The use of medicines outside of licensed indications - so-called ‘off-label prescribing’ - is common in general medicine Reference Radley, Finkelstein and Stafford1 and even more frequent in psychiatry where it involves agents from across the psychotropic spectrum. 2-Reference Hodgson and Belgamwar4 It typically involves four main categories (the four Ds) relating to the disorder treated, patient demographics, dose and duration of treatment. Reference Baldwin and Kosky5
Off-label prescribing represents a continuum of behaviour from deliberate, targeted activity where some supporting evidence of effectiveness is the motivation for use (e.g. combination antidepressant therapy) v. inadvertent off-label activity where the prescriber is unaware that the practice is not licensed. Although the use of agents for licensed indications does not guarantee efficacy or safety, use beyond licensed indications does not imply an absence of evidence and much off-label prescribing is advocated within evidence-based guidelines. Reference Taylor, Paton and Kerwin6 Moreover, off-label prescribing allows for innovative practice that is targeted to the specific needs of an individual. Reference Stafford7 However, studies have highlighted the risks associated with off-label prescribing Reference Jonville-Béra, Béra and Autret-Leca8 emphasising that it is frequently not supported by a robust evidence base. Reference Radley, Finkelstein and Stafford1 In view of these concerns, the Royal College of Psychiatrists has made recommendations to guide practice involving unlicensed use of agents in psychiatric practice. 2 We examined the prevalence of off-label prescribing for a common psychiatric condition (recurrent depressive disorder) in a community mental health service. Relevant participant and illness factors were also explored.
Method
The study was conducted in the St Anne's Community Mental Health Service, which provides a general adult psychiatry service for a catchment area of approximately 50 000 in south-east Limerick. To determine the frequency and clinical factors relevant to unlicensed use of psychotropic agents in people with recurrent depressive disorder, we collected data from the files of all individuals attending over a 3-day period in autumn 2006 regarding demographics, prescribing practice, patterns of clinic attendance (duration, frequency of attendance and missed appointments), and multidisciplinary team contacts (including duration since last contact with responsible consultant psychiatrist). Shared care was defined as attendance with two or more multidisciplinary team members. Diagnoses were made according to ICD-10 criteria by a single consultant psychiatrist (D.M.). 9 The characteristics of the service and population have been described in detail previously. Reference Meagher, O'Brien, Pullela, Oshun and Brosnan10
Health and social functioning were assessed with the Health of the Nation Outcome Scales (HoNOS). Reference Wing, Beevor, Curtis, Park, Hadden and Burns11 These assessments were also made by a single clinician (D.M.) to avoid issues with interrater reliability. Reference Bebbington, Brugha, Hill, Marsden and Window12 The HoNOS has twelve items and four subscales that rate behaviour, impairment, symptoms and social functioning.
Five categories of off-licence prescribing in individuals with recurrent depressive disorder without a history of psychosis were examined: high-dose antidepressant treatment (i.e. beyond doses provided within the British National Formulary 13 ); antidepressant polypharmacy; use of antipsychotic agents; maintenance prescribing (>4 weeks) of benzodiazepine agents; and prescribing outside of the recommended age range. In addition, the responsible consultant psychiatrist was asked to review all cases of off-label prescribing and indicate whether this was part of a deliberate and documented treatment plan or undesirable/inadvertent practice.
Statistical analysis was conducted using SPSS 14 for Windows. We compared individuals receiving off-label prescribing with those who were not, individuals with deliberate v. inadvertent off-label prescribing, and specific aspects of off-label prescribing (receiving v. not-receiving). Independent t-tests (age), Mann-Whitney U-tests (HoNOS scores, duration and frequency of attendance/non-attendance, duration since last review by the Royal College of Psychiatrists) and chi-squared tests (gender, frequency of shared care) were used.
Results
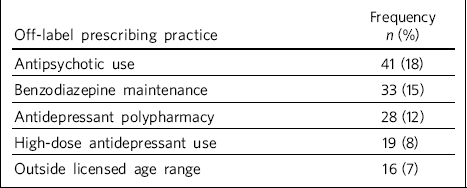
Of the 226 people with recurrent depressive disorder attending the clinic, 87 were treated with off-label prescribing of some sort (38%), involving 137 instances of off-label prescribing (Table 1). High-dose use (>225 mg) of venlafaxine extended release accounted for all high-dose antidepressant use (19 individuals). Antidepressant polypharmacy consisted mostly (80%; 23/29) of mirtazapine combined with either venlafaxine (n = 19) or duloxetine (n = 4). The use of quetiapine accounted for over 50% (21/41) of antipsychotic use, of which 17 people were prescribed a dose of 100 mg a day or less. Other atypicals accounted for most (17/20) of the remainder. Out-of-age-range prescribing involved 2 individuals younger than 18 years and 14 over 65 years of age. Thirty-three people were treated with several types of off-label prescribing simultaneously (range 1-4).
Table 1 Frequency of various off-label prescribing practices (n = 226)
| Off-label prescribing practice | Frequency n (%) |
|---|---|
| Antipsychotic use | 41 (18) |
| Benzodiazepine maintenance | 33 (15) |
| Antidepressant polypharmacy | 28 (12) |
| High-dose antidepressant use | 19 (8) |
| Outside licensed age range | 16 (7) |
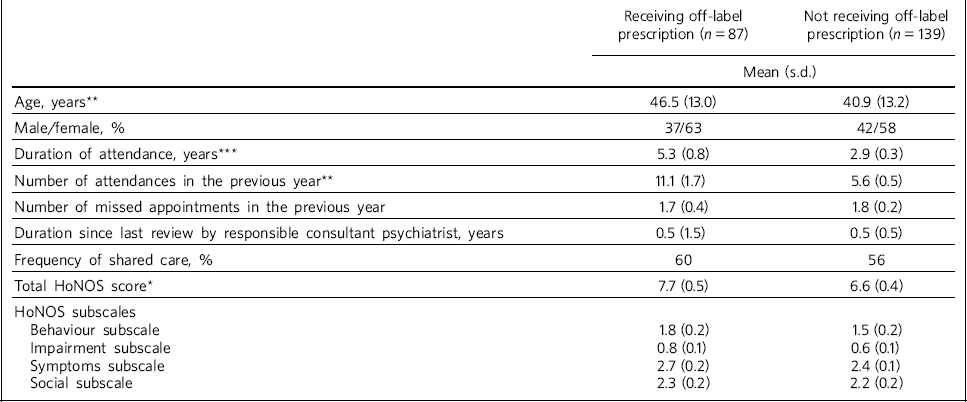
Characteristics of those receiving off-label prescriptions are compared with the remaining individuals with recurrent depressive disorder inTable 2. Significant differences were found in relation to older age, longer duration of attendance and more frequent attendance in the off-label prescribing group, as well as higher total HoNOS scores. There were no significant differences in relation to gender, frequency of non-attendance, HoNOS subscale scores, frequency of shared care and duration since last review by the responsible consultant psychiatrist.
Table 2 Comparison of demographic, service and clinical characteristics of those receiving and those not receiving off-label prescribing
| Receiving off-label prescription (n = 87) | Not receiving off-label prescription (n = 139) | |
|---|---|---|
| Mean (s.d.) | ||
| Age, years** | 46.5 (13.0) | 40.9 (13.2) |
| Male/female, % | 37/63 | 42/58 |
| Duration of attendance, years*** | 5.3 (0.8) | 2.9 (0.3) |
| Number of attendances in the previous year** | 11.1 (1.7) | 5.6 (0.5) |
| Number of missed appointments in the previous year | 1.7 (0.4) | 1.8 (0.2) |
| Duration since last review by responsible consultant psychiatrist, years | 0.5 (1.5) | 0.5 (0.5) |
| Frequency of shared care, % | 60 | 56 |
| Total HoNOS score* | 7.7 (0.5) | 6.6 (0.4) |
| HoNOS subscales | ||
| Behaviour subscale | 1.8 (0.2) | 1.5 (0.2) |
| Impairment subscale | 0.8 (0.1) | 0.6 (0.1) |
| Symptoms subscale | 2.7 (0.2) | 2.4 (0.1) |
| Social subscale | 2.3 (0.2) | 2.2 (0.2) |
When we considered the relationship between specific aspects of off-label prescribing and participant characteristics, we found that in comparison with the other participants with recurrent depressive disorder those individuals treated with polypharmacy were significantly older (P = 0.01) and had a higher frequency of attendance (P = 0.02). Antipsychotic use was associated with a longer duration (P = 0.006), higher frequency of attendance (P<0.001) and higher total HoNOS and behaviour subscale scores (both P = 0.02). The prescription of maintenance benzodiazepines was more common in females (P = 0.03) and was associated with a longer duration (P = 0.02), higher frequency of attendance (P = 0.02), and higher total HoNOS (P = 0.01) and behaviour subscale scores (P = 0.008). High-dose antidepressant treatment and use of antidepressants beyond the appropriate age category were not associated with a significant difference in any of the parameters.
Off-label prescribing was further divided into deliberate (n = 91; 68%) v. undesirable/inadvertent (n = 46) according to whether the practice was part of a documented treatment plan. Forty-four people had at least one aspect of prescribing that was inadvertently off label and this subgroup is compared with those who had exclusively deliberate off-label prescribing in Table 3. These subgroups were largely similar in relation to demographic and clinical variables but with significantly higher scores for total HoNOS (P = 0.03) and a trend for greater behavioural problems (P = 0.07) in the inadvertent group.
Table 3 Comparison of demographic, service and clinical characteristics of those receiving deliberate and inadvertent off-label prescribing

| Deliberate off-label prescribing (n = 43) | Inadvertent off-label prescribing (n = 44) | |
|---|---|---|
| Mean (s.d.) | ||
| Age, years | 44.7 (11.7) | 48.3 (14.1) |
| Male/female, % | 25/18 | 30/14 |
| Duration of attendance, years | 5.1(6.9) | 5.5 (7.1) |
| Number of attendances in the previous year | 9.8 (9.9) | 13.1 (10.8) |
| Number of missed appointments in the previous year | 1.8 (2.5) | 1.5 (1.5) |
| Duration since last review by responsible consultant psychiatrist, years | 0.4 (1.5) | 0.7 (1.2) |
| Frequency of shared care, % | 58 | 62 |
| Total HoNOS score* | 6.7 (4.9) | 8.6 (4.3) |
| HoNOS subscales | ||
| Behaviour subscale, | 1.4 (1.5) | 2.2 (2.0) |
| Impairment subscale | 0.6 (1.1) | 1.0 (1.4) |
| Symptoms subscale | 2.5 (1.4) | 2.9 (1.4) |
| Social subscale | 2.2 (2.0) | 2.5 (1.9) |
Discussion
Frequency of off-label prescribing
This study focused on five aspects of off-label prescribing for the commonest clinical diagnosis (recurrent depressive disorder) occurring in people attending a community mental health service. We found that off-label prescribing was common and related to a range of patient demographic and clinical variables. It was frequently part of a documented and deliberate treatment plan but in a significant minority of cases reflected inadvertent or undesirable practice.
Previous work in this area has focused on prescribing in acute in-patient settings and/or the use of specific drugs in particular populations. Douglas-Hall and colleagues Reference Douglas-Hall, Fuller and Gill-Banham14 studied prescribing patterns in acute psychiatric in-patients from 14 National Health Service trusts and identified that 7.5% of prescriptions involved off-label use of drugs, whereas Hodgson & Belgamwar Reference Hodgson and Belgamwar4 found that use of antipsychotics was off label in 40% of those treated within specialist psychiatry services. European studies in community-based settings suggest that one-half to two-thirds of antipsychotic use is off label. Reference Barbui, Ciuna, Nosé, Patten, Stegagno and Burti15,Reference Weiss, Hummer, Koller, Ulmer and Fleischhacker16 Similarly, in one study from the USA off-label prescribing in combat veterans comprised 43% of atypical antipsychotic use. Reference Rosenheck, Leslie and Sernyak17
Factors associated with off-label prescribing
Diagnostic factors are a key determinant of drug use, such that the licensed indications are often dictated by the initial use of an agent even though evidence can emerge for use in other unlicensed indications. Innovation in pharmacotherapy requires wider clinical use but often remains off label because there is limited benefit for manufacturers to go through the onerous process of seeking additional licensed indications, especially with agents that have a limited remaining duration of patent. As such, a common disorder such as recurrent depressive disorder that is prone to treatment resistance and often requires innovative approaches to pharmacotherapy commonly results in off-label use of drugs. Leslie and colleagues found that common diagnoses for off-label use of antipsychotics were post-traumatic stress disorder (42%) and minor (39%) or major (23%) depression. Reference Leslie, Mohamed and Rosenheck18
A number of previous studies have identified higher rates of off-label prescribing in older age groups, Reference Chen, Reeves, Fincham, Kennedy, Dorfman and Martin19,Reference Kamble, Sherer, Chen and Aparasu20 perhaps reflecting greater treatment resistance or more chronic illness. In our study we also found a positive correlation between older age and off-label prescribing in general and specifically in relation to the frequency of polypharmacy. The increased frequency of these practices in older people is a cause for concern because associated physical risks are more pronounced in older individuals.
A strength of this work is the inclusion of a measure of symptom profile within the diagnostic category of recurrent depressive disorder. Previous work has focused on diagnostic categories as predictors of off-label prescribing but without consideration of the role of particular clinical variants within these categories. Reference Hodgson and Belgamwar4,Reference Leslie, Mohamed and Rosenheck18 Other work linked particular practices such as polypharmacy to illness severity. Reference Mojtabai and Olfson21 The observed cross-sectional associations do not imply causality as effective use of off-label prescribing is likely to diminish symptoms, although some of the observed associations are symptoms that could conceivably be caused by off-label prescribing (e.g. paradoxical disinhibition with benzodiazepines). Nevertheless, it was apparent that off-label prescribing occurred more frequently in individuals with a greater burden of clinical problems (as per the HoNOS) and that, in particular, off-label use of antipsychotics and benzodiazepines was related to higher scores on the behavioural subscale of the HoNOS that includes problems with agitation, deliberate self-harm and substance misuse. It seems more likely that the use of these agents represents a response to more complex presentations of depressive illness that are complicated by behavioural disturbances and/or comorbid substance misuse.
Categories of off-label prescribing
The relative frequency of different aspects of off-label prescribing is uncertain but is likely to vary considerably according to treatment settings and populations studied. In a postal survey of psychiatrists, Lowe-Ponsford & Baldwin found that off-label prescribing was as a result of use of agents in diagnoses without a licensed indication in 50% of cases, with variations in dose (19%) and age (12%) also common. Reference Lowe-Ponsford and Baldwin22 Our work highlights a broad range of reasons for prescribing off label within a specific diagnostic category, reflecting the particular challenges of individuals with comorbid anxiety and treatment-resistant illness where high-dose or combination/augmentation strategies were applied.
Antipsychotics
The use of antipsychotic agents in the management of depressive illness has become increasingly popular in recent years as a result of a gathering evidence base. Nelson & Papakostas conducted a meta-analysis of 16 randomised controlled trials of atypical antipsychotics’ use in treatment-resistant, non-psychotic, unipolar major depression and found significant benefits compared with placebo but with higher discontinuation rates due to adverse effects. Reference Nelson and Papakostas23 Hodgson & Belgamwar found that 18% of off-label use antipsychotics in secondary care attenders in north Staffordshire was for affective disorder diagnoses. Reference Hodgson and Belgamwar4 Similarly, Leslie et al Reference Leslie, Mohamed and Rosenheck18 studied off-label use of antipsychotics in the US Department of Veteran Affairs healthcare system and found that affective disorders and post-traumatic stress disorder were the commonest diagnoses associated with 23% of off-label prescribing in people with major depressive disorder. Doses were typically lower than those suggested for psychotic illness.
The prevalence of off-label use of antipsychotics in our cohort was similar (18%) to these studies. We were also able to document the agents used, which included the use of low-dose quetiapine as an hypnotic as well as antipsychotic augmentation for more resistant illness.
Antidepressant polypharmacy and use in high dose
Although the use of antidepressants in combination is not highlighted as unlicensed use in the Royal College of Psychiatrists report, 2 it is not included within licensed indications and warrants the same approach to patient information and documentation of risk/benefit ratio as other practices with uncertain prescribing status. Moreover, although there is a gathering literature to support such strategies in treatment-resistant illness, they can convey an increased potential for adverse drug interactions. Future guidelines regarding the use of agents beyond licensed indications would benefit from inclusion of such practices. Previous studies have not explored the frequency of such practice but the noradrenergic and specific serotonergic antidepressant/serotonin and noradrenaline reuptake inhibitor combination is a particular strategy that has been used to effect in our service. Reference Meagher, Hannan and Leonard24,Reference Hannan, Hamzah, Akinpeloye and Meagher25
Similarly, high-dose venlafaxine treatment is supported by an evidence base and reflects deliberate prescribing. Reference Thase, Shelton and Khan26 However, the licensed dose range for the slow-release form has only recently been increased to that of the regular venlafaxine. The slow-release form is preferred within our service due to superior tolerability but because of this licensing issue it was technically off label. Recent increases in the licensed dose range for the slow-release form means that much of the high-dose treatment documented in this work would no longer be considered off label, highlighting the evolving nature of licensed prescribing.
Benzodiazepines
Benzodiazepines are licensed for the short-term treatment of anxiety but are not advocated in longer-term treatment or in mood disorders because of the risks of dependence, daytime sedation, cognitive impairment and falls. The reduction of benzodiazepine use has been a specific target within the St Anne's service for many years, Reference Raju and Meagher27-Reference Meagher, Pullela, Meisinger, Geaney and O'Brien29 such that the documented rate in this study may be lower than elsewhere. However, similar to others, Reference Haw and Stubbs30 we have found that for a small percentage of individuals maintenance use of benzodiazepines persists despite our best efforts to manage with other approaches. Maintenance benzodiazepine use occurred in individuals with higher scores on the behavioural subscale of the HoNOS, perhaps indicating that benzodiazepines are being used for more complex and challenging presentations of recurrent depressive disorder.
Management of off-label prescribing
The high rate of off-label prescribing (38% of individuals), with over two-thirds of cases indentified as deliberate and goal directed reflects the shortcomings of licensing norms in addressing the needs of real-world patients. However, although much off-label prescribing was targeted, there remained a considerable amount of inadvertent practice, highlighting the need for continued vigilance and education about such practices. In contrast, Radley et al Reference Radley, Finkelstein and Stafford1 studied treatment patterns for 160 commonly prescribed drugs with the National Disease and Therapeutics Index and found that off-label prescribing comprised 21% of prescribing and that the vast majority (96%) of off-label use of psychotropics had little or no scientific support.
Royal College of Psychiatrist guidelines suggest that where off-label prescribing occurs it should be closely monitored. 2 It was reassuring that people receiving off-label prescribing attended more frequently and did not have a higher frequency of missed appointments. This may indicate an elevated level of monitoring of off-label prescribing and/or more frequent attendance because of higher morbidity levels in those receiving off-label prescribing. More frequent attendance in those receiving maintenance benzodiazepines may reflect a reluctance of clinicians to provide long-term prescriptions or greater motivation for clinic attendance because of the addictive qualities of these agents. Whatever the reason, greater supervision of such individuals is highly desirable.
Limitations
Although many aspects of the St Anne's service make it a relatively representative population of individuals attending a community-based service, the population have previously participated in studies focused on the quality of prescribing practices, including the use of benzodiazepines, high-dose medications and polypharmacy. As a consequence, there may be a disproportionate awareness among clinicians of off-label prescribing in this service. Reference Raju and Meagher27–Reference Meagher, Pullela, Meisinger, Geaney and O'Brien29 In addition, the inclusion of clinical ratings is a valuable aspect of this work but the cross-sectional design of the study limits the interpretation of HoNOS scores such that they may not be causally related to prescribing practice. Future work addressing the clinical and economic effectiveness of off-label prescribing can further illuminate this issue.
Acknowledgements
We thank the Community Mental Health Team at St Anne's Day Hospital, all of whom contributed to data collection and analysis.






eLetters
No eLetters have been published for this article.