Understanding the mechanisms behind our choices to undertake actions detrimental to health is one key to developing ways to effectively prevent these choices. The hazards of cigarette smoking are numerous and well documented (Prasad et al., Reference Prasad, Kabir, Dash and Das2009). Yet, why some individuals partake in this activity while others do not is still a topic of debate. The present study focused on the interplay between two known suspects: peer relationships in adolescence and genetic influences (Kobus, Reference Kobus2003; Munafò & Johnstone, Reference Munafò and Johnstone2008).
Peer Relationships
Association with cigarette smoking peers has been shown to increase the likelihood that one will also smoke (Alexander et al., Reference Alexander, Piazza, Mekos and Valente2001; Hoffman et al., Reference Hoffman, Sussman, Unger and Valente2006; Holliday et al., Reference Holliday, Rothwell and Moore2010; Pollard et al., Reference Pollard, Tucker, Green, Kennedy and Go2010; Vink et al., Reference Vink, Willemsen, Engels and Boomsma2003). For example, belonging to peer networks composed of at least half smokers or having best friends who smoked made individuals twice as likely to smoke (Alexander et al., Reference Alexander, Piazza, Mekos and Valente2001). This observed homogeneity in smoking behavior between individuals and their peer groups was reviewed by Kobus (Reference Kobus2003), who summarized the work done to pinpoint the specifics of how peers are related to smoking behavior, but concluded that further work should be done to illuminate the ‘subtleties’ behind the social dynamics of this contribution.
Two potential mechanisms may influence the correlation in smoking among peers — homophily and peer influence. Homophily is defined as the propensity to associate with more similar than dissimilar individuals (McPherson et al., Reference McPherson, Smith-Lovin and Cook2001). McPherson et al. noted that it was originally assumed that peer groups directly influenced an individual's behavior. However, the surge of longitudinal data led to a shift toward recognition of the importance of homophily: individuals may actually select membership into groups that share one's initial behavioral characteristics. Thus, in the context of homogeneity of smoking between individuals and peer groups, arise two distinct, yet non-mutually exclusive, possibilities: (1) peer group may directly influence smoking behavior (peer influence), and (2) those with a propensity for smoking behavior may self-select into groups with similar characteristics (homophily).
The relative contribution of each of these possibilities, however, remains a matter of debate. Arnett (Reference Arnett2007) rejected the assumption that the association between peer and individual smoking is a result of direct peer influence, and suggested that selection of friends based on a number of factors leads to peer-group selection that creates a pathway to peer context variables such as group expectations, identity, and opportunities that may influence smoking behavior. Simons-Morton and Farhat (Reference Simons-Morton and Farhat2010), on the other hand, conclude that both homophily and peer influence are important. Most reports emphasize that the magnitude of each influence remains unclear (Dishion & Owen, Reference Dishion and Owen2002; Go et al., Reference Go, Green, Kennedy, Pollard and Tucker2010; Hall & Valente, Reference Hall and Valente2007; Mercken et al., Reference Mercken, Snijders, Steglich, Vertiainen and de Vries2010; White et al., Reference White, Hopper, Wearing and Hill2003).
Peer Relationships in the Context of Genetic Influences
Genetic factors have also been associated with smoking behavior (Munafò & Johnstone, Reference Munafò and Johnstone2008) and should be examined in terms of how they may connect individuals to particular peer groups. The interface between genetics and peer relationships has been demonstrated in results illustrating that the relationship between peer smoking and substance use depends on genetic liability (Agrawal et al., Reference Agrawal, Balasubramanian, Smith, Madden, Bucholz, Heath and Lynskey2010; Harden et al., Reference Harden, Hill, Turkheimer and Emery2008; Johnson et al., Reference Johnson, Chen, Breslau, Hatsukami, Robbins, Saccone and Bierut2010). Genetics may also play a direct role in friend selection, as it has been demonstrated that more genetically similar siblings have more similar peer groups (Harakeh et al., Reference Harakeh, Neiderhiser, Spotts, Engels, Scholte and Reiss2008) and that genetic factors may regulate exposure to peer substance use (Cleveland et al., Reference Cleveland, Wiebe and Rowe2005).
There is also evidence that the genetic contribution to smoking behavior may depend on the stage of smoking progression, from initiation to regular use and dependence. For example, Sullivan and Kendler (Reference Sullivan and Kendler1999) demonstrated that shared environment may be more important for smoking initiation, while genetics may be more important in the transition from initiation to regular smoking and dependence. Numerous other studies have attempted to understand the degree to which the genetics and environmental factors influencing each of these phenotypes are related, but the general consensus is that there are factors that are both common and unique to each stage of the smoking process (Koopmans et al., Reference Koopmans, Slutske, Heath, Neale and Boomsma1999; Madden et al., Reference Madden, Heath, Pedersen, Kaprio, Koskenvuo and Martin1999; Maes et al., Reference Maes, Sullivan, Bulik, Neale, Prescott, Eaves and Kendler2004). However, little has been done to explore the degree to which peers and the potential genetic implications of these peers may influence different stages of smoking.
Loehlin (Reference Loehlin2010) further illuminated the complex relationship among peers, genes, and substance use by examining whether more shared friends were associated with similarity in drinking behavior using the National Merit Twin Study (Loehlin & Nichols, Reference Loehlin and Nichols1976). Fewer shared friends were related to greater differences in alcohol drinking behaviors and, in females, the dizygotic (DZ) correlation was stronger than the monozygotic (MZ) correlation. Thus, genetic differences in DZ twins may result in different choices of friends that are related to differences in behavior for DZ, but not MZ, twins.
One mechanism that could contribute to such observed patterns of MZ and DZ correlations is homophily based on liability to smoking — including the overt phenotype of smoking — along with the risk and protective factors associated with smoking. When genes influence peer selection, the friends of MZ pairs will be more similar than those of DZ pairs along all genetically influenced domains of homophily, including smoking.
A popular model among behavioral geneticists combines genetically influenced homophily with peer influence. Here genetic and environmental influences on smoking-related homophily variables lead individuals to associate with similarly inclined peer groups. Individuals within a group then mutually influence one another's behavior. Afterwards, genetic values for smoking become statistically correlated with environmental values for smoking, or active gene–environment correlation (rGE) (Eaves et al., Reference Eaves, Last, Martin and Jinks1977; Plomin et al., Reference Plomin, DeFries and Loehlin1977; Scarr & McCartney, Reference Scarr and Mccartney1983).
Given expanded data sets on twins, adoptees, and biological relatives raised apart, it would be possible to assess and quantify the model described above. However, in this article, we have the more modest goal of exploring the relationship between similarity in smoking status and similarity in peer groups for MZ and DZ twins raised together. The paper is effectively a ‘clone’ of Loehlin's (Reference Loehlin2010) analysis of peer-group similarity and twin similarity for alcohol use. The only difference is the phenotype.
Thus, using the same National Merit Twin Study dataset as Loehlin (Reference Loehlin2010), we predicted that (1) more shared friends should be associated with less differences in smoking behavior, and (2) this negative correlation between shared friends and smoking behavior should be stronger for DZ twins. Given Loehlin's differential findings for each gender on the latter, we examined gender differences in the degree to which genetics and shared environment influence smoking behavior. We also examined the degree to which these effects exist when our smoking variable is separated to assess either smoking initiation or persistence.
Methods
Participants
The sample consisted of 850 twin pairs (514 MZ and 336 DZ same-sex twins) who participated in the 1962 National Merit Scholarship Qualifying Test as high school juniors (Loehlin & Nichols, Reference Loehlin and Nichols1976). Exclusions and missing values reduced this sample to 509 MZ twin pairs (216 males and 293 females) and 330 same-sex DZ twin pairs (135 males and 195 females).
Items of Interest and Scoring
Zygosity assignment was made on the basis of a questionnaire on reported similarities of a twin pair (Loehlin & Nichols, Reference Loehlin and Nichols1976). The original survey contained the following three questions on smoking:
1. How much do you smoke? Responses: (1) never smoked, (2) used to or occasionally smoke, (3) 1 to 19 cigarettes a day, and (4) greater than 20 cigarettes a day.
2. If you smoke, do you inhale the smoke into your lungs? Responses: (1) don't smoke, (2) rarely or never inhale, (3) sometimes inhale, and (4) usually inhale.
3. (How often have you) smoked a cigarette or cigar before breakfast? Responses: (1) frequently, (2) occasionally, and (3) not at all.
The participants reported on the frequency of the said action, and a composite score was assigned for each individual in the following manner. Individuals who had never smoked were given a score of 1 (63% of the individuals); individuals who occasionally smoked, but had never inhaled were given a score of 2 (10% of the individuals); current or former smokers who had inhaled were given a score of 3 (14% of the sample); current or former smokers who had both inhaled and smoked before breakfast were given a score of 4 (3% of the sample).
For the initiation part of our analysis, the composite score was used to dichotomize individuals into categories of initiation status: a binary variable of having never smoked (composite score of 1) or having initiated smoking behavior (composite score > 1). To assess smoking persistence, we only included twin pairs where each twin had initiated (27% of the sample), and persistent smoking behavior was analyzed using the composite score (scores 2 through 4) described above.
Individuals missing scores on any of the above items were assigned scores based on responses to the other smoking items. For example, a respondent who omitted an answer to ‘How much do you smoke’ but reported smoking before breakfast was assigned a score of 4. Such assignments involved only a small proportion of the sample (1.50%) and were made without knowledge of zygosity and twin's smoking status.
For the measure of shared friends, the participants were asked the following: ‘Do you and your twin have the same or different friends?’ Responses were on an ordinal scale ranging from a score of 1 (all shared friends) to 4 (few to no shared friends). As in Loehlin (Reference Loehlin2010), we reverse-scored this item for ease of interpretation. Thus, a score of 1 indicated few shared friends between twins, and a score of 4 indicated complete sharing of friends between twins. To get a shared friend's score for each twin pair, we averaged the two twins’ shared friends’ scores. In the case of a missing shared friend score for one twin, we assigned that twin the score given by the other twin in the pair. If both twins had no score, this pair was excluded from the shared friends portion of the analysis.
Evaluation of Heritability and Gender Differences
All four gender-by-zygosity groups were fitted to a multi-factorial threshold model, which assumed a threshold imposed on an underlying continuous distribution of factors related to liability of the smoking composite. The first question of interest was whether there was a sex difference in the additive genetic and environmental influences of the composite smoking variable that may account for differences in results for male and female twin pairs. Here we tested whether the parameters of an A (additive genetics) C (common environment) E (unique environment) (ACE) model could be equated across genders.
To examine the relationship between smoking and number of friends in common, we followed Loehlin (Reference Loehlin2010) and correlated the absolute value of the difference in pair smoking scores with the measure of common friends. This was done for the composite, initiation, and persistent smoking measures.
All statistical and model fitting analyses were conducted in R version 2.12.0 and OpenMx version 1.0.3-1505 (Boker et al., Reference Boker, Neale, Maes, Wilde, Spiegel, Brick and Fox2011).
Results
For simplicity, we report descriptive and univariate heritability measures for the composite smoking score that includes both components of the initiation and persistence measures.
Gender Differences in Shared Friends and Composite Smoking
Tables 1 and 2 present descriptive statistics for the gender-by-zygosity groups. Gender and zygosity effects were tested using a two-by-two ANOVA with an interaction term. No interactions were significant, so here we report the marginal differences.
TABLE 1 Mean and Standard Deviation on Smoking Composite and Absolute Difference in Smoking Composite Between Twins for the Four Samples

TABLE 2 Mean and Standard Deviation for Mean Shared Friends for the Four Samples
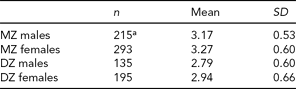
aOne missing value.
Males (M = 1.68, SD = 0.80) had higher smoking composite scores than females (M = 1.57, SD = 0.66), but the difference was only marginally significant, t(837) = 1.95, p = .05. Females (M = 3.14, SD = 0.65) reported more shared friends than males (M = 3.02, SD = 0.59, t(836) = −2.67, p = .008).
Zygosity Differences in Shared Friends and Smoking
Across sex, MZ and DZ twin pairs only marginally differed in their smoking composite scores, with DZ twin pairs (M = 1.68, SD = 0.81) having slightly higher composite scores than MZ twin pairs (M = 1.57, SD = 0.79, t(837) = −2.01, p = .05). However, DZ twin pairs (M = 0.49, SD = 0.76) were significantly more divergent in their smoking behaviors than MZ twin pairs (M = 0.31, SD = 0.60, t(837) = −3.62, p = .0003). MZ twin pairs (M = 3.22, SD = 0.57) also shared significantly more friends than DZ twin pairs (M = 2.87, SD = 0.64, t(836) = 8.03, p < .001).
Heritability of Smoking Composite
Table 3 presents the biometrical genetic model for the smoking composite measure. Thresholds could be equated across twin pair (Δχ2(8) = 3.19, p = .92) and zygosity (Δχ2(4) = 6.23, p = .18), but not gender (Δχ2(2) = 12.95, p = .002). The ACE model with equal parameters across gender did not significantly reduce fit compared with the model that allowed these parameters to vary separately for each sex (Δχ2(3) = 4.61, p = .20). Therefore, analysis was continued jointly for male and female twin pairs. Both the Akaike Information Criterion (AIC) and the likelihood ratio tests suggested that the model containing all three variance components is to be preferred. Compared with a full model, the CE model (Δχ2(1) = 14.46, p < .001) and AE model (Δχ2(1) = 8.98, p = .003) could be rejected convincingly.
TABLE 3 Smoking Composite Model Fit Statistics and Variance Component Estimates With 95% Confidence Intervals for ACE and Nested AE and CE Models

aThreshold values: male thresholds: t1: 0.25, t2: 0.54; female thresholds: t1: 0.48, t2: 0.43.
Differences Between MZ and DZ Correlations
In order to evaluate the possibility of active rGE, we examined the polychoric correlation between absolute differences in smoking between twins and the mean amount of shared friends that each twin reported (Table 4).
TABLE 4 Polychoric Correlations Between Absolute Difference in Twin Pair Smoking Behavior and Average Shared Friends for Composite, Initiation, and Persistence Smoking Measures

aAll correlations met assumption of bivariate normality.
*p < .05.
For the smoking composite, both the correlations for MZ males and MZ females did not significantly differ from 0. However, the DZ correlations were significant for both males and females. Hence, for DZ but not MZ twins, more shared friends predicted greater twin similarity in smoking. However, the difference between the MZ and DZ correlations was not significant for female (Δχ2(1) = 2.44, p = .12), but marginally significant for male (Δχ2(1) = 3.49, p = .06) twin pairs.
For smoking initiation, polychoric correlations between shared friends and differences in smoking for MZ males and females did not significantly differ from 0. Yet for DZ males and females, the correlation between shared friends and absolute difference in smoking initiation was significant. Further, for both males (Δχ2(1) = 6.15, p = .01) and females (Δχ2(1) = 4.48, p = .03), the DZ correlation between smoking and shared friends was significantly stronger than the MZ correlation. Therefore, the initiation phenotype gave the same results as the composite measure.
For smoking persistence, however, the previous pattern of correlations was not observed. Correlations for MZ males (n = 61), MZ females (n = 76), DZ males (n = 44), and DZ females (n = 48) did not significantly differ from 0. Thus, shared friends did not predict similarity in smoking status beyond initiation.
Discussion
From the results, two major findings are highlighted. First, in DZ, but not MZ, twin pairs there was a relationship between the number of shared friends and the similarity of the smoking composite and initiation score. Thus, these results were consistent with the possibility that genetic differences within DZ twin pairs may influence the choice of friends with characteristics that correlate with each twin's unique genetic predispositions, which in turn may explain corresponding differences in the pair's smoking.
Second, when the smoking variable was reduced to include only individuals who had initiated for the smoking persistence part of the analysis, we did not find this same pattern. This was unsurprising, especially given the small sample size of roughly 40 to 80 twin pairs per group. The two items used to define smoking persistence, whether one inhales or smokes before breakfast, may not have been the most optimal or relevant measure of smoking persistence in an adolescent sample (Heatherton et al., Reference Heatherton, Kozlowski, Frecker and Fagerstrom1991). Therefore, our composite smoking measure may more accurately assess initiation rather than smoking persistence, and while our results for initiation may be valid, limitations of the current dataset may be unable to conclude on the effect of shared peers on smoking persistence past the stage of initiation.
However, before substantive interpretation of these findings, it is important to first rule out other mechanisms that could contribute to our observed pattern of correlations.
One potential issue is the possibility that peer influence may violate a cardinal assumption of the twin method, namely, that the correlations in latent, trait-relevant environmental values are equal for MZ and DZ pairs. Similar peer groups for MZ twins may partly arise from the extra attention these twins receive by being together in a group. Kendler and Gardner (Reference Kendler and Gardner1998) evaluated whether this mechanism could play a role for both smoking initiation and nicotine dependence. They reported that twins with higher ‘co-socialization’ scores, a factor based upon items related to how often twins socialized together, resembled each other more with respect to smoking initiation but not nicotine dependence. However, this mechanism would also predict high correlations between peer-group differences and within-pair smoking differences for both MZ and DZ twins. Thus, our results are inconsistent with this being a strong mechanism behind differences in correlations between MZ and DZ twins.
The second mechanism that could influence the relationship between peers’ smoking behavior is passive assortment, or peer associations based on background variables that are correlated with smoking. Family socio-economic status is a clear example. In nationally representative twin samples, simple geography, ethnicity, culture, and religious affiliation may influence both peer similarity and smoking behavior (Degenhardt et al., Reference Degenhardt, Chiu, Sampson, Kessler and Anthony2007). However, neither Loehlin's (Reference Loehlin2010) nor our results were consistent with pure passive assortment as the pre-eminent mechanism for the correlation between self- and peer-drug use behaviors. Friendship groups based on background factors correlated with smoking should be the same for MZ and DZ twins, leading to identical correlations for MZ and DZ twins in peer-group differences and within-pair smoking differences.
Hence, the most likely factors contributing to the observed homogeneity in peer-group smoking are a combination of homophily and, possibly, peer influence. Unfortunately, we could not quantify the precise contribution of each mechanism with the current data because there were no data on smoking for the twin's friends. The data, however, are not consistent with a strong role for peer influence. If peer influence were very important then we should have observed at least a trend toward significance in the correlations between smoking status and peer-group similarity in MZ twins. Yet, with the exception of smoking persistence, all MZ correlations in Table 4 are very close to 0.
Hence, the pattern of results definitely supported homophily as an important mechanism. In contrast to peer influence alone, homophily predicts that peer groups should be more similar for MZ than DZ twins. Hence, our results could be consistent with active rGE as an explanation to why individuals and their peer groups tend to share smoking habits if this homophily is accompanied by peer influence. However, we cannot definitively conclude that both of these requirements for rGE are taking place, given the limitations of a cross-sectional, twin dataset. Future analysis on more expanded datasets that include twins, adoptees, and siblings raised apart may illuminate the specific contribution of rGE, especially with longitudinal data from adolescence until early adulthood.
A further caveat was the age of the participants. The participants were only evaluated at the single time point of juniors in high school (~17 years of age). However, longitudinal analyses have suggested that genetic and environmental components related to peer influences might vary across different ages within the span of early adolescence to young adulthood (Vink et al., Reference Vink, Willemsen, Engels and Boomsma2003; White et al., Reference White, Hopper, Wearing and Hill2003).
In addition, given only a single time point of evaluation, it is important to take into consideration cohort differences in the etiology of smoking behavior. Boardman et al. (Reference Boardman, Blalock and Pampel2010) demonstrated the dynamic nature of heritability for regular smoking behavior across a series of cohorts born in the United States, finding rather negligible genetic influences for those born in the 1940s, the cohort of the National Merit Twins (Loehlin & Nichols, Reference Loehlin and Nichols1976). Boardman et al. (Reference Boardman, Blalock and Pampel2010) reasoned that social pressures might have pushed the popularity of smoking to a level in which genetically vulnerable individuals were no more likely to smoke than those without genetic predispositions toward smoking. Kendler et al. (Reference Kendler, Thornton and Pedersen2000) examined the heritability of regular smoking by birth cohort for males and females separately in Sweden and found that heritability for women was actually greatest for those born after 1940. The reasoning in this case was not so different from Boardman et al.'s (Reference Boardman, Blalock and Pampel2000), but appears to have quite a different effect on the manifestation of genetic influences for women: Decline in the social stigma of women smoking may have allowed women to partake in behaviors aligned with their genetic propensities. Given the evidence of these changes in the etiology of smoking behavior over time, it becomes difficult to generalize both the genetic and peer-influence components of our model to the present era. Therefore, given that we only had the participants at a single age from a single point in time, it is possible that our results illustrated only a snapshot of the true mechanisms by which smoking behavior may be regulated through the influence of genes on peer choice.
Further issues discussed by Loehlin (Reference Loehlin2010) regarding this sample included the ability of the questionnaire to accurately assess the number of shared friends and the key behavior (smoking in this study). Also addressed was the fact that variance in the key behavior may have been restricted by a select sample of high-achieving participants.
Thus, our findings are consistent with Loehlin's (Reference Loehlin2010) for alcohol-related behavior in that there appeared to be a pathway to smoking through both genes and peer groups. However, the contribution of active rGE that requires both genetically based homophily and additional peer influence remains unclear. Yet, given evidence in the literature for the dual contribution of selection and peer influence to smoking behavior (Simons-Morton & Farhat, Reference Simons-Morton and Farhat2010), rGE might be a likely scenario. Thus, future investigations should specifically test for the contribution of rGE using datasets more amenable to quantifying the alternative contributions of unequal twin environments, passive assortment, or peer influence/homophily that may produce similar results.
In conclusion, our results further evidenced the contribution of genetics on exposure to environments that may influence our behaviors. However, given the unclear effect of sex and age on such findings as well as the inconclusive evidence as to the full range mechanisms at work, the next step is to further illuminate both the ways by which rGE might specifically be at work and what other factors may contribute to homogeneity between individuals and their peer groups in smoking behavior. Particularly, gaining a clearer understanding of this association will allow future research to examine ways toward effective prevention of the problems associated with cigarette smoking.
Acknowledgment
Amanda G. Wills has been supported by institutional training grants from the National Institute of Child Health and Human Development (T32 HD007289, Michael C. Stallings) and the National Institute on Drug Abuse (T32 DA017637, John K. Hewitt).






