Depression is common in old age and is associated with cognitive impairment, reduced quality of life and increased disability. Reference Reppermund, Brodaty, Crawford, Kochan, Slavin and Trollor1,Reference Beekman, Deeg, Braam, Smit and Van Tilburg2 Neuroimaging studies have suggested white matter hyperintensities, in particular in subcortical prefrontal and temporal brain areas, as one of the primary neurobiological substrates of geriatric depression. Reference O'Brien, Desmond, Ames, Schweitzer, Harrigan and Tress3-Reference de Groot, de Leeuw, Oudkerk, Hofman, Jolles and Breteler5 A number of studies have shown that white matter changes predict depressive symptoms in older, community-dwelling adults. Reference Teodorczuk, Firbank, Pantoni, Poggesi, Erkinjuntti and Wallin6-Reference Godin, Dufouil, Maillard, Delcroix, Mazoyer and Crivello8 The authors explain their findings on the basis of the vascular hypothesis of depression in elderly people. According to this hypothesis, cerebrovascular pathologies cause depressive symptoms through disruption of frontosubcortical connections. Reference Alexopoulos, Meyers, Young, Campbell, Silbersweig and Charlson9 However, other studies have not confirmed an association between white matter hyperintensities and the development of depressive symptoms. Reference Versluis, van der Mast, van Buchem, Bollen, Blauw and Eekhof10
Diffusion tensor imaging (DTI) may offer information about the structural integrity of white matter areas and their longitudinal relationship to depressive symptoms. DTI is a relatively new, sensitive and reliable technique for examining white matter microstructure in vivo, thus providing information that can estimate the integrity of pathways within relevant neural networks. A commonly used metric in DTI studies is fractional anisotropy, which estimates the degree to which tissue organisation limits diffusion of water molecules in white matter fibre tracts. Reference Chua, Wen, Slavin and Sachdev11 Older patients with major depression demonstrate lower fractional anisotropy values mainly in frontal Reference Bae, MacFall, Krishnan, Payne, Steffens and Taylor12,Reference Abe, Yamasue, Kasai, Yamada, Aoki and Inoue13 and temporal brain regions. Reference Abe, Yamasue, Kasai, Yamada, Aoki and Inoue13 Tract-based spatial statistics (TBSS) are designed for examining fibre tracts of the whole brain. This method has superior objectivity and interpretability of DTI analyses than the regions of interest (ROI) method. Reference Smith, Jenkinson, Johansen-Berg, Rueckert, Nichols and Mackay14 The TBSS method analyses the entire brain for group differences or correlations, so it is not restricted to specific brain areas.
Colloby et al Reference Colloby, Firbank, Thomas, Vasudev, Parry and O'Brien15 investigated white matter microstructural changes with TBSS in a sample of patients with late-life depression. They found lower fractional anisotropy in frontal, temporal and midbrain regions compared with healthy controls. However, after correction for multiple comparisons, these differences were no longer statistically significant. Although Bezerra et al Reference Bezerra, Pereira, Cendes, Jackowski, Nakano and Moscoso16 did not discover significant differences between currently depressed and non-depressed elderly patients using TBSS, two other studies report loss of white matter integrity in the cingulate area and in widespread regions of the frontal and temporal lobes. Reference Alves, Karakaya, Fusser, Kordulla, O'Dwyer and Christl17,Reference Mettenburg, Benzinger, Shimony, Snyder and Sheline18 Two recent meta-analyses Reference Liao, Huang, Wu, Yang, Kuang and Du19,Reference Murphy and Frodl20 and a systematic review on DTI in depression Reference Sexton, Mackay and Ebmeier21 identified reduced fractional anisotropy in seven brain areas: superior frontal gyrus (left and right), genu of corpus callosum (right), inferior fronto-occipital fasciculus (right), posterior thalamic radiation (right), body of corpus callosum (left), inferior longitudinal fasciculus (right) and superior longitudinal fasciculus (left and right). We report the first prospective study assessing associations between DTI parameters using the TBSS method and depressive symptoms over a 2-year period in a large community sample of older adults. The aims of the present study were to assess whether community-dwelling elderly participants with depressive symptoms demonstrate white matter microstructure abnormalities and whether a lifetime history of depression is associated with white matter microstructure abnormalities. A further aim was to assess whether white matter microstructure abnormalities predict depressive symptoms in a 2-year follow-up period. We expected reduced fractional anisotropy in individuals with depressive symptoms and with a history of depression compared with healthy controls. We further hypothesised that a higher degree of white matter microstructure abnormalities predicts the incidence of depression at follow-up.
Method
Sample
Participants were recruited from the electoral roll of the Eastern suburbs of Sydney, Australia between 2005 and 2007 as part of an ongoing longitudinal study to examine the prevalence, longitudinal course and risk and protective factors of cognitive impairment and decline in older, community-dwelling individuals (Sydney Memory and Ageing Study, MAS). Detailed methods and recruitment process are published elsewhere. Reference Sachdev, Brodaty, Reppermund, Kochan, Trollor and Draper22 Briefly, 1037 participants aged between 72 and 92 years were assessed using a detailed neuropsychological and medical assessment. Exclusion criteria were dementia (according to DSM-IV criteria 23 ), developmental disabilities, psychotic symptoms, schizophrenia or bipolar disorder, multiple sclerosis, motor neuron disease, progressive malignancy and inadequate English to complete a psychometric assessment. The cohort is followed up every 2 years with comprehensive face-to-face assessments. The current study reports data from the first and second follow-up (2 and 4 years after baseline, respectively). All participants without contraindications (pacemaker, metallic implant or foreign bodies, cochlear implants, ferromagnetic homeostatic clips, claustrophobia) were invited to undergo magnetic resonance imaging (MRI) scans. A total of 414 participants agreed to and were eligible for MRI brain scans. Participants who did not agree to or were ineligible for an MRI were slightly older than participants who had an MRI scan done (81.1 years (s.d. = 4.7) v. 79.8 years (s.d.= 4.6), t = 4.4, P<0.001). There were no differences between these groups with regard to gender, education or depressive symptoms (all P>0.05). Of the 414 participants with an MRI scan, 14 had missing Geriatric Depression Scale - 15 item (GDS) Reference Yesavage, Brink, Rose, Lum, Huang and Adey24 data and 19 participants had too many missing data to classify them as having mild cognitive impairment (MCI), resulting in a total sample of 381 participants. Consensus diagnoses of MCI were made by a panel of psychogeriatricians, neuropsychiatrists, clinical and research neuropsychologists using current international consensus criteria Reference Winblad, Palmer, Kivipelto, Jelic, Fratiglioni and Wahlund25 and 167 participants were classified as having MCI. At the 2-year follow-up, GDS data were available for 353 participants. The study protocol was approved by the ethics committees of the University of New South Wales and the South Eastern Sydney and Illawarra Area Health Service and written informed consent was obtained from each participant.
MRI data acquisition
All participants underwent MRI scanning on a Philips 3T Achieva Quasar Dual MRI scanner (Philips Medical System, Best, The Netherlands). For each participant, a 32-directional DTI (b1 = 1000, one b0) scan was acquired with a single-shot, spin-echo, echo-planar imaging sequence: echo time = 68 ms, repetition time = 7767 ms, flip angle = 90°, matrix size = 240 × 240, field of view = 240 × 240 mm, yielding in-plane resolution of 1 × 1 mm, 55 2.5 mm contiguous axial slices without gap. Each DTI scan was repeated twice to increase the signal-to-noise ratio.
DTI processing
DTI data were preprocessed and analysed with the FMRIB’s Diffusion Toolbox of the FMRIB’s Software Library (FSL) version 4.1.7 (http://www.Fmrib.ox.ac.uk/fsl, Oxford Centre for Functional Magnetic Resonance Imaging of the Brain, Oxford University, UK). First, each participant’s raw DTI data were co-registered and merged together using the FMRIB’s linear image registration. Correction for head movement and eddy current distortions was performed on the merged DTI data by linearly registering each diffusion-weighted volume to the first non-diffusion b0 image. Two pre-processed DTI data-sets of each participant were averaged to create a single DTI image. A binary brain mask was created to remove the non-brain tissue using the Brain Extraction Tool in FSL. Subsequently, a diffusion tensor model was fitted to each imaging voxel of the preprocessed DTI data to derive a fractional anisotropy map.
Voxel-wise statistical analyses of fractional anisotropy images were performed using tract-based spatial statistics, Reference Smith, Jenkinson, Johansen-Berg, Rueckert, Nichols and Mackay14 version 1.2 within FSL. First, each participant’s fractional anisotropy map was non-linearly aligned to a 1 × 1 × 1 fractional anisotropy target image (FMRIB58_FA) in Montreal Neurological Institute standard space. Second, all aligned fractional anisotropy images were averaged to generate a mean fractional anisotropy image that was then thinned to create a mean tract skeleton. The tract skeleton was further thresholded at fractional anisotropy >0.2 to minimise the partial volume effect and cross-participant misregistration. Third, each participant’s fractional anisotropy data were projected onto the tract skeleton to create a skeletonised fractional anisotropy map.
Depression measures
Current depressive symptoms were assessed with the 15-item short form of the GDS, Reference Yesavage, Brink, Rose, Lum, Huang and Adey24 a self-rating questionnaire shown to be reliable and valid for the assessment of depressive symptoms in elderly people. A higher score indicates more symptoms of depression and a cut-off of six has been established to indicate clinically relevant symptoms of depression. Reference Herrmann, Mittmann, Silver, Shulman, Busto and Shear26 The GDS does not include somatic and sexual items, and has been validated for use in individuals with mild impairment of cognition. Reference Yesavage, Brink, Rose, Lum, Huang and Adey24 In the MAS we used the GDS with item 9 as described in Brink (here item 12). Reference Brink27 As the GDS is a self-rated questionnaire, there were some missing data. Provided that 80% or more of the questions were answered, scores were prorated (raw score/items completed × total number of items). This was done for 41 participants (10.8%) at time 1 (T 1) and for 36 participants (10.2%) at the 2-year follow-up.
A history of depression was defined as one or more self-reported depressive episodes that had required the attention of a general practitioner, psychologist or psychiatrist and/or previous prescription of antidepressants. A total of 39 participants (10.2%) were taking antidepressants at time T 1 and all of these individuals were either in the currently depressed group and/or in the history of depression group
Cardiovascular Risk Factor Index
The Cardiovascular Risk Factor Index (CVR) is a computed variable based on the research of the Framingham Stroke Study. Reference D'Agostino, Vasan, Pencina, Wolf, Cobain and Massaro28 It is based on a regression model using current smoking status, diabetic status, systolic blood pressure, total cholesterol level, high-density-lipoprotein level and use of antihypertensive medication. A higher score indicates a higher 10-year risk prediction of a cardiovascular event (coronary death, myocardial infarction, coronary insufficiency, angina, ischemic stroke, hemorrhagic stroke, transient ischemic attack, peripheral artery disease and heart failure). The CVR was available for 369 participants (96.9 % of the sample).
Statistical analysis
Statistical analyses included independent t-tests and chi-squared tests for comparison of demographic and clinical measures using SPSS 20 for Windows. Voxel-wise statistical analyses were performed using a permutation-based inference program called ‘randomise’ (with 1000 permutations), which is part of FSL. Reference Nichols and Holmes29 Group differences in fractional anisotropy between currently depressed and non-depressed elderly people were assessed using ANOVA implemented in the randomise tool, controlling for age, gender, years of education, MCI and CVR score. Correlation analyses between fractional anisotropy and GDS scores in the whole sample and for individuals who developed depressive symptoms at follow-up were performed using linear regression in the randomise program, adjusting for age, gender, years of education, MCI and CVR score.
Multiple logistic regression was used to analyse whether decreased fractional anisotropy in certain brain areas predicted depression at 2-year follow-up, adjusting for age, gender, years of education, MCI and CVR score. Fractional anisotropy values were converted into z-scores. Multiple comparison correction controlling for the family-wise error (FWE) rate was applied to the resulting raw statistical images, using the threshold-free cluster enhancement method implemented in the randomise tool. Reference Smith and Nichols30 The significance threshold was set at P<0.05 on the FWE-corrected statistical images. The anatomical location of significant skeletal regions was labelled using the Johns Hopkins University white matter atlas. Reference Wakana, Jiang, Nagae-Poetscher, van Zijl and Mori31
Results
Of the total sample, 6.6% (n = 25) were classified as currently depressed at T 1 based on a GDS score of 6 or above. The currently depressed group and the non-depressed groups did not differ in age, gender, education, rate of MCI, rate of a lifetime history of depression and CVR score (Table 1).
Group differences in white matter microstructure
Voxel-wise comparison of the brains of these currently depressed and non-depressed groups of older people revealed significant clusters of reduced fractional anisotropy following permutation-based multiple comparison correction and adjustment for age, gender, education, MCI and CVR score. Online Fig. DS1 and online Table DS1 show brain areas with significantly decreased fractional anisotropy values in the currently depressed group relative to the non-depressed control group. Compared with the non-depressed group, the currently depressed group had widespread reduced fractional anisotropy in 45 different regions.
Table 1 Sample characteristics at baseline
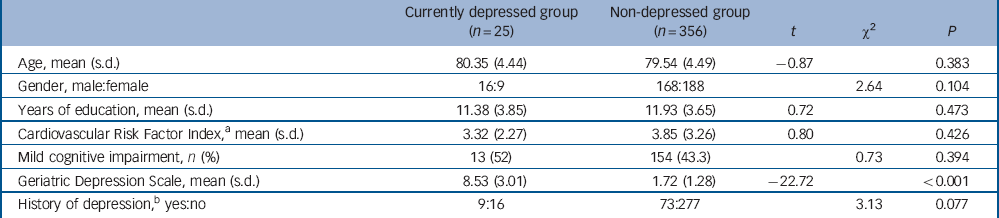
| Currently depressed group (n = 25) |
Non-depressed group (n = 356) |
t | χ2 | P | |
|---|---|---|---|---|---|
| Age, mean (s.d.) | 80.35 (4.44) | 79.54 (4.49) | –0.87 | 0.383 | |
| Gender, male:female | 16:9 | 168:188 | 2.64 | 0.104 | |
| Years of education, mean (s.d.) | 11.38 (3.85) | 11.93 (3.65) | 0.72 | 0.473 | |
| Cardiovascular Risk Factor Index,Footnote a mean (s.d.) | 3.32 (2.27) | 3.85 (3.26) | 0.80 | 0.426 | |
| Mild cognitive impairment, n (%) | 13 (52) | 154 (43.3) | 0.73 | 0.394 | |
| Geriatric Depression Scale, mean (s.d.) | 8.53 (3.01) | 1.72 (1.28) | –22.72 | <0.001 | |
| History of depression,Footnote b yes:no | 9:16 | 73:277 | 3.13 | 0.077 |
a. Data available for 369 participants only.
b. Data available for 375 participants only.
Online Fig. DS2 illustrates correlations between reduced fractional anisotropy and GDS scores for the whole sample. There were no significant fractional anisotropy reductions in older individuals with a lifetime history of depression (n = 82) compared with older individuals without a lifetime history of depression (n = 293; all P>0.05).
At follow-up, GDS data were available for 353 individuals. There was no significant change in the mean GDS score for the whole sample (2.18 (s.d. = 2.25) v. 2.37 (s.d. = 2.30), t(d.f. = 352) = –1.60, P = 0.110). A total of 26 individuals had clinically significant symptoms of depression (GDS score of 6 or above) at follow-up. The total number included in the statistical analyses was 317, as 12 had missing CVR data and 24 individuals were currently depressed at T 1. Of the 317 individuals included in the analyses, 13 developed depression at follow-up.
Voxel-wise comparison of the brains of those who developed depression at follow-up (i.e. GDS score ⩾6) v. those who did not develop depression revealed no significant results following permutation-based multiple comparison correction and adjustment for age, gender, education, MCI and CVR score. This was most likely because of the small number of individuals who developed depression at follow-up, increasing the risk of type II errors. We repeated the analysis using the GDS as a continuous variable to assess whether fractional anisotropy reduction at T 1 was associated with an increase in depressive symptoms at follow-up, corrected for baseline GDS scores. Reduced fractional anisotropy values with the largest clusters in the frontal lobe, projection fibres and commissural fibres were significantly associated with depressive symptoms 2 years later (see online Table DS2).
In a post hoc analysis, we examined whether reduced fractional anisotropy in the brain areas identified in two recent meta-analyses Reference Liao, Huang, Wu, Yang, Kuang and Du19, Reference Murphy and Frodl20 and a systematic review Reference Sexton, Mackay and Ebmeier21 predicted depression (GDS score ⩾6) at follow-up. We used logistic regression with currently depressed v. not depressed at follow-up as the dependent variable and eight brain areas as predictor variables, adjusted for age, gender, years of education, MCI and CVR score. Table 2 shows the results. Reduced fractional anisotropy in the superior frontal gyrus (left and right), posterior thalamic radiation (right), superior longitudinal fasciculus (left) and body of corpus callosum (left) predicted depression at follow-up.
Discussion
Comparison with findings from other studies
The results of this study confirm reduced white matter integrity in currently depressed older, community-dwelling individuals compared with non-depressed individuals. This is in line with previous studies in older patients with depression Reference Bae, MacFall, Krishnan, Payne, Steffens and Taylor12,Reference Alves, Karakaya, Fusser, Kordulla, O'Dwyer and Christl17,Reference Mettenburg, Benzinger, Shimony, Snyder and Sheline18,Reference Nobuhara, Okugawa, Sugimoto, Minami, Tamagaki and Takase32-Reference Yang, Huang, Hong and Yu35 and in population-based samples of older individuals. Reference Lamar, Charlton, Morris and Markus36 Most of the aforementioned studies used the ROI approach and four studies used TBSS. Reference Colloby, Firbank, Thomas, Vasudev, Parry and O'Brien15-Reference Mettenburg, Benzinger, Shimony, Snyder and Sheline18 In contrast to the widely used ROI approach, which involves manually placing an ROI over a predefined area and is susceptible to user bias, TBSS includes the whole brain and reduces the problem of misalignment. Previous TBSS study samples comprised selected patients with depression, whereas our study is the first to use TBSS in a community sample of older adults with and without depressive symptoms. Findings from previous TBSS studies in older patients with depression are inconclusive. Whereas two did not find significant differences in white matter integrity between currently depressed and non-depressed elderly people, Reference Colloby, Firbank, Thomas, Vasudev, Parry and O'Brien15,Reference Bezerra, Pereira, Cendes, Jackowski, Nakano and Moscoso16 two reported decreased fractional anisotropy in the corpus callosum, cingulate bundle and corticospinal tract. Reference Alves, Karakaya, Fusser, Kordulla, O'Dwyer and Christl17,Reference Mettenburg, Benzinger, Shimony, Snyder and Sheline18 Our findings show more widespread fractional anisotropy reduction in addition to the aforementioned areas. One explanation could be that our sample was significantly older, with an age range of 72-92 years compared with 59 years and above in other samples. Reference Colloby, Firbank, Thomas, Vasudev, Parry and O'Brien15-Reference Mettenburg, Benzinger, Shimony, Snyder and Sheline18 However, we adjusted our analyses for age and still found significant fractional anisotropy reduction in many brain regions in the currently depressed group.
Table 2 Multiple logistic regression results for predicting depression v. no depression at follow-up
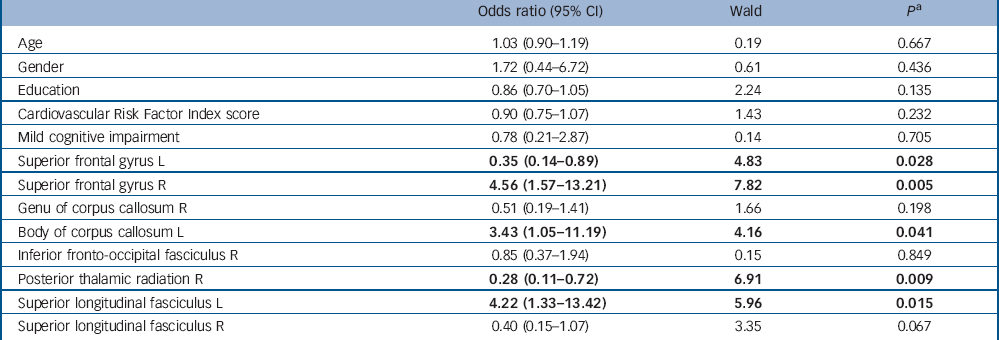
| Odds ratio (95% CI) | Wald | P Footnote a | |
|---|---|---|---|
| Age | 1.03 (0.90-1.19) | 0.19 | 0.667 |
| Gender | 1.72 (0.44-6.72) | 0.61 | 0.436 |
| Education | 0.86 (0.70-1.05) | 2.24 | 0.135 |
| Cardiovascular Risk Factor Index score | 0.90 (0.75-1.07) | 1.43 | 0.232 |
| Mild cognitive impairment | 0.78 (0.21-2.87) | 0.14 | 0.705 |
| Superior frontal gyrus L | 0.35 (0.14-0.89) | 4.83 | 0.028 |
| Superior frontal gyrus R | 4.56 (1.57-13.21) | 7.82 | 0.005 |
| Genu of corpus callosum R | 0.51 (0.19-1.41) | 1.66 | 0.198 |
| Body of corpus callosum L | 3.43 (1.05-11.19) | 4.16 | 0.041 |
| Inferior fronto-occipital fasciculus R | 0.85 (0.37-1.94) | 0.15 | 0.849 |
| Posterior thalamic radiation R | 0.28 (0.11-0.72) | 6.91 | 0.009 |
| Superior longitudinal fasciculus L | 4.22 (1.33-13.42) | 5.96 | 0.015 |
| Superior longitudinal fasciculus R | 0.40 (0.15-1.07) | 3.35 | 0.067 |
L, left; R, right.
a. Results in bold are significant.
A systematic review of DTI studies in affective disorders Reference Sexton, Mackay and Ebmeier21 and a recent meta-analysis of voxel-based DTI studies in major depression Reference Liao, Huang, Wu, Yang, Kuang and Du19 suggest that depression can be regarded as a disconnection syndrome within frontal and subcortical areas. Price & Drevets Reference Price and Drevets37 suggest that the synaptic transmission through the limbic-cortical-striato-pallido-thalamic circuit related to the medial and orbital prefrontal networks is affected in major depression and this can produce the pathological emotional symptoms.
In contrast to current depressive symptoms, a lifetime history of depression was not associated with reduced fractional anisotropy. However, reduced fractional anisotropy in the superior frontal gyrus (left and right), posterior thalamic radiation (right), superior longitudinal fasciculus (left) and body of corpus callosum (left) predicted depression (GDS score ⩾6) at follow-up. In addition, reduced fractional anisotropy mainly in the frontal lobe, projection fibres and commissural fibres were associated with increased depressive symptoms at follow-up. These findings indicate that reduced white matter integrity in certain brain areas may precede depression. Previous studies reported associations between structural brain abnormalities and poor outcomes of late-life depression Reference Alexopoulos, Murphy, Gunning-Dixon, Latoussakis, Kanellopoulos and Klimstra38 and population-based studies showed that white matter changes can predict depressive symptoms after 1-4 years. Reference Teodorczuk, Firbank, Pantoni, Poggesi, Erkinjuntti and Wallin6-Reference Godin, Dufouil, Maillard, Delcroix, Mazoyer and Crivello8 Godin et al Reference Godin, Dufouil, Maillard, Delcroix, Mazoyer and Crivello8 found, in addition, that severity of white matter lesions was associated with lifetime depression and that white matter lesion increase in the frontal lobe was significantly higher over 4-year follow-up in participants with lifetime depression compared with those without lifetime depression. A possible explanation for the different findings is that the number of participants with a history of depression was larger than in our study and the authors used a standardised interview to diagnose lifetime depression. More longitudinal TBSS studies with larger sample sizes including participants with acute depressive symptoms and those who are in remission are needed to confirm the findings.
TBSS did not reveal significant results for the whole brain comparison between individuals who developed depression at follow-up v. those who did not after correction for multiple comparisons. A possible reason for the lack of significant associations is the relatively small number of currently depressed individuals at follow-up, increasing the risk of type II errors. Only 13 individuals developed clinically significant symptoms of depression (a GDS score of 6 or above) at the 2-year follow-up. A longer follow-up period might reveal significant associations even after correction for multiple comparisons. However, reduced fractional anisotropy values at T 1, mainly in the frontal lobe, projection fibres and commissural fibres, were associated with an increase in depressive symptoms at follow-up.
Link with cardiovascular risk factors and other neuropathological mechanisms
There is evidence for a link between depression and cardiovascular risk factors. Reference Godin, Dufouil, Maillard, Delcroix, Mazoyer and Crivello8 The vascular depression hypothesis links vascular risk factors/vascular disease to late-life depression. Reference Valkanova and Ebmeier39 According to this hypothesis, white matter lesions are caused by cerebrovascular disease disrupting fibre tracts within frontostriatal circuits. Reference Alexopoulos, Meyers, Young, Campbell, Silbersweig and Charlson9 There was no significant difference in the cardiovascular risk profile between currently depressed and non-depressed individuals in our sample. A recent systematic review and meta-analysis suggests that vascular risk factors such as smoking or hypertension, without the actual acute vascular disease, might not precede depression. Reference Valkanova and Ebmeier39 White matter lesions increase with age even in the absence of major cerebrovascular risk factors. Reference Chowdhury, Nagai, Bokura, Nakamura, Kobayashi and Yamaguchi40 Our findings indicate widespread white matter changes in older, currently depressed individuals after adjusting for cardiovascular risk factors and for age.
Other possible neuropathological mechanisms causing reduced white matter integrity in depression are (a) elevated cytokine levels that have been associated with reduced neurogenesis and (b) dysregulation of the hypothalamic-pituitary-adrenal axis that leads, with an associated increase in glucocorticoid secretion, to cell death in the hippocampus and to an elimination of activity-dependent increases in brain-derived neurotrophic factor (see Murphy & Frodl Reference Murphy and Frodl20 for a review). Glucocorticoids can induce microglial modulation in the central nervous system with consequences such as the release of proinflammatory mediators and disruption of brain networks. Reference Kato, Hayakawa, Monji and Kanba41
Limitations
One limitation of this study is the relatively small number of individuals with clinically significant depressive symptoms. We assessed depressive symptoms with the self-rated GDS and not with a standardised clinical interview that would have enabled a clinical diagnosis of depression. However, depressive symptoms not fulfilling rigorous diagnostic criteria are highly prevalent in elderly people and their consequences are similar to those of major depression. Reference Reppermund, Brodaty, Crawford, Kochan, Slavin and Trollor1,Reference Beekman, Deeg, Braam, Smit and Van Tilburg2 There is evidence from previous research that white matter pathology is associated not only with clinical depression but also with the endorsement of depressive symptoms in an older population-based cohort of euthymic individuals, Reference Lamar, Charlton, Morris and Markus36 supporting our findings. Another limitation is that history of depression was measured by self-report during a medical history interview with a trained research assistant. Inevitably, all self-reported data are susceptible to bias and are potentially not as reliable as data derived from clinical records. Finally, the prevalence of MCI in our sample was relatively high. The classification of MCI is susceptible to alterations of criteria for cognitive impairment and the difficulty in operationalising MCI is a limitation of all population-based studies.
In conclusion, our findings demonstrate widespread reduction of white matter integrity in elderly individuals with current depression and suggest that fractional anisotropy reductions precede depressive symptoms. Tract-based spatial statistics might be a useful technique to predict depression in elderly people and may help to explain the pathogenesis of late-life depression.
Acknowledgements
The authors thank the many research assistants and administrative assistants who contributed to data gathering. We are grateful to the participants for their enthusiastic support.





eLetters
No eLetters have been published for this article.