Published online by Cambridge University Press: 30 November 2005
The heating rate effects in simulated liquid Al2O3 have been investigated by Molecular Dynamics (MD) method. Simulations were done in the basic cube under periodic boundary conditions containing 3000 ions with Born-Mayer type pair potentials. The temperature of the system was increasing linearly in time from the zero temperature as $T(t)=T_0 +\gamma t$ 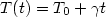 , where $\gamma $
, where $\gamma $ 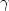 is the heating rate. The heating rate dependence of density and enthalpy of the system was found. Calculations show that static properties of the system such as the coordination number distributions and bond-angle distributions slightly depend on $\gamma $
is the heating rate. The heating rate dependence of density and enthalpy of the system was found. Calculations show that static properties of the system such as the coordination number distributions and bond-angle distributions slightly depend on $\gamma $ 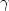 . Structure of simulated amorphous Al2O3 model with the real density at the ambient pressure is in good agreement with Lamparter's experimental data. The heating rate dependence of dynamics of the system has been studied through the diffusion constant, mean-squared atomic displacement and comparison of partial radial distribution functions (PRDFs) for 10% most mobile and immobile particles with the corresponding mean ones. Finally, the evolution of diffusion constant of Al and O particles and structure of the system upon heating for the smallest heating rate was studied and presented. And we find that the temperature dependence of self-diffusion constant in the high temperature region shows a crossover to one which can be described well by a power law, $D\propto (T-T_c )^\gamma $
. Structure of simulated amorphous Al2O3 model with the real density at the ambient pressure is in good agreement with Lamparter's experimental data. The heating rate dependence of dynamics of the system has been studied through the diffusion constant, mean-squared atomic displacement and comparison of partial radial distribution functions (PRDFs) for 10% most mobile and immobile particles with the corresponding mean ones. Finally, the evolution of diffusion constant of Al and O particles and structure of the system upon heating for the smallest heating rate was studied and presented. And we find that the temperature dependence of self-diffusion constant in the high temperature region shows a crossover to one which can be described well by a power law, $D\propto (T-T_c )^\gamma $ 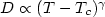 . The critical temperature T c is about 3500 K and the exponent $\gamma $
. The critical temperature T c is about 3500 K and the exponent $\gamma $ 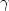 is close to 0.941 for Al and to 0.925 for O particles. The glass phase transition temperature T g for the Al2O3 system is at anywhere around 2000 K.
is close to 0.941 for Al and to 0.925 for O particles. The glass phase transition temperature T g for the Al2O3 system is at anywhere around 2000 K.