Although deficits in episodic memory characterise Alzheimer's disease, there is increasing evidence that working memory is also impaired at the earliest stages of the disease. Reference Huntley and Howard1 Working memory refers to the ability to hold and manipulate information over short periods and is essential for the successful performance of many cognitive processes. Working memory deficits in Alzheimer's disease have been associated with difficulties in everyday tasks, and executive control of working memory is particularly sensitive to the effects of the disease. Reference Belleville, Chertkow and Gauthier2 Strategic encoding of information is critical to working memory performance and learning. There is some evidence that strategic encoding is impaired at the mild stage of Alzheimer's disease but may be preserved at an earlier ‘minimal’ stage of the disease. Reference Germano, Kinsella, Storey, Ong and Ames3
Chunking is an effective form of strategic encoding that involves the recoding of a set of data into a compressed, efficient form and can extend working memory capacity in healthy individuals. Reference Bor, Duncan, Wiseman and Owen4 A series of verbal and spatial working memory tasks have been developed that present structured sequences to encourage the reorganisation of information into higher-level chunks. Studies have demonstrated that structured stimuli significantly encourage chunking, lessening working memory demand and significantly improving working memory performance. Reference Bor, Duncan, Wiseman and Owen4,Reference Bor, Cumming, Scott and Owen5 Training in the use of chunking strategies can lead to significant increases in working memory capacity. Reference Ericcson, Chase and Faloon6 Studies of cognitive training using working memory tasks in healthy young people have demonstrated significant improvements on measures of working memory and general cognitive function, and chunking has been postulated to be a major strategy underlying these successful cognitive training regimens. Reference Olesen, Westerberg and Klingberg7,Reference McNab, Varrone, Farde, Jucaite, Bystritsky and Forssberg8 It is not known, however, whether the ability to use chunking within working memory is preserved at the early stages of Alzheimer's disease. This is important, as impairment in the use of chunking may contribute to the reduction of working memory capacity noted as the disease progresses to the mild to moderate stage. Further, preserved chunking ability would provide a potential therapeutic target for cognitive training among people with Alzheimer's disease.
In this study we investigated the use of chunking strategies to improve working memory performance in people with early Alzheimer's disease. Given the evidence for preserved strategic encoding at the very mild (minimal) stage of the disease but not at the mild stage, we hypothesised that both healthy elderly participants and patients at the very mild stage of Alzheimer's disease, defined as a score above 24 on the Mini-Mental State Examination (MMSE), Reference Folstein, Folstein and McHugh9 would be able to use chunking strategies to improve working memory capacity, but patients with mild Alzheimer's disease (MMSE score 18–24) would be impaired in the use of chunking strategies and therefore show no improvement in working memory capacity when given opportunities to use this strategy.
Method
Twenty-eight patients with Alzheimer's disease were recruited from the dementia case register and community mental health team (CMHT) caseloads of the South London and Maudsley National Health Service Foundation Trust. Prior to recruitment, a clinical diagnosis of Alzheimer's disease was made according to ICD-10 criteria 10 by a senior consultant psychiatrist in conjunction with a multidisciplinary team assessment. Patients also fulfilled the criteria of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association (NINCDS-ADRDA Alzheimer's Criteria) for probable or possible Alzheimer's disease. Reference McKhann, Drachman, Folstein, Katzman, Price and Stadlan11 All patients had demonstrated at least 2 years of deterioration in memory and some impairment of activities of daily living. Ten of 13 of the ‘very mild’ group and 6 of 15 of the ‘mild’ group were recruited from the dementia case register or had been assessed previously by researchers in the old age psychiatry department at the Institute of Psychiatry, London, and a diagnosis of Alzheimer's disease had been made prior to the study using a range of cognitive assessments, including the cognitive subscale of the Alzheimer's Disease Assessment Scale and the Logical Memory II subtest of the Wechsler Memory Scale. Reference Rosen, Mohs and Davis12,Reference Wechsler13 The remaining 3 participants in the ‘very mild’ group and 9 participants in the ‘mild’ group had all been under the care of the CMHT for at least 2 years and demonstrated progressive impairment in memory, word-finding difficulties or executive deficits and impaired activities of daily living. All participants in the two disease groups had neuroimaging (computed tomography or magnetic resonance imaging) consistent with a diagnosis of Alzheimer's disease. Patients were excluded if there was a history of head injury, cerebrovascular event, epilepsy, other major medical illness, depression or psychosis. A control group of 15 healthy individuals matched for age, gender and years of education was also recruited. Data recorded for the sample included scores on the Geriatric Depression Scale, Reference Yesavage, Brink, Rose, Lum, Huang and Adey14 years of education, IQ score derived from the National Adult Reading Test, Reference Nelson and Willison15 and MMSE score. All participants provided written informed consent before taking part in the study, which had been approved by the Bexley and Greenwich research ethics committee.
Tasks
Two working memory span tasks were used. These were based on tasks used in previous studies investigating chunking in normal participants, Reference Bor, Duncan, Wiseman and Owen4,Reference Bor, Cumming, Scott and Owen5 and adapted for use in people with Alzheimer's disease.
Digit span task
In the digit span task a sequence of digits to be memorised appeared on a computer screen. On each trial each digit was presented for 1000 ms with a 500 ms interval between digits. At the end of the sequence a command to recall prompted the participant to recall the sequence verbally in the correct order. The recalled sequence was then typed into the computer by the experimenter, and accuracy data were collected. Trials began with a three-digit sequence and either increased by one digit if no error was made or decreased by one digit following an unsuccessful trial. In this way, sequence length tended to oscillate around the participant's maximum span capacity. In each case, average span capacity was calculated as the mean length of sequences presented in all trials. Five practice trials were given in all cases, and a total of 20 structured and 20 unstructured trials were presented in counterbalanced blocks. In the structured trials sequences to be learned were presented as runs of ascending or descending adjacent even or odd numbers, thus encouraging the recoding of digit sequences into higher-order chunks. Unstructured sequences followed no such pattern and were designed to be as random as possible (see Appendix). Previous studies have demonstrated that such structured sequences significantly encourage chunking owing to the mathematical associations inherent in structured stimuli. Reference Bor and Owen16
Spatial span task
Participants performed a variation of Corsi's spatial span task, Reference Milner17 in which they were required to memorise sequences of locations illuminated on a 4 × 4 grid, presented on a touch-sensitive screen. Specifically, on each trial a sequence of red squares flashed blue, each square changing colour for 1000 ms with a 500 ms interval between squares. At the end of the sequence a short tone prompted participants to respond by touching the same series of locations with the index finger of their dominant hand. Accuracy and reaction time data were collected, although the participants were only instructed to reproduce the sequences as accurately as possible. At least ten unstructured practice trials were performed to ensure participants were able to perform the task. Twenty structured and 20 unstructured trials were then performed in counterbalanced blocks. Trials began with a three-location sequence and then either increased by one square following a successful trial or decreased by one square following an unsuccessful trial. In this way, sequence length tended to oscillate around the participant's maximum span capacity. In each case, average span capacity was calculated as the mean length of sequences presented in all 20 trials.
In the structured condition, all of the sequences followed a structured rule such that every location was in either the same column, row or diagonal as the location preceding it. In the unstructured condition two successive locations were never in the same column, row or diagonal. The result of this manipulation was subtle, in that the structured sequences tended to contain more familiar shape components, involving symmetry and parallel sides, and were thus more easily organised into higher-level patterns (see Appendix,Fig. A1). Previous studies of normal individuals have demonstrated that spatially structured trials encourage chunking into higher-level patterns. Reference Bor, Duncan, Wiseman and Owen4
Statistical analysis
General linear model repeated-measures analysis was used, with structured and unstructured scores as within-participant variables and group as a between-participant variable.
Results
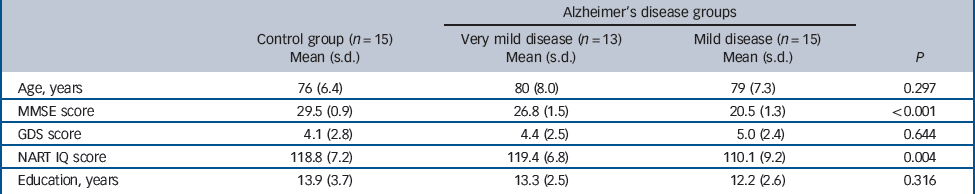
Demographic information on all participants is shown inTable 1. One-way analyses of variance (ANOVAs) demonstrated no significant difference in age, Geriatric Depression Scale score or years of education between the groups. There was a significant difference in IQ scores between the mild disease group and the control and very mild disease groups. The potential significance of this difference between groups is discussed below.
Table 1 Demographic information
| Alzheimer's disease groups | ||||
|---|---|---|---|---|
| Control group (n = 15) Mean (s.d.) | Very mild disease (n = 13) Mean (s.d.) | Mild disease (n = 15) Mean (s.d.) | P | |
| Age, years | 76 (6.4) | 80 (8.0) | 79 (7.3) | 0.297 |
| MMSE score | 29.5 (0.9) | 26.8 (1.5) | 20.5 (1.3) | <0.001 |
| GDS score | 4.1 (2.8) | 4.4 (2.5) | 5.0 (2.4) | 0.644 |
| NART IQ score | 118.8 (7.2) | 119.4 (6.8) | 110.1 (9.2) | 0.004 |
| Education, years | 13.9 (3.7) | 13.3 (2.5) | 12.2 (2.6) | 0.316 |
Task performance between groups
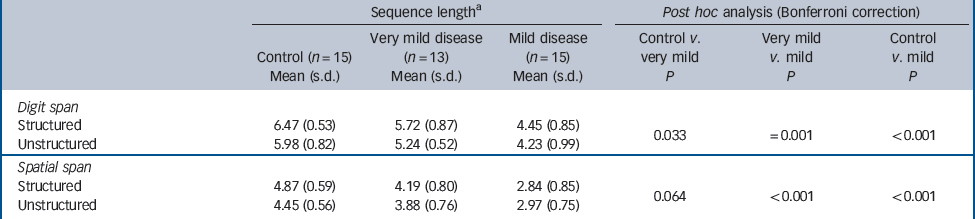
The participants’ performance on the two tasks is summarised inTable 2.
Table 2 Performance on span tasks
| Sequence lengtha | Post hoc analysis (Bonferroni correction) | |||||
|---|---|---|---|---|---|---|
| Control (n = 15) Mean (s.d.) | Very mild disease (n = 13) Mean (s.d.) | Mild disease (n = 15) Mean (s.d.) | Control v. very mild P | Very mild v. mild P | Control v. mild P | |
| Digit span | ||||||
| Structured | 6.47 (0.53) | 5.72 (0.87) | 4.45 (0.85) | 0.033 | =0.001 | <0.001 |
| Unstructured | 5.98 (0.82) | 5.24 (0.52) | 4.23 (0.99) | |||
| Spatial span | ||||||
| Structured | 4.87 (0.59) | 4.19 (0.80) | 2.84 (0.85) | 0.064 | <0.001 | <0.001 |
| Unstructured | 4.45 (0.56) | 3.88 (0.76) | 2.97 (0.75) | |||
Digit span task
General linear model repeated-measures analysis revealed a main effect of group (F = 24.8, P<0.001). Post hoc Bonferroni analysis demonstrated a significant difference between all groups (control v. very mild, P = 0.033; control v. mild, P<0.001; very mild v. mild, P = 0.001).
Spatial span task
Repeated-measures analysis revealed a main effect of group (F = 24.9, P<0.001). Post hoc Bonferroni analysis demonstrated no significant difference between the control and very mild disease groups, although this approached significance (P = 0.064). There was a significant difference between the mild disease group and the control (P<0.001) and very mild (P<0.001) disease groups.
Use of chunking
Digit span task
Mean span scores for digit span tasks are presented inFig. 1. The repeated-measures analysis revealed a main effect for structured v. unstructured trial type (F = 24.0, P<0.001) and no interaction between group and trial type (F = 1.2, P = 0.309).
Spatial span task
Mean span scores for spatial span tasks are presented inFig. 2. Repeated-measures analysis revealed a main effect for structured v. unstructured trial type (F = 8.3, P = 0.006) and a significant interaction between group and trial type (F = 6.5, P = 0.004). Post hoc analysis was conducted by calculating the differences between structured and unstructured trial performance for each group. Independent t-tests were then conducted which revealed a significant difference between the mild disease group and both the control group (P = 0.003) and the very mild disease group (P = 0.012), with no difference between the control and very mild disease groups (P = 0.498).
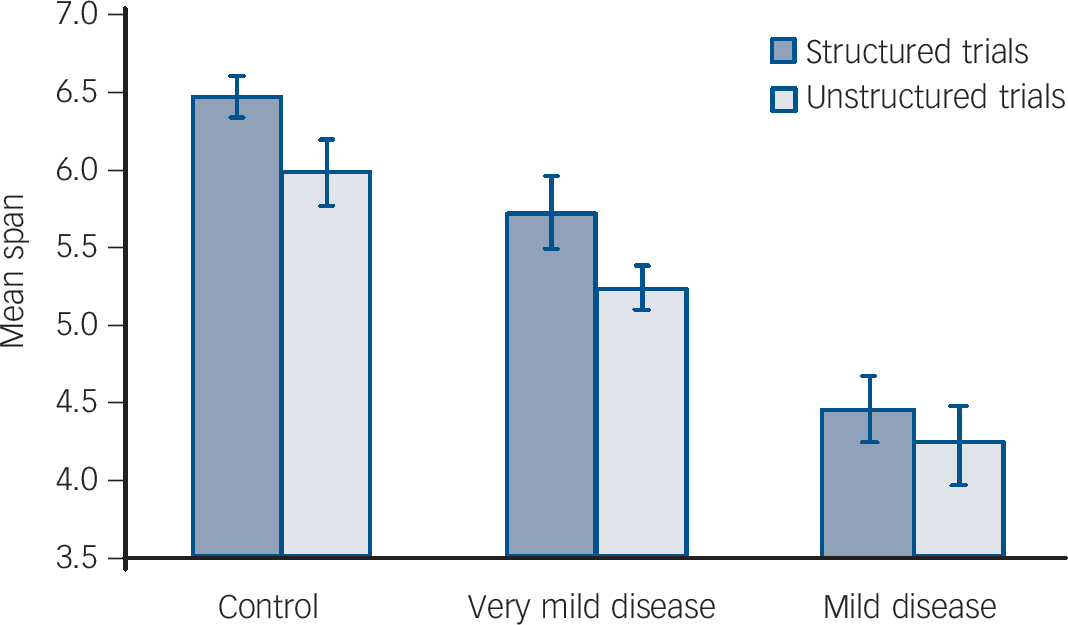
Fig. 1 Results of chunking strategy on performance of the digit span task. Error bars indicate standard errors of the means.
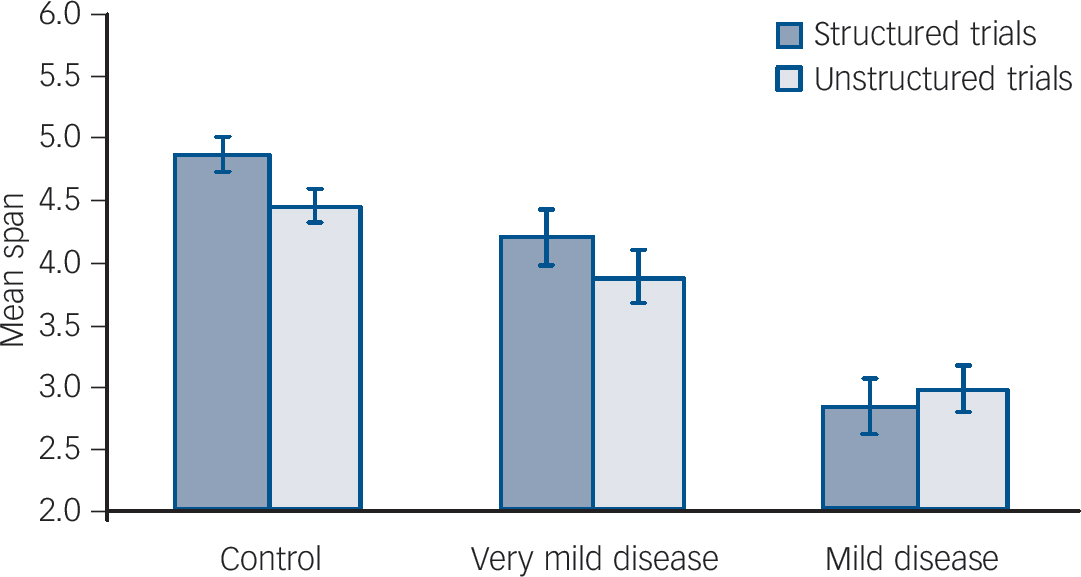
Fig. 2 Results of chunking strategy on performance of the spatial span task. Error bars indicate standard errors of the means.
Discussion
The main aim of this study was to identify whether patients with early Alzheimer's disease are able to use chunking to improve their working memory. The control and very mild disease groups showed improved span performance with structured sequences compared with unstructured sequences in both tasks. They were therefore able to use chunking strategies to improve verbal and spatial working memory performance. The mild disease group also demonstrated an improvement in digit span with structured compared with unstructured trials, but no advantage with structured trials in the spatial span task. This suggests that participants in the mild disease group were able to use chunking to improve digit span but not spatial span performance.
The ability to use strategic encoding strategies such as chunking in early Alzheimer's disease has been attributed to the ‘episodic buffer’ within Baddeley's working memory model. Reference Baddeley18 A previous study examining the differential performance on recall of clustered v. unclustered word lists, whereby associations between words in clustered lists allowed chunking, attributed the differential performance to episodic buffer functioning. Reference Germano, Kinsella, Storey, Ong and Ames3 The modulation of working memory by recoding data into higher-level chunks in our study requires the identification of mathematical rules or geometrical patterns to recode data. This in itself involves a number of higher cognitive functions including holding data in working memory, identifying mathematical associations between numbers, accessing knowledge from long-term memory stores of known shapes or mathematical rules, strategically using these associations to recode the data into a new representation in working memory and inhibiting interference from data that do not share associations with already chunked information. The finding that this ability to chunk information is impaired at the mild stage of Alzheimer's disease in spatial working memory is unsurprising given the relatively high executive demands involved. There is evidence that performance on executively demanding tasks is impaired at an early stage of Alzheimer's disease, Reference Perry and Hodges19 with spatial span considered a more executively demanding task than digit span. Reference Carlesimo, Fadda, Lorusso and Caltagirone20 However, contrary to our hypothesis, participants with mild Alzheimer's disease also benefited from the use of chunking in the digit span task. This result is encouraging, as it suggests that the use of mnemonic strategies in the verbal domain may remain preserved at the early stage of the disease, and may therefore be a potential target for cognitive training.
Animal and human studies have demonstrated that encoding, storage and retrieval of information in working memory are associated with activity in the prefrontal cortex and posterior parietal cortex. Reference Goldman-Rakic, Roberts, Robbins and Weiskrantz21–Reference Cabeza and Nyberg24 Functional magnetic resonance imaging (fMRI) studies have demonstrated the importance of the prefrontal cortex in working memory tasks requiring executive control. Reference Owen, McMillan, Laird and Bullmore25 A confounding factor in such studies has been the observed increase in prefrontal cortex activity with increasing task difficulty. Reference Duncan and Owen26 A series of fMRI studies using verbal and spatial chunking tasks similar to those in our study have overcome this effect of task difficulty, demonstrating activation of prefrontal cortex and posterior parietal cortex during the use of chunking strategies, despite reduced working memory demand in normal young individuals. Reference Bor, Duncan, Wiseman and Owen4,Reference Bor, Cumming, Scott and Owen5 There is evidence that neuropathological changes in Alzheimer's disease result in reduced effective connectivity between brain regions, including prefrontal and parietal regions Reference Delbeuck, Van der Linden and Collette27 identified as being involved in chunking. Functional imaging studies have implicated a range of areas in spatial working memory, including frontal, posterior parietal and occipital cortex. Reference Smith and Jonides28 Such disconnectivity between these areas may therefore underlie the impairment observed in the use of spatial chunking strategies at an early stage of Alzheimer's disease.
Group performance comparison
In the mild Alzheimer's disease group, performance on verbal and spatial working memory span tasks was impaired compared with the control and very mild disease groups. Similar results have been reported from previous studies of verbal and spatial working memory in mild Alzheimer's disease, Reference Becker29,Reference Hodges and Patterson30 although some studies have found no impairment in mild disease. Reference Lines, Dawson, Preston, Reich, Foster and Traub31,Reference Perry, Watson and Hodges32 At the very mild stage of Alzheimer's disease performance on spatial span tasks was not significantly impaired, although this approached significance (P = 0.064), whereas digit span performance was impaired in these participants (P = 0.033). Previous studies of people with ‘minimal’ Alzheimer's disease and minor cognitive impairment have suggested that performance on span tasks remains intact at the preclinical stage. Reference Perry, Watson and Hodges32,Reference Traykov, Raoux, Latour, Gallo, Hanon and Baudic33 Our findings that our very mild disease group were impaired on digit span performance might be due to their greater cognitive impairment compared with preclinical samples in other studies.
According to the model of working memory proposed by Baddeley & Hitch, successful performance on working memory span tasks relies on the functioning of subsidiary ‘slave’ systems (phonological loop for verbal information, and visuospatial sketchpad for visuospatial information) and central executive functioning. Reference Baddeley, Hitch and Bower34 Central executive functioning is impaired earlier in Alzheimer's disease than the subsidiary systems, which may remain relatively intact until the disease is at the mild to moderate stage. Reference Collette, Van der Linden, Bechet and Salmon35 Our finding that performance on verbal and spatial working memory tasks is significantly impaired by the mild stage of Alzheimer's disease may therefore reflect impairments in central executive functioning rather than in the subsidiary systems.
Chunking in cognitive training
Training in the use of chunking strategies can significantly increase working memory capacity. Reference Ericcson, Chase and Faloon6 We observed that some participants in the control and very mild disease groups were not explicitly aware of using chunking strategies, despite successfully doing so. Following initial testing, a small subset of four patients with very mild Alzheimer's disease and one with mild Alzheimer's disease were explicitly informed about chunking techniques and retested on the structured digit span task, to examine whether working memory performance improved in a single session. This was the case for those with very mild disease – the mean digit span improved from 5.4 (s.d. = 1.27) to 6.1 (s.d. = 0.8; P = 0.08) – but not for the patient with mild disease. This finding, together with evidence of the efficacy of chunking in cognitive training in normal young individuals, Reference Olesen, Westerberg and Klingberg7,Reference McNab, Varrone, Farde, Jucaite, Bystritsky and Forssberg8 suggests that simple training in chunking techniques may be a useful cognitive training strategy for improving working memory performance in people with very mild Alzheimer's disease.
Benefits of cognitive training:
A recent large study of brain training in healthy adults (aged 18–60 years) demonstrated no evidence that such training led to any generalised improvement in cognitive functioning; significant improvements were seen on the trained tasks but generalisation did not occur even to untrained tasks that required similar cognitive functions. Reference Owen, Hampshire, Grahn, Stenton, Dajani and Burns36 However, there is some evidence that cognitive training is effective in older people. Reference Willis, Tennstedt, Marsiske, Ball, Elias and Koepke37 There is also some evidence of benefit from cognitive training in people with Alzheimer's disease or mild cognitive impairment. Reference Bier, van der Linden, Gagnon, Desrosiers, Adam and Louveaux38,Reference Belleville39 It may be the case that, whereas healthy young people already perform at their full capacity, elderly individuals or those with cognitive impairment would benefit from learning or relearning specific strategies to help compensate for their underlying cognitive deterioration and loss of inherent strategic abilities. There is evidence that cognitive training using restorative strategies may be more efficacious than compensatory strategies in both cognitive domains and everyday functioning. Reference Sitzer, Twamley and Jeste40 Many uncertainties remain as to the benefits of cognitive training in Alzheimer's disease. It is unclear how sustained any benefits of cognitive training would be in the face of continued neurodegeneration; however, our results provide a case for studies that investigate the use of and training in well-defined mnemonic strategies such as chunking in the growing population of individuals with early Alzheimer's disease.
Methodological issues
We divided our Alzheimer's disease patients into two groups according to their MMSE score. The MMSE is commonly used as a screening tool for dementia, and has been used in several other studies as a marker for severity of Alzheimer's disease. Reference Germano, Kinsella, Storey, Ong and Ames3,Reference Perry, Watson and Hodges32 However, objections have been raised as to whether it is suitable for this purpose. Reference Greene, Baddeley and Hodges41 Individuals with a high premorbid level of functioning and educational achievement can score relatively well on the MMSE despite having significant dementia. More detailed neuropsychological assessment would undoubtedly have been useful in classifying the severity of dementia in our participants. Although we acknowledge this, the MMSE served as a useful clinical marker of Alzheimer's disease severity to test our hypothesis. We are not suggesting that our very mild disease group represents a homogeneous clinical entity, but rather that in this study it represented a group of patients who were at an earlier stage of disease progression than those in our mild disease group. We were interested in examining whether patients with mild Alzheimer's disease were able to use chunking to improve working memory performance and whether this ability was lost during the early stages of the disease. We therefore used MMSE scores as a pragmatic cut-off to test this hypothesis, not as a diagnostic marker or to suggest a homogeneous clinical population.
Our finding that chunking ability appears preserved in patients who score above 24 on the MMSE is in keeping with other studies that examined executive function at such an early stage in the disease; Reference Germano, Kinsella, Storey, Ong and Ames3 however, further larger studies incorporating more detailed information about specific attentional and executive deficits would be helpful.
IQ scores
Although all participants were recruited from the same geographical area and matched for age, gender and years of education, analysis of NART scores revealed a significant difference between the mild disease group and the very mild disease and control groups. Subsequent repeated-measures analysis using IQ as a covariable revealed no significant interaction between structured v. unstructured trial type and IQ (F = 0.6, P = 0.438) for either the digit or the spatial span task. Bivariate correlation analysis revealed a highly significant correlation between IQ and MMSE scores (Pearson correlate 0.528, P<0.01). Although the NART is often used to assess premorbid IQ, its validity in dementia has been questioned, as the cognitive deficits may lead to poor performance on this measure. It has been demonstrated that NART score is impaired even at mild stages of the disease, Reference McFarlane, Welch and Rodgers42 and this is reflected in the high correlation in our study between IQ and MMSE scores. The difference in IQ scores seen in our study between the mild disease group and the other two groups is therefore likely to reflect the cognitive impairment caused by Alzheimer's disease, rather than representing a true difference in premorbid IQ between the groups.
Study implications
We conclude that healthy elderly people and people with very mild Alzheimer's disease are able to use chunking strategies to improve working memory in both verbal and spatial domains, whereas those with mild Alzheimer's disease remain able to benefit from chunking in verbal but not spatial working memory. Chunking is a well-established form of strategic encoding that has been shown to improve working memory capacity and underlie improvements seen in cognitive training programmes. Our study opens up the possibility that training in the use of chunking strategies might be a simple but effective tool to help maintain working memory at the early stages of Alzheimer's disease.
Appendix
Examples of verbal and spatial span tasks
The following examples of verbal and spatial span tasks illustrate the differences between structured trials, which encourage chunking, and unstructured trials.
Verbal span task
Structured sequence:
2 4 6 9 7 5
Unstructured sequence:
8 1 6 2 9 4
Spatial span task
An example of a structured sequence is shown inFig. A1(a) and an unstructured form inFig. A1(b).
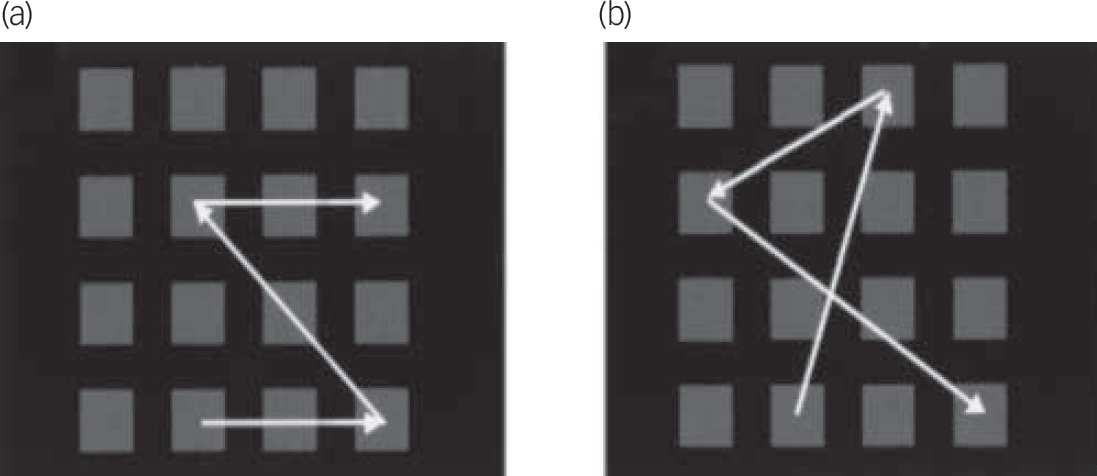
Fig. A1 Spatial span task: (a) structured; (b) unstructured. Reprinted from Bor et al Reference Bor, Duncan, Wiseman and Owen4 with permission from Elsevier.








eLetters
No eLetters have been published for this article.