikos, Zafrakas and Bontis2005).
Note: Reprinted with permission from Elsevier from Athanasiadis, A. P. et al. (Reference Athanasiadis, Tzannatos, Mikos, Zafrakas and Bontis2005). A unique case of conjoined triplets. American Journal of Obstetrics and Gynecology, 192, 2084–2087.
Sesquizygotic Twinning is Not a New Twinning Mechanism
A unique and rare form of MZT, named sesquizygotic twinning (SZT), has been reported only twice. In these cases, both twin pairs developed from the one single zygote and shared between 50% and 100% of genetic identity rather than 100%, which is speculated to be caused by dispermic fertilization (DF; Golubovsky, Reference Golubovsky2002).
SZT was first reported by Souter et al. in 2007, when a pair of newborn monozygotic twins received attention for the ambiguous external genitalia in one. One twin was true hermaphroditism (TH) and the other was a normal male. The TH individual had ambiguous external genitalia, and diagnostic laparoscopy revealed the presence of a hemi-uterus, fallopian tube and bilateral gonads. Histological examination revealed both gonads to be ovotestes, with the coexistence of follicles, oocytes and ovarian stroma, and sertoli cells and spermatogonia were also observed. Both twins were 46,XX/46,XY chimeric individuals. The gonad of the TH twin was composed of XX[42%]/XY[57%]/X[1%] (right gonad) and XX[35%]/XY[55%]/X[10%] (left gonad). The gonad of the male twin was composed of XX[8%]/XY[77%]/X[15%] (right gonad) and XX[31%]/XY[59%]/X[9%] (left gonad). The twins retained a single maternal genetic contribution and two paternal genetic contributions and shared 100% of maternal alleles and approximately 50% of paternal alleles (Souter et al., Reference Souter, Parisi, Nyholt, Kapur, Henders, Opheim, Gunther, Mitchell, Glass and Montgomery2007). Another case was reported by Gabbett et al. (Reference Gabbett, Laporte, Sekar, Nandini, McGrath, Sapkota, Jiang, Zhang, Burgess, Montgomery, Chiu and Fisk2019) when a pair of monochorionic twins with sex-discordance caught the attention of researchers. Both twins were 46,XX/46,XY chimera with no evidence of sexual ambiguity. The female twin underwent a prophylactic oophorectomy and amputation because of gonadal dysgenesis and brachial artery thromboembolism. Analysis of single nucleotide polymorphisms (SNPs) revealed that they shared 100% of the maternal alleles and approximately 77.7% of the paternal alleles (Gabbett et al., Reference Gabbett, Laporte, Sekar, Nandini, McGrath, Sapkota, Jiang, Zhang, Burgess, Montgomery, Chiu and Fisk2019).
DF resulting in sex-chromosome discordant chimerism (XX/XY chimerism) was first reported in the 1960s (Gartler et al., Reference Gartler, Waxman and Giblett1962). XX/XY chimeras possess a higher hazard of gonadal malformation comprising gonadal dysgenesis and different degrees of ambiguous genitalia, and it is also possible to have a normal reproductive system (Giltay et al., Reference Giltay, Brunt, Beemer, Wit, Ploos van Amstel, Pearson and Wijmenga1998; Kawamura et al., Reference Kawamura, Kato, Miyai, Suzuki, Naru, Kato, Tanaka, Nagasaka, Tsutsumi, Inagaki, Ioroi, Yoshida, Nao, Conlin, Iijima, Kurahashi and Taniguchi-Ikeda2020). Typically, DF results in a triploid zygote, which seems to be one of the constant chromosomal errors responsible for cleavage and implantation failure with an incidence of nearly 1% in all conceptions in humans (Golubovsky, Reference Golubovsky2003). However, Angell et al. (Reference Angell, Templeton and Messinis1986) observed that an immediate diploidization event may arise in triploid zygotes and eventually turn triploid cells into diploid cells; then the diploid chimeric embryo may survive carrying different genomes (Angell et al., Reference Angell, Templeton and Messinis1986; Plachot & Crozet, Reference Plachot and Crozet1992). The only two cases reported have revealed that DF could be the origin of the phenotypic disparity between twins; however, no evidence has been found to prove that embryo splitting was associated with DF, chromosomal abnormalities or chimeric embryos, so the author believes that SZT is still a special form of MZT instead of the new twinning mechanism. In conclusion, SZT implies that two rare high-risk incidents arise in embryonic development. The existence of other similar twins have been ignored due to their normal phenotype, and possibly have never been identified.
Author contribution statement
The entire work was completed by Tan Yuge alone.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Declaration of interest
The author has no competing or conflicting interests to declare.








