The simultaneous presence of two fetuses in the uterus exposes both to a higher risk of complications compared to singleton gestations. Specifically, growth restriction and amniotic fluid disorders reflect compromised fetal wellbeing. It is estimated that approximately 29% of twin pregnancies (TP) present with growth restriction, although this is more common in monochorionic (MC) than in dichorionic (DC) twins, affecting 42% and 25% of MC and DC gestations, respectively. Perinatal mortality is three to seven times higher in TP than in singleton pregnancies (SP; Sherer, Reference Sherer2000).
Nevertheless, there is no consensus on the best parameters to use to evaluate the wellbeing of the fetus in TP; specifically, the most accurate way to evaluate the amniotic fluid volume using ultrasound (Schrimmer & Moore, Reference Schrimmer and Moore2002; Sherer, Reference Sherer2000). The most commonly used methods for fetal monitoring in TP (ultrasound, Doppler velocimetry, fetal biophysical profile, fetal movement assessment) have not been evaluated in a prospective, randomized way and stratified according to chorionicity (Sherer, Reference Sherer2000). To date, modalities validated in SP have been extrapolated to evaluate TP, typically disregarding the need to evaluate each fetus separately in multiple pregnancies, particularly in complicated situations such as twin-to-twin transfusion syndrome (TTTS; Schrimmer & Moore, Reference Schrimmer and Moore2002). In addition, there are technical difficulties in estimating the amniotic fluid volume in TP due to the coexistence of two fetuses in the uterus.
Considering the high potential for complications, MC gestations require the most rigorous surveillance in most obstetric services. However, there are insufficient data to determine ideal frequency control periodicity. Sueters et al. (Reference Sueters, Middeldorp, Lopriore, Oepkes, Kanhai and Vandenbussche2006) tested a regime consisting of two ultrasound examinations per week in MC after measuring nuchal translucency, with an emphasis on the evaluation of the amniotic fluid discrepancy, fetal bladder volumes, and Doppler (Sueters et al., Reference Sueters, Middeldorp, Lopriore, Oepkes, Kanhai and Vandenbussche2006). This protocol resulted in timely diagnoses for all cases that were complicated by TTTS. According to the authors, a timely diagnosis was defined as diagnosis before the occurrence of serious complications, such as premature rupture of membranes, delivery between 24 and 32 weeks, fetal hydrops, and intrauterine death (Sueters et al., Reference Sueters, Middeldorp, Lopriore, Oepkes, Kanhai and Vandenbussche2006).
In the absence of methods that offer specific information on fetal wellbeing in TG, fetal urine production rate (FUPR) may provide a functional parameter of fetal (hemodynamics) homeostasis. This could be a novel and promising variable through which to evaluate fetal wellbeing (Peixoto-Filho et al., Reference Peixoto-Filho, Sa, Lopes, Velarde, Marchiori and Ville2007).
Sumie et al. (Reference Sumie, Nakata, Murata, Miwa and Sugino2009) reported two TTTS cases in which hourly FUPR evaluation after laser treatment contributed to a diagnosis of reverse transfusion syndrome. Even before the amniotic fluid volume was altered, the FUPR already exhibited alteration in both fetuses; the new receiver showed an increased FUPR before a larger vertical polyhydramnios pocket was evident. This finding underlines the hypothesis that FUPR can reflect fetal hypo- and hypervolemia more accurately than amniotic fluid measurements do (Sherer, Reference Sherer2000; Sumie et al., Reference Sumie, Nakata, Murata, Miwa and Sugino2009).
The goal of this study was to evaluate a model that better explains the FUPR in TP and to establish reference patterns for the study population.
Methods
A cross-sectional study was performed in 41 consecutive twin pregnancies with gestational ages ranging from 20 to 34 weeks between June 2009 and December 2010. The study population included dichorionic and monochorionic pregnancies with gestational age confirmed by first-trimester ultrasonography with no complications at the time of the ultrasound. Cases with poorly defined bladders and/or VFB2 lower than VFB1 were excluded.
Ultrasound
We began by performing biometric measurements for both fetuses, including biparietal diameter (BPD), head circumference (HC), abdominal circumference, and femur and humerus length. UPR was measured using a 3D US virtual organ computer-aided analysis (VOCAL) system supplied by Medison X8 (Medison, Seoul, Korea). This instrument is equipped with 4-8 MHZ convex electronic volumetric transducer, which enables the acquisition of more than 16 volumes per second. The slowest scan duration (high quality 2) and harmonic tissue imaging were utilized to improve contrast between images of adjacent structures. The 3D volume of fetal bladder (FB) was acquired during fetal rest, preferably in a mid-sagittal position with the belly positioned anteriorly to avoid the spine and pelvic bones. However, due to fetal presentation, this was not possible in all cases. The box volume was adjusted to contain only the FB, with clearly defined limits. Measurements of UPR were performed offline by a single investigator.
The VOCALTM technique was used to obtain a set of six consecutive planes for each FB after sequential rotations of 30o. The internal contour of the FB was determined manually by excluding adjacent structures. All cases with undefined FB limits were excluded.
The UPR (in ml/hour) was estimated during the filling phase using equation 1:
in which, VFB is the FB volume and indices 2 and 1 represent the second and first measurement events, respectively. When VFB2 was smaller than VFB1, the value for VFB2 was assigned to the first measurement and the bladder volume was measured again within 1–5 min.
Statistic Analysis
The results were analyzed using non-parametric tests. Comparisons among gestational ages were made separately in the samples of monochorionic and dichorionic twins using Wilcoxon's test, with a level of 0.05.
Initially, the relationship between UPR and the gestational age was evaluated through graphic representation to identify the model that best represented the relationship between variables. We also tested the model proposed by Peixoto Filho et al. for single gestations immediately after this evaluation; this model was not adapted for TG.
A model was developed using linear regression between the gestational age (GA) and the UPR logarithm (LnFUPR.) Finally, the model was adjusted based on LnFUPR, fetal biometry (femur, biparietal diameter), and the chorionicity to obtain a linear regression analysis that best fit the normal range in the period from 20 to 34 weeks of TP.
The criteria for choosing the best regression were: correlation coefficient (r 2), level of significance (p) and residuals analysis for the estimated curve, accepting a significance level of p < .05. Statistical analyses were performed using SPSS version 12.0.1 (Chicago, IL, USA).
Results
We recruited 41 women with twin pregnancies, of which 10 were monochorionic and 31 were dichorionic, with an average gestational age of 27.9 weeks. After evaluating the FUPR, 11 cases were excluded due to technical difficulties in measuring FB volume, imprecise bladder profile, or the observation of a bladder-emptying period in one or both fetuses. Of the 30 gestations included in the study, 24 were dichorionic, and 6 were monochorionic. The youngest gestational age evaluated was 21 weeks; and the oldest was 34 weeks (mean of 27.6 weeks and standard deviation of 3.9 weeks).
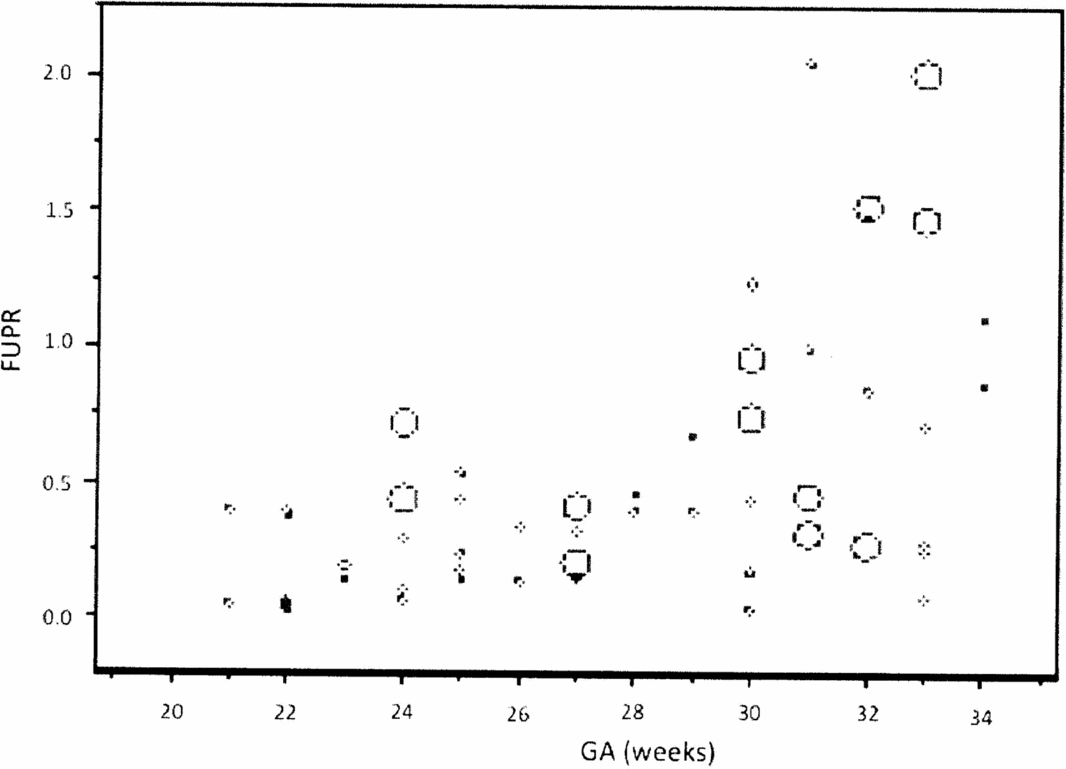
The analysis of the FUPR dispersion graph per gestational age (GA) clearly shows an exponential relationship, and the FUPR variance increases with the increase in GA (Figure 1). To solve both problems, a logarithmic FUPR function was used [Ln(FUPR)] to represent the linear relationship with GA.

FIGURE 1 Graphic representation of the relationship between the fetal urinary production rate (FUPR) and gestational age (GA), showing an exponential distribution.
The FUPR linear regression model proposed by Peixoto Filho et al. (Reference Peixoto-Filho, de Sa, Velarde, Lopes and Ville2011) for single gestations was tested. It was unsuitable, revealing the need to create a specific regression model for twin gestations (Peixoto-Filho et al., Reference Peixoto-Filho, de Sa, Velarde, Lopes and Ville2011). The graphical analysis of the values for FUPR found in our sample of twin pregnancies against the prediction model by gestational age described by Peixoto-Filho in singleton pregnancies demonstrated overestimated values of FUPR for twins. This observation led us to propose a novel prediction model for TPUF by fetal biometry (Peixoto-Filho et al., Reference Peixoto-Filho, de Sa, Velarde, de Castro Mocarzel, Lopes and Ville2013).
Linear regression analyses of FUPR as a function of GA and fetal biometry generated the curves expressed by the following equations and their respective coefficients of correlation (R 2).
The linear regression analysis of LnFUPR with GA generated the curve expressed by equation 2:
 \begin{eqnarray}
&&{\rm GA:}\,{\rm ln(UPR)} = - 4.8201 + 0.1291\nonumber\\ &&\quad\times {\rm GA}({\rm weeks})(R^2 = 0.245p\,{\rm value} < .001)\end{eqnarray}
\begin{eqnarray}
&&{\rm GA:}\,{\rm ln(UPR)} = - 4.8201 + 0.1291\nonumber\\ &&\quad\times {\rm GA}({\rm weeks})(R^2 = 0.245p\,{\rm value} < .001)\end{eqnarray}The linear regression analysis of LnFUPR associated with GA and IG2 generated the curve expressed by equation 3, which represented a weak adjustment;
 \begin{eqnarray}
&&{\rm GA}^2 :\ln \,({\rm UPR}) = - 9.9787 + (0.5111 \times {\rm IG})\nonumber\\
&&\quad- (0.0069 \times {\rm IG}^{\rm 2} )(R^2 = 0.252{\rm }p{\rm value} = .4376)\quad\end{eqnarray}
\begin{eqnarray}
&&{\rm GA}^2 :\ln \,({\rm UPR}) = - 9.9787 + (0.5111 \times {\rm IG})\nonumber\\
&&\quad- (0.0069 \times {\rm IG}^{\rm 2} )(R^2 = 0.252{\rm }p{\rm value} = .4376)\quad\end{eqnarray}The linear regression analysis of LnFUPR associated with biparietal diameter (BPD) generated the curve expressed by equation 4:
 \begin{eqnarray}
&&{\rm Biparietal}\,{\rm diameter}({\rm BPD}):{\rm LnFUPR} = - 5.0121\nonumber\\
&&\quad + 0.0548\times {\rm BPD}({\rm mm})(R^2 = 0.3386p\,{\rm value} < .001)\nonumber\\
&&\end{eqnarray}
\begin{eqnarray}
&&{\rm Biparietal}\,{\rm diameter}({\rm BPD}):{\rm LnFUPR} = - 5.0121\nonumber\\
&&\quad + 0.0548\times {\rm BPD}({\rm mm})(R^2 = 0.3386p\,{\rm value} < .001)\nonumber\\
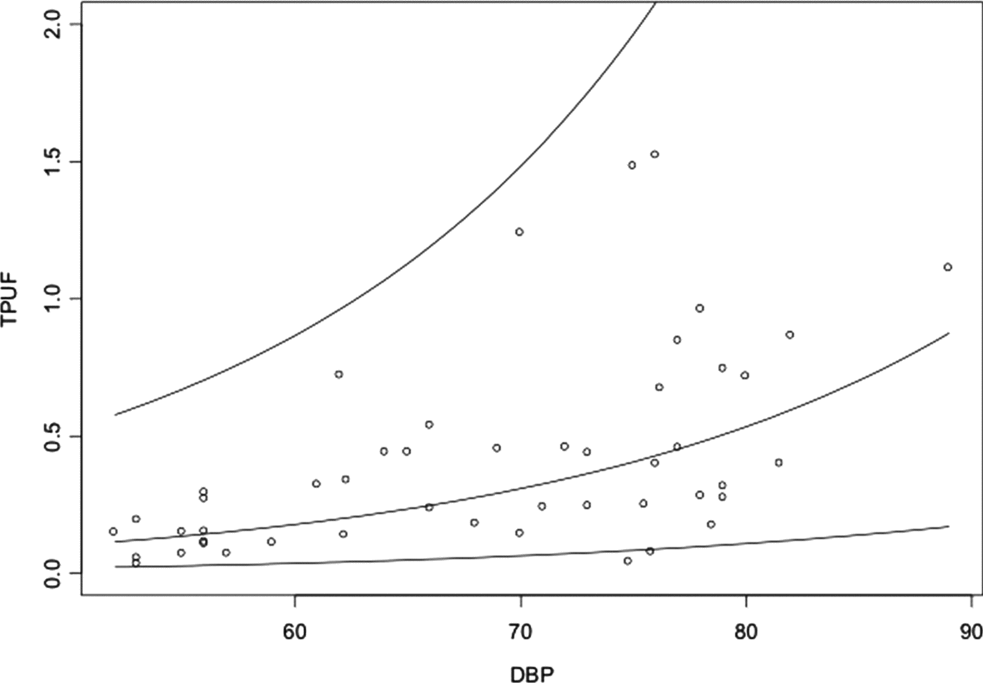
&&\end{eqnarray}This regression model was best adapted for the FUPR analysis in multiple gestations. We chose to follow up the studies of regression models that used BPD (Figure 2).

FIGURE 2 Graphic representation of the relationship between the fetal urinary production rate (FUPR) and biparietal diameter (BPD).
The linear regression analysis of LnFUPR associated with BPD and chorionicity generated the curve expressed by equation 5, with a value of 0 (zero) assigned to monochorionic gestations and a value of 1 assigned to dichorionic gestations. Chorionicity did not significantly affect FUPR.
 \begin{eqnarray}
\hspace*{-5pt}&&{\rm Chorionicity:}\,{\rm LnFUPR} = - 4.9528 + (0.0525 \times {\rm BPD})\nonumber\\
\hspace*{-5pt}&&\hspace*{6pt} + (0.4648 \times {\rm chorionicity) (R}^{\rm 2} {\rm = 0}.3793,\,p\,{\rm value}\quad .0925)\nonumber\\
\hspace*{-5pt}&&\end{eqnarray}
\begin{eqnarray}
\hspace*{-5pt}&&{\rm Chorionicity:}\,{\rm LnFUPR} = - 4.9528 + (0.0525 \times {\rm BPD})\nonumber\\
\hspace*{-5pt}&&\hspace*{6pt} + (0.4648 \times {\rm chorionicity) (R}^{\rm 2} {\rm = 0}.3793,\,p\,{\rm value}\quad .0925)\nonumber\\
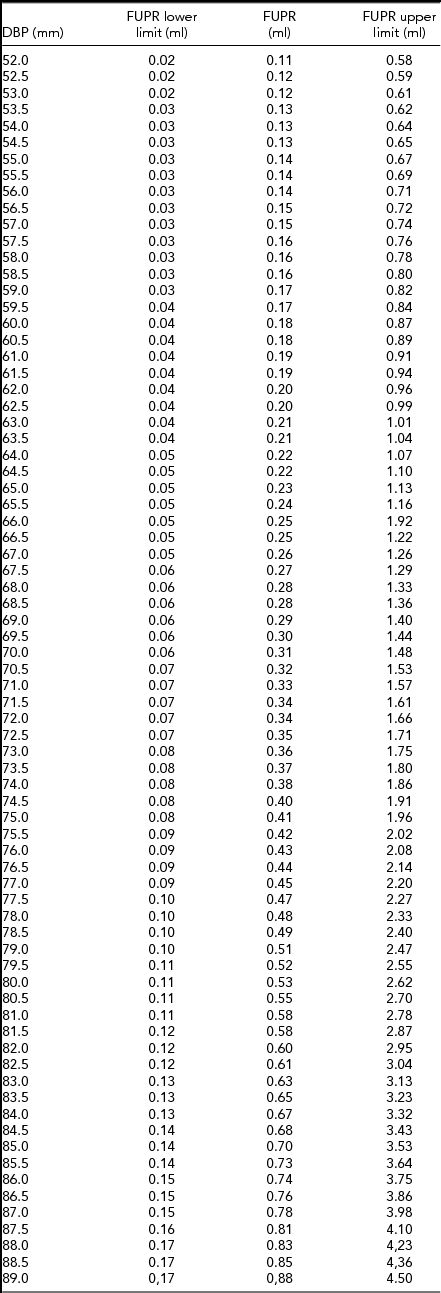
\hspace*{-5pt}&&\end{eqnarray}An FUPR reference table was developed for both fetuses according to the BPD. We observed that for every 1 mm of BPD increase there was 5% increase in FUPR (Table 1).
TABLE 1 Fetal Urinary Production Rate (FUPR) Reference Table According to Fetal Biparietal Diameter (BPD) in Twin Gestations

Discussion
The FUPR was first studied with bidimensional ultrasonography (2D US; Campbell et al., Reference Campbell, Wladimiroff and Dewhurst1973; Hedriana & Moore, Reference Hedriana and Moore1994; Rabinowitz et al., Reference Rabinowitz, Peters, Vyas, Campbell and Nicolaides1989) in models using fetal cadavers; however, the measures overestimated urinary production from 40% to 70%. The 3D Vocal® technology has been evaluated as adequate to determine FUPR in single gestations that presented an intra- and inter-observer correlation with 15°- and 30°-rotation angles (Peixoto-Filho et al., Reference Peixoto-Filho, Sa, Lopes, Velarde, Marchiori and Ville2007).
3D Vocal® technology was used to develop a curve of normal FUPR values in single gestations, initially in populations of Korean and French fetuses (Lee et al., Reference Lee, Park, Shim, Jun, Park and Syn2007; Touboul et al., Reference Touboul, Boulvain, Picone, Levaillant, Frydman and Senat2008). Peixoto Filho et al. (Reference Peixoto-Filho, Sa, Lopes, Velarde, Marchiori and Ville2007) proposed a curve of normal values for the Brazilian population with values ranging from 8.03 to 55.3 ml/h at 24 and 38 weeks, respectively. A comparison of the three curves reveals that the main differences in the values obtained appeared at the end of the third quarter; it is likely that the biometric characteristics of the fetuses contributed to these findings (Lee et al., Reference Lee, Park, Shim, Jun, Park and Syn2007; Peixoto-Filho et al., Reference Peixoto-Filho, Sa, Lopes, Velarde, Marchiori and Ville2007; Touboul et al., Reference Touboul, Boulvain, Picone, Levaillant, Frydman and Senat2008).
The 30°-rotation angle used in our study was based on work published by the same group that evaluated singleton pregnancies (Peixoto-Filho et al., Reference Peixoto-Filho, Sa, Lopes, Velarde, Marchiori and Ville2007). Considering that Yamamoto et al. (Reference Yamamoto, Essaoui, Nasr, Malek, Takahashi, Moreira de Sa and Ville2007) observed that the interval between V1 and V2 acquisitions must be less than those described for single gestations, we chose an interval from 1 to 5 min to avoid capturing the urination phase in one or both.
We are aware of two limitations of this study. In some instances, there were technical difficulties in evaluating vesicle profile due to fetal presentation, which would influence FUPR estimates. Therefore, we excluded those five cases to avoid misinterpretations. The second problem was fetal urination; six cases were excluded in the initial phase of data collection because VFB2 was lower than VFB1.
BPD was the variable that was most closely correlated to FUPR in twin gestation. For every 1 mm of BPD increase, the FUPR increased by 5%. The GA was not found to be an adequate variable in these gestations. This may be justified by the growth pattern of multiple gestations that, as of 30 weeks, tend to exhibit a slower pace of weight gain than that observed in single gestations (Blickstein, Reference Blickstein2004). In general, twins are born at weights that are 15–20% less than those of singletons born at the same gestational age. We must emphasize that TG by itself, particularly MC, is frequently associated with clinical complications that can lead to growth deficits in one or both fetuses. Thus, when the GA of the fetuses is considered separately, the fetuses could present FUPR values similar to fetuses with normal growth and the same GA.
Chorionicity did not influence the FUPR, which enabled the construction of a reference standard for MC and DC. This observation should motivate closer studies of placental hemodynamics; if the production of urine is not affected by the sharing of the same placenta mass, then factors other than chorionicity must have an influence in cases of oligohydramnios and/or polyhydramnios.