Glycogen synthase kinase-3 (GSK-3) is a serine/threonine kinase known to be involved in multiple cellular processes, including signalling transduction, gene transcription, translation, cytoskeletal organisation in cell-cycle progression and survival. Because of its multiple functions, GSK-3 plays important roles in many human disorders such as cancer, immune disorders, metabolic disorders, neurodegenerative diseases and neuropsychiatric diseases. Reference Takahashi-Yanaga1 Consequently, GSK-3 inhibitors are speculated to be potential agents for treatment. The role of GSK-3 in cancer development remains complex and controversial. Reference McCubrey, Davis, Abrams, Montalto, Cervello and Basecke2 GSK-3 may play a positive role in cell proliferation and its aberrant expression as a tumour promoter. Several studies have shown that GSK-3 is overexpressed in various tumour types, such as colon, liver, ovarian and pancreatic tumours. Reference McCubrey, Steelman, Bertrand, Davis, Sokolosky and Abrams3 However, GSK-3 may also be a tumour suppressor. GSK-3 can suppress the Wnt/β-catenin pathway by phosphorylating β-catenin, which results in the degradation of β-catenin. Reference McCubrey, Davis, Abrams, Montalto, Cervello and Basecke2,Reference McCubrey, Steelman, Bertrand, Davis, Sokolosky and Abrams3 Many target genes of Wnt/β-catenin signalling are proto-oncogenes that have been directly implicated in cancer development. Furthermore, increased β-catenin levels have been reported to be linked to various types of cancers, including colorectal cancer, melanoma, hepatocellular and ovarian cancers, whereas conflicting data exist for prostate cancer. Another pathway related to cancer development is aberrant activation of the Hedgehog signalling pathways. It has been reported that the Hedgehog pathway is linked to basal cell carcinomas, medulloblastoms and rhabdomyosarcomas. Reference Takahashi-Yanaga1 Lithium, primarily used for bipolar disorder and as augmentation therapy for refractory depression through two possible mechanisms of action, was discovered as the first inhibitor of GSK-3 in 1996. Reference Brown and Tracy4 Lithium is an excellent mood stabiliser for the treatment of bipolar disorder (the gold standard), and provides several benefits: prevents both manic and depressive episodes and exerts acute antimanic and antisuicidal effects. Reference Severus, Taylor, Sauer, Pfennig, Ritter and Bauer5 A hospital-based cohort study has reported a slightly, but not statistically significant, higher total cancer prevalence rate in participants taking lithium compared with those in the control group. Reference Cohen, Chetrit, Cohen, Sirota and Modan6 However, in their analysis based on histopathological category, a lower but non-significant risk of developing non-epithelial tumours was found in the lithium group compared with the control group. We found very few clinical studies that have investigated the association between cancer risk and lithium. The aim of our study was to investigate lithium usage and cancer risk in the population. Additionally, we estimated the dose–response relationship of lithium usage with its cumulative defined daily dose, cumulative prescription days and average daily dose.
Method
Data sources
A retrospective cohort study was conducted based on Taiwan's National Health Insurance Research Database (NHIRD). The single-payer National Health Insurance programme was launched in Taiwan in 1995, and it has enrolled more than 99% of the 23 million people in Taiwan's population. The NHIRD contains claim records of the beneficiaries, such as demographic data, prescriptions and expenditure for healthcare services. All investigators are required to sign an agreement that guarantees patient confidentiality before using the database. The accuracy of diagnoses of major diseases in the NHIRD, such as stroke, has been validated. Reference Cheng, Kao, Lin, Lee and Lai7 We used the Longitudinal Health Insurance Database 2005 (LHID 2005), a subset of the NHIRD, which comprises a randomly sampled representative database of 1 000 000 people from the entire NHI enrolees who were alive in 2005. There were no statistically significant differences in the distribution of gender and age or average insured payroll-related amount between the sample group of the LHID and the original NHIRD. 8 This study was approved by the Institutional Review Board of Kaohsiung Medical University Hospital, Kaohsiung, Taiwan (KMUHIRB-EXEMPT(I)-20150034).
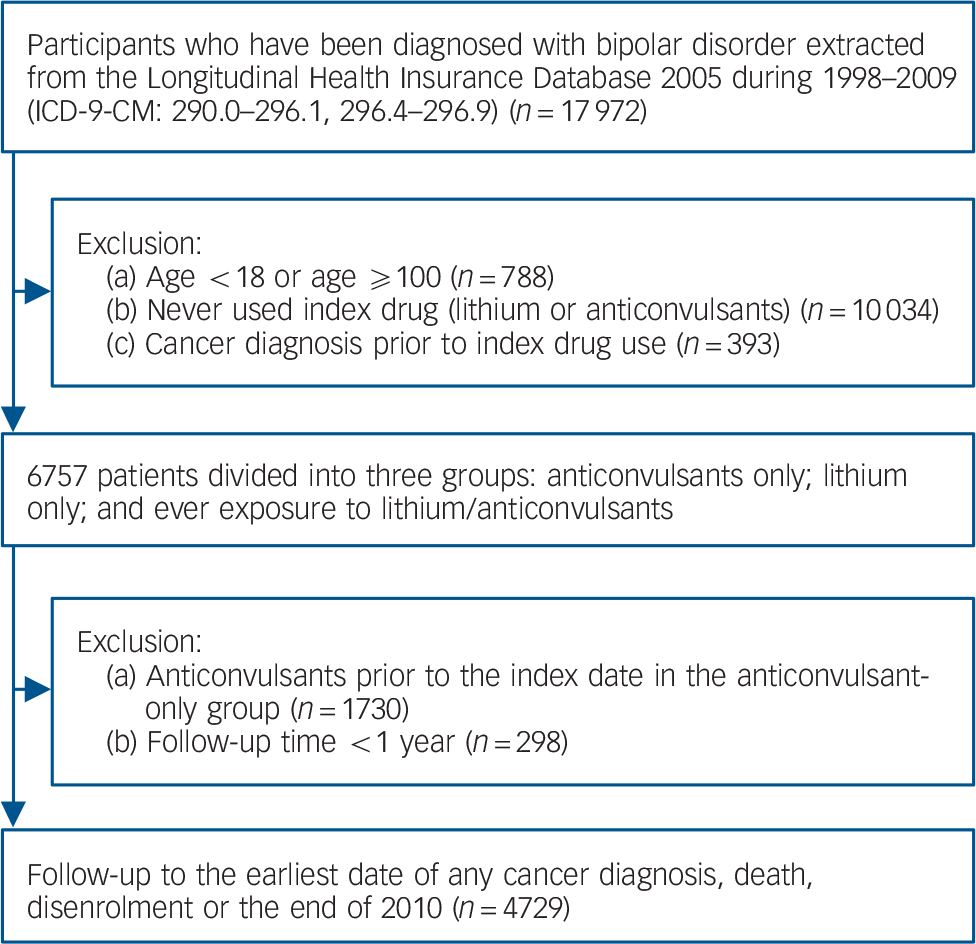
Fig. 1 Diagram for identifying study participants.
Study cohort
Patients were identified by a diagnosis of bipolar disorder (ICD-9-CM codes 296.0, 296.1, and 296.4–296.9) between 1998 and 2009. 9 Lithium was the primary index drug. To identify a comparison group with the same indications for lithium, users of anticonvulsants were selected as controls because of their comparable antimanic and antidepressant properties, and reimbursement period in the Taiwan NHI programme. Reference Young10,Reference Bowden11 We further excluded patients younger than 18 years old, the extremely elderly (older than 100 years), those with less than 1 year of follow-up and those with a diagnosis of cancer before the index date. The earliest date of lithium or anticonvulsant exposure was defined as the index date. Study cohorts were divided into three groups: anticonvulsants only, lithium only and ever exposure to anticonvulsants and lithium. The end of the observation period was the date of cancer diagnosis, death, disenrolment from the NHI programme or the end of 2010, whichever occurred first.
Drug exposure and outcome measurement
Anatomical therapeutic chemical classification system (ATC) codes were used to identify medications (lithium: N05AN01; anticonvulsants: N03AF01, N03AF02, N03AG01, N03AX09, N03AX11, N03AX12). 12 The defined daily dose (DDD) recommended by the World Health Organization (WHO) is the assumed average maintenance dose per day for a drug used for its main indication in adults. Cumulative DDD (cDDD) was calculated by summing the total DDD during the study period. Cumulative prescription days were measured by accumulating the days of lithium prescriptions for all visits within the study period. Average daily dose was expressed as cDDD divided by cumulative prescription days. Dose–response relationships regarding incidence were evaluated in three categories based on the amount of cDDD and average daily DDD: low (less than 33rd percentile), medium (between 33rd and 66th percentile) and high (above the 66th percentile).
As the ICD-9-CM diagnosis may be coded for disease-screening purposes in the NHI medical claims database, cancer diagnosis was confirmed by the Registry for Catastrophic Illness (ICD-9-CM codes for malignant neoplasm: 140–208). To be included in the Registry, patient medical records have to be reviewed and approved according to pathology evaluation and/or cytology evidence for exemption from copayments for the treatment of the disease. It includes approximately 90–92% of cancer patients in the Taiwan Cancer Registry.
Covariates
Analysis covariates included gender, age at the index date, comorbidities and comedications. Baseline comorbidities were defined as any appearance of corresponding ICD-9-CM codes in the in-patient or out-patient records during the 12 months prior to the index date. Diseases included those listed in the Deyo–Charlson Comorbidity Index, which summarises 17 diagnostic categories Reference Deyo, Cherkin and Ciol13 (online Table DS1). Some diseases with a higher prevalence in patients with bipolar disorder than in the general population were also considered, including other acute or chronic ischaemic diseases, hypertension, arrhythmias, dyslipidaemia and obesity. In addition, psychiatric comorbidities comprised schizophrenia, other psychosis, depression, anxiety disorders, alcohol-related disorders and substance-related disorders Reference Gerhard, Devanand, Huang, Crystal and Olfson14 (online Table DS1).
Baseline comedications were identified by prescriptions dispensed during the 6 months prior to the index date, including metformin, sulfonylureas, aspirin, non-steroidal anti-inflammatory drugs (NSAIDs), cyclo-oxygenase-2 (COX-2) inhibitors, statins, systemic steroids, angiotensin-converting enzyme inhibitors (ACEIs), angiotensin II receptor blockers (ARBs), antidepressants (tricyclic antidepressants (TCAs), selective serotonin reuptake inhibitors (SSRIs) and other antidepressants), first-generation antipsychotics (FGAs), second-generation antipsychotics (SGAs) and anxiolytics/hypnotics Reference Pottegard, Friis, Andersen and Hallas15 (online Table DS2).
Statistical analysis
For between-group comparisons, t-tests or ANOVAs were used for continuous variables, and chi-squared tests or Fisher's exact tests were used for nominal variables.
Logistic regression was used to estimate the predicated probability (propensity score) of the treatment drug for each patient, including the covariates of baseline comorbidities and comedication (online Tables DS1 and DS2). Reference Brookhart, Wyss, Layton and Sturmer16 Inverse probability of treatment weights (IPTW) were calculated as the inverse of the estimated propensity score for the lithium group and the inverse of one minus the estimated propensity score for the control group. Reference Austin17 The IPTW was applied to the regression analysis to create a pseudo-population with a similar covariate distribution of individual treatment groups. The Cox regressions were used to estimate hazard ratios (HRs) and their 95% confidence intervals for the occurrence of cancer and for the various dose–response categories. Two models were implemented to investigate effects from various groups of covariates. Model I was weighted by IPTW. Model II additionally included cancer-risk-related drugs. All the analysis variables in the Cox regressions were examined for possible violation of proportional hazards assumptions, and time-dependent covariates were added if the assumption was not met.
The crude incidence for different types of cancer was calculated for individuals on lithium with and without anticonvulsant use and for anticonvulsant-only users. The numerator for the incidence was number of patients with each type of cancer during follow-up, and the denominator was the number of person-years of follow-up. All data management and statistical analyses were performed using SAS 9.4 software. All statistical tests in this study were two-sided. P<0.05 was considered statistically significant. A post hoc power analysis was also conducted using the POWER procedure in SAS.
Sensitivity and subgroup analyses
We also conducted several sensitivity analyses. First, we broadened the definition for cancer diagnosis to at least one ICD-9-CM code in the claims database. Although cancer development may need a longer time than 10 years, and although sometimes ICD-9 coding was given for screening purposes, this definition could be a better representation of suspected early cancer signs. Second, we only included prescriptions of lithium or anticonvulsants prescribed by psychiatrists. Because anticonvulsants may have other indications, such as epilepsy and neuropathic pain, this restriction reduces confounding. Third, patients with an epilepsy diagnosis prior to the index date were excluded to investigate the potential confounding of other indications for anticonvulsants. Reference Kaae, Carstensen, Wohlfahrt, Melbye and Boyd18
Combined therapies are common in bipolar disorder treatments. They are often used to discontinue or shift to another medication if the patient cannot tolerate the side-effects of the drug. Although the association between valproate and cancer risk is still uncertain, Reference Singh, Bell, Driever and Sander19–Reference Kang, Gillespie, Goodman, Brodie, Brandes and Ribeiro21 the fourth sensitivity analysis was conducted to evaluate cancer risk of therapies involving lithium and valproate prescribed alone, in combination, or one of these two drugs followed sequentially by the other. Fifth, a number of studies have investigated the relationship between cancer and psychiatric agents, such as TCAs, SSRIs and SGAs, Reference Pottegard, Friis, Andersen and Hallas15,Reference Boursi, Lurie, Mamtani, Haynes and Yang22–Reference Fond, Macgregor, Attal, Larue, Brittner and Ducasse25 therefore, these psychiatric agents may have confounded our results. We performed backward selection to find the most and least significant psychiatric drug variables in the full model, and we analysed the cancer risk in each permutation.
To assess whether the overall risk of cancer varied across patient characteristics, we performed analyses stratified by subgroups of various characteristics of the patients, including age and the Charlson Comorbidity Index (CCI).
Results
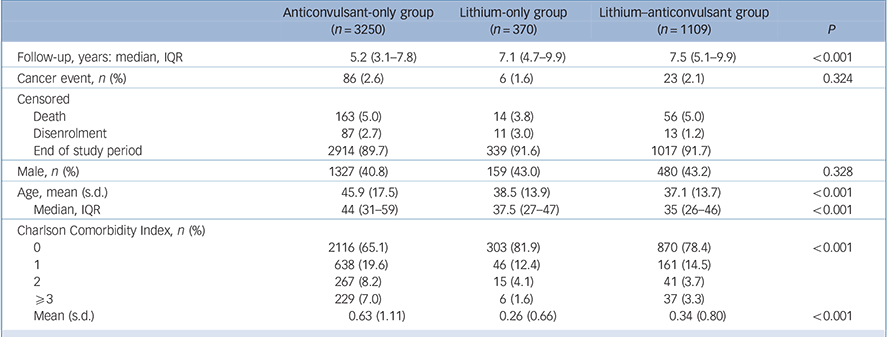
We identified 4729 patients with bipolar disorder, of which 3250 (68.7%) were anticonvulsant-only users, 370 (7.8%) were lithium-only users and 1109 (23.5%) were lithium and anticonvulsants users. Median follow-up time for the lithium-only group was 7.1 (interquartile range (IQR) = 4.7–9.9) years, for the lithium and anticonvulsants group it was 7.5 (IQR = 5.1–9.9) years and for the anticonvulsant-only group it was 5.2 (IQR = 3.1–7.8) years. The majority (97.6%) of patients were censored by the end of the study period, followed by death and disenrolment (Table 1). In total 115 cases of cancer were identified, and the mean (s.d.) age of cancer incidence in our study cohort was 60.7 (15.3) years. There were 86 individuals (2.65%) with newly diagnosed cancer during follow-up (4.74 cases per 1000 person-years) in the anticonvulsant-only group and 29 individuals (1.96%) with newly diagnosed cancer during follow-up (2.66 cases per 1000 person-years) in the combined lithium with or without anticonvulsants group (online Table DS3).
Table 1 Baseline characteristics of study groups a
| Anticonvulsant-only group (n = 3250) |
Lithium-only group (n = 370) |
Lithium–anticonvulsant group (n = 1109) |
P | |
|---|---|---|---|---|
| Follow-up, years: median, IQR | 5.2 (3.1–7.8) | 7.1 (4.7–9.9) | 7.5 (5.1–9.9) | <0.001 |
| Cancer event, n (%) | 86 (2.6) | 6 (1.6) | 23 (2.1) | 0.324 |
| Censored | ||||
| Death | 163 (5.0) | 14 (3.8) | 56 (5.0) | |
| Disenrolment | 87 (2.7) | 11 (3.0) | 13 (1.2) | |
| End of study period | 2914 (89.7) | 339 (91.6) | 1017 (91.7) | |
| Male, n (%) | 1327 (40.8) | 159 (43.0) | 480 (43.2) | 0.328 |
| Age, mean (s.d.) | 45.9 (17.5) | 38.5 (13.9) | 37.1 (13.7) | <0.001 |
| Median, IQR | 44 (31–59) | 37.5 (27–47) | 35 (26–46) | <0.001 |
| Charlson Comorbidity Index, n (%) | ||||
| 0 | 2116 (65.1) | 303 (81.9) | 870 (78.4) | <0.001 |
| 1 | 638 (19.6) | 46 (12.4) | 161 (14.5) | |
| 2 | 267 (8.2) | 15 (4.1) | 41 (3.7) | |
| ⩾3 | 229 (7.0) | 6 (1.6) | 37 (3.3) | |
| Mean (s.d.) | 0.63 (1.11) | 0.26 (0.66) | 0.34 (0.80) | <0.001 |
a. See online Table DS4 for a version of this table that includes a larger number of variables.
Baseline characteristics are compared in Table 1 (see online Table DS4 for a more detailed version of this table). Patients taking lithium were younger than the anticonvulsant-only group (P<0.001). The CCI indicated lower scores for those taking lithium (P<0.001). Although the prevalence rates of physical comorbidities were lower in those taking lithium, the prevalence rates of psychiatric comorbidities were higher in individuals with lithium exposure (except for anxiety) (online Table DS4).
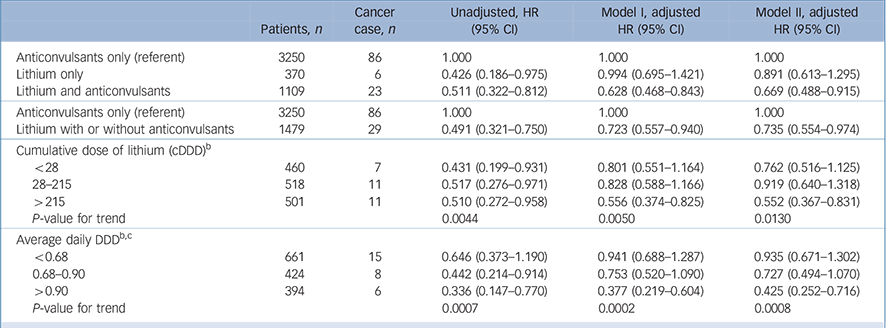
Separate Cox regressions were implemented to investigate the hazard ratios of cancer risks in the groups (Table 2). The unadjusted hazard ratios were 0.426 (95% CI 0.186–0.975) in the lithium-only group and 0.511 (95% CI 0.322–0.812) in the lithium and anticonvulsant group, using the anticonvulsant-only group as the reference. Because the sample size of the lithium-only group was small, we also computed the hazard ratio for the combined group using lithium with or without anticonvulsants; the hazard ratio was 0.491 (95% CI 0.321–0.750). After being weighted using the IPTW approach (Model I) and being additionally adjusted for other chemoprevention drugs (Model II), only the hazard ratio of the lithium and anticonvulsant group (Model I: 0.628, 95% CI 0.468–0.843; Model II: 0.669, 95% CI 0.488–0.915) and the combined lithium with or without anticonvulsants group (Model I: 0.723, 95% CI 0.557–0.940; Model II: 0.735, 95% CI 0.554–0.974) remained statistically significant. Although the number of cancer cases was not large in Table 2, we conducted post hoc power analyses of significant hazard ratios and found that most powers were higher than 80%; four hazard ratios (0.723, 0.735, 0.556 and 0.552) were between 71 and 78%. For site-specific cancer, lithium users showed trends of decreased cancer risks, except for bone, skin, and connective and other soft tissue cancer risks (HR = 3.012, 95% CI 0.798–11.365) and genitourinary cancer risk (HR = 1.014, 95% CI 0.472–2.179, online Table DS3).
Table 2 Lithium exposure and hazard ratios for overall cancer risk a
| Patients, n | Cancer case, n |
Unadjusted, HR (95% CI) |
Model I, adjusted HR (95% CI) |
Model II, adjusted HR (95% CI) |
|
|---|---|---|---|---|---|
| Anticonvulsants only (referent) | 3250 | 86 | 1.000 | 1.000 | 1.000 |
| Lithium only | 370 | 6 | 0.426 (0.186–0.975) | 0.994 (0.695–1.421) | 0.891 (0.613–1.295) |
| Lithium and anticonvulsants | 1109 | 23 | 0.511 (0.322–0.812) | 0.628 (0.468–0.843) | 0.669 (0.488–0.915) |
| Anticonvulsants only (referent) | 3250 | 86 | 1.000 | 1.000 | 1.000 |
| Lithium with or without anticonvulsants | 1479 | 29 | 0.491 (0.321–0.750) | 0.723 (0.557–0.940) | 0.735 (0.554–0.974) |
| Cumulative dose of lithium (cDDD) b | |||||
| <28 | 460 | 7 | 0.431 (0.199–0.931) | 0.801 (0.551–1.164) | 0.762 (0.516–1.125) |
| 28–215 | 518 | 11 | 0.517 (0.276–0.971) | 0.828 (0.588–1.166) | 0.919 (0.640–1.318) |
| >215 | 501 | 11 | 0.510 (0.272–0.958) | 0.556 (0.374–0.825) | 0.552 (0.367–0.831) |
| P-value for trend | 0.0044 | 0.0050 | 0.0130 | ||
| Average daily DDD b,c | |||||
| <0.68 | 661 | 15 | 0.646 (0.373–1.190) | 0.941 (0.688–1.287) | 0.935 (0.671–1.302) |
| 0.68–0.90 | 424 | 8 | 0.442 (0.214–0.914) | 0.753 (0.520–1.090) | 0.727 (0.494–1.070) |
| >0.90 | 394 | 6 | 0.336 (0.147–0.770) | 0.377 (0.219–0.604) | 0.425 (0.252–0.716) |
| P-value for trend | 0.0007 | 0.0002 | 0.0008 | ||
HR, hazard ratio; DDD, defined daily dose.
a. Model I: weighted by inverse probability of treatment weights (IPTW); Model II: weighted by IPTW and adjusted for the usage of non-steroidal anti-inflammatory drugs, aspirin, cyclo-oxygenase-2 inhibitors, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, metformin, sulfonylurea, systemic steroid and statin.
b. Referent group: never-users of lithium.
c. Average daily DDD is the cumulative defined daily dose divided by the cumulative prescription days.
When considering the dose–response of lithium usage, there were significant trends of reduced overall cancer risk with increasing cumulative dose of lithium (P-value for trends <0.05; Table 2). Users of lithium were divided into three tertiles by cumulative dose of lithium (cDDD) and average daily DDD. Significantly lower risks were found in the third tertile of cDDD (cDDD>215, HR = 0.552, 95% CI 0.367–0.831) and average daily DDD (DDD>0.9, HR = 0.425, 95% CI 0.252–0.716).
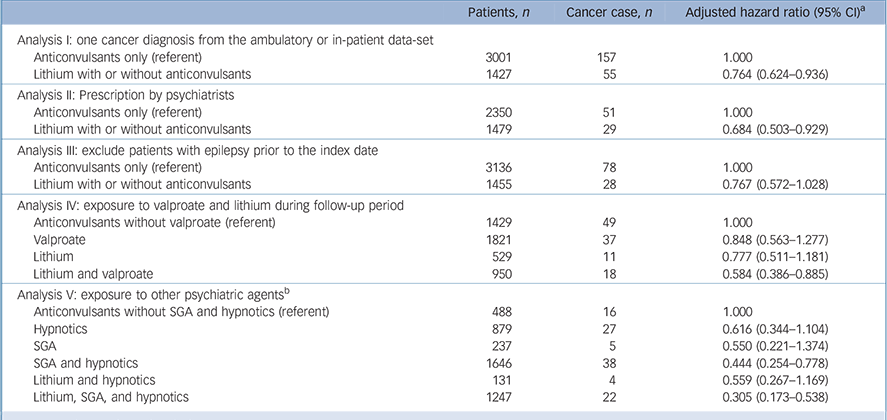
The sensitivity analyses consistently showed a reduced risk of overall cancer for lithium users v. anticonvulsant-only users (Table 3). In the broader definition of cancer diagnosis, the hazard ratio was close to the primary analysis (HR = 0.764, 95% CI 0.624–0.936). After restricting the analysis to those with psychiatrists' prescriptions in both drug groups, the HR estimates were slightly lower than those of the primary analysis (HR = 0.684, 95% CI 0.503–0.929) (Table 3). In the analysis excluding patients with epilepsy prior to the index date, lithium users showed a trend of decreased cancer risk, but it was non-significant (HR = 0.767, 95% CI 0.572–1.028). In the fourth analysis, valproate users or lithium users had similarly lower cancer risks (HR = 0.848, 95% CI 0.563–1.277 for valproate users; HR = 0.777, 95% CI 0.511–1.181 for lithium users). There was a significantly decreased overall cancer risk in the lithium and valproate combined or sequential therapy group (HR = 0.584, 95% CI 0.386–0.885). In the fifth analysis, we chose to analyse the cancer risk related to lithium, SGA and hypnotics because patients had exposure to these agents. The hazard ratio of cancer risk for both SGA/hypnotics and lithium/SGA/hypnotics combinations had statistically lower hazard ratios (HR = 0.444, 95% CI 0.254–0.778 for SGA/hypnotics; HR = 0.305, 95% CI 0.173–0.538 for lithium/SGA/hypnotics). The hazard ratio for the lithium/SGA/hypnotics treatment was lower than that for SGA/hypnotics treatment, supporting our finding that lithium has a protective effect on overall cancer risk.
Table 3 Sensitivity analyses
| Patients, n | Cancer case, n | Adjusted hazard ratio (95% CI) a | |
|---|---|---|---|
| Analysis I: one cancer diagnosis from the ambulatory or in-patient data-set | |||
| Anticonvulsants only (referent) | 3001 | 157 | 1.000 |
| Lithium with or without anticonvulsants | 1427 | 55 | 0.764 (0.624–0.936) |
| Analysis II: Prescription by psychiatrists | |||
| Anticonvulsants only (referent) | 2350 | 51 | 1.000 |
| Lithium with or without anticonvulsants | 1479 | 29 | 0.684 (0.503–0.929) |
| Analysis III: exclude patients with epilepsy prior to the index date | |||
| Anticonvulsants only (referent) | 3136 | 78 | 1.000 |
| Lithium with or without anticonvulsants | 1455 | 28 | 0.767 (0.572–1.028) |
| Analysis IV: exposure to valproate and lithium during follow-up period | |||
| Anticonvulsants without valproate (referent) | 1429 | 49 | 1.000 |
| Valproate | 1821 | 37 | 0.848 (0.563–1.277) |
| Lithium | 529 | 11 | 0.777 (0.511–1.181) |
| Lithium and valproate | 950 | 18 | 0.584 (0.386–0.885) |
| Analysis V: exposure to other psychiatric agents b | |||
| Anticonvulsants without SGA and hypnotics (referent) | 488 | 16 | 1.000 |
| Hypnotics | 879 | 27 | 0.616 (0.344–1.104) |
| SGA | 237 | 5 | 0.550 (0.221–1.374) |
| SGA and hypnotics | 1646 | 38 | 0.444 (0.254–0.778) |
| Lithium and hypnotics | 131 | 4 | 0.559 (0.267–1.169) |
| Lithium, SGA, and hypnotics | 1247 | 22 | 0.305 (0.173–0.538) |
SGA, second-generation antipsychotics.
a. Model weighted by inverse probability of treatment weights (IPTW) and adjusted for the usage of non-steroidal anti-inflammatory drugs, aspirin, cyclo-oxygenase-2 inhibitors, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, metformin, sulfonylurea, systemic steroid and statin.
b. Medication groups with less than 100 patients were not included.
In the subgroup analysis of age groups, lithium users between 18 and 44 years of age (HR = 0.570, 95% CI 0.339–0.959) were associated with decreased cancer risk (Table 4). Similarly, patients without comorbidities (CCI = 0) had a significant protective effect with lithium usage.
Table 4 Subgroup analyses
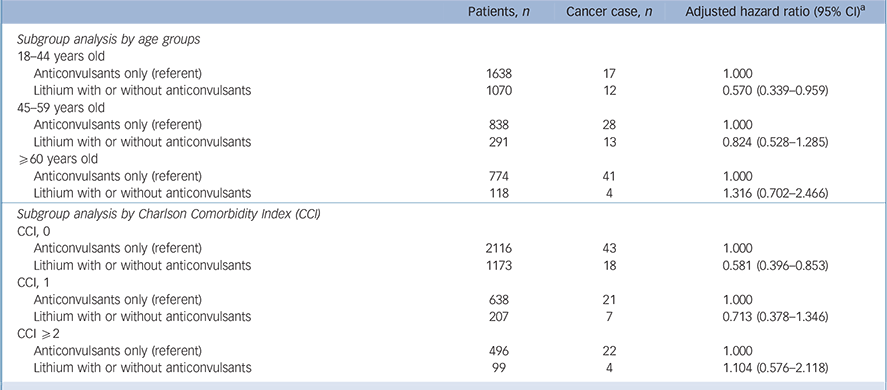
| Patients, n | Cancer case, n | Adjusted hazard ratio (95% CI) a | |
|---|---|---|---|
| Subgroup analysis by age groups | |||
| 18–44 years old | |||
| Anticonvulsants only (referent) | 1638 | 17 | 1.000 |
| Lithium with or without anticonvulsants | 1070 | 12 | 0.570 (0.339–0.959) |
| 45–59 years old | |||
| Anticonvulsants only (referent) | 838 | 28 | 1.000 |
| Lithium with or without anticonvulsants | 291 | 13 | 0.824 (0.528–1.285) |
| ⩾60 years old | |||
| Anticonvulsants only (referent) | 774 | 41 | 1.000 |
| Lithium with or without anticonvulsants | 118 | 4 | 1.316 (0.702–2.466) |
| Subgroup analysis by Charlson Comorbidity Index (CCI) | |||
| CCI, 0 | |||
| Anticonvulsants only (referent) | 2116 | 43 | 1.000 |
| Lithium with or without anticonvulsants | 1173 | 18 | 0.581 (0.396–0.853) |
| CCI, 1 | |||
| Anticonvulsants only (referent) | 638 | 21 | 1.000 |
| Lithium with or without anticonvulsants | 207 | 7 | 0.713 (0.378–1.346) |
| CCI ⩾2 | |||
| Anticonvulsants only (referent) | 496 | 22 | 1.000 |
| Lithium with or without anticonvulsants | 99 | 4 | 1.104 (0.576–2.118) |
a. Model weighted by inverse probability of treatment weights (IPTW) and adjusted for the usage of non-steroidal anti-inflammatory drugs, aspirin, cyclo-oxygenase-2 inhibitors, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, metformin, sulfonylurea, systemic steroid and statin.
Discussion
Main findings
Previous studies have shown that patients with bipolar disorder are at increased risk for cancer. Reference Lin, Chen, Kuo, Jaiteh, Wu and Lo26,Reference Hung, Yang, Huang, Lung, Lin and Chen27 In the Taiwan cancer registry, the crude cancer incidence rates during our study duration (1999–2010) were 2.55 to 4.00 per 1000 person-years. 28 The cancer incidence rates in our study cohort of 4.74 cases per 1000 person-years in the anticonvulsant-only group and 2.66 cases per 1000 person-years in the lithium with or without anticonvulsants group (online Table DS3) are consistent with previous reports that patients with bipolar disorder are at increased risk for cancer. We demonstrate that lithium usage in patients with bipolar disorder is associated with a 26.5% lower risk (HR = 0.735, 95% CI 0.554–0.974) of overall cancer, and a cDDD of more than 215 is associated with a 44.8% lower risk (HR = 0.552, 95% CI 0.367–0.831). To our knowledge, this is the first population-based study to demonstrate overall reduced cancer risk in patients with bipolar disorder taking lithium.
Lithium was described in 1996 as an inhibitor of GSK-3. Reference Brown and Tracy4 Previous research regarding the association of GSK-3 with cancer was limited in in vivo and in vitro studies. Although it may not be feasible to conduct clinical studies to investigate the association of lithium usage and cancer development, the availability of large-scale population-based data has become a useful resource to investigate possible chemoprevention effects in the population. One previous hospital-based study involving psychiatric patients showed a lower but non-significant risk (OR = 0.79, 95% CI 0.17–3.60) of cancer of non-epithelial origin in the lithium group. Reference Cohen, Chetrit, Cohen, Sirota and Modan6 Although we were able to report hazard ratios from different types of cancer, one still needs to interpret results with caution because the number of specific cancer cases was low and there may not be enough statistical power. The duration of bipolar disorder treatment is generally from 6 months to 3 years, depending on individual clinical considerations. Reference Grunze, Vieta, Goodwin, Bowden, Licht and Moller29,Reference Bai, Chang, Tsai, Chen, Hsiao and Li30 Long-term exposure is an important drug safety issue. Our study showed that the protective effect of lithium only exists when the cDDD in the highest tertile is reached. Nevertheless, we did not see a similar finding for cumulative prescription days. We suspected that there are two exposure situations, higher daily dose with shorter duration and lower daily dose with longer duration. An average daily dose assessment was further performed and the dose–response relationship was proven. However, more research is still needed to investigate the cumulative dose and days to reach the effect.
As lithium users were younger and had lower CCIs, age and CCI may be important confounders in our results. Only in the youngest stratification and lowest CCI stratification were the positive effects of lithium for cancer risk shown. This may result from there not being enough power in the subgroup analysis. Nevertheless, the hazard ratio of every subgroup showed a trend of lower overall cancer risk, except for patients older than 60 years.
As there are several medication options for bipolar disorder treatment, it is important to evaluate whether they differ with regard to the risk of cancer. Valproate is one of the anticonvulsants used for bipolar disorder. The effects of valproate on GSK-3 have been extensively studied, but there are no consistent conclusions. This discrepancy possibly results from minor differences in substrates or assay conditions. Reference Rowe, Wiest and Chuang31 Valproate has been reported to act as a histone deacetylase (HDAC) inhibitor, which may be associated with several haematological malignancies. Several preclinical studies support the anticarcinogenic effects of valproate, although conclusions in epidemiological studies are inconsistent. Reference Singh, Bell, Driever and Sander19–Reference Kang, Gillespie, Goodman, Brodie, Brandes and Ribeiro21 We restricted the analysis of anticonvulsants to valproate and evaluated the effect of valproate in our sensitivity study. Lithium–valproate exposure has an additional effect for lower overall risk of cancer compared with either lithium or valproate exposure in patients with bipolar disorder. Our finding supports the idea that lithium has some protective effect regardless of valproate. There were also many other psychiatric agents that were prescribed during the follow-up period that may interfere with the results. We found that SGA and hypnotics use in the follow-up period reduced overall cancer risk. Moreover, lithium/SGA/hypnotic users had a lower risk than SGA/hypnotics users. These findings enhanced the idea of a protective effect from lithium despite the fact that a protective effect may also be derived from other psychiatric agents.
Limitations
The present study has some limitations that warrant attention. First, possible misclassification may occur when extracting patients only from records of an ambulatory and in-patient care database. In this study, the diagnoses of cancer were confirmed by linking to the catastrophic illness database. The registration of cancer as a catastrophic illness is very rigorous and has to be approved by evaluating pathology and/or cytology evidence. Hence, the catastrophic illness database has been widely used in previous studies to ascertain cancer events. Reference Lin, Chen, Kuo, Jaiteh, Wu and Lo26,Reference Hung, Yang, Huang, Lung, Lin and Chen27 Although bipolar disorder may be misdiagnosed, the probability was less likely because all prescriptions should have a corresponding diagnosis in the rigorous claims review system, which is the main indicator of lithium for bipolar disorder. Anticonvulsants have a very diverse range of indications. Two sensitivity analyses (limited to prescriptions by psychiatrists and excluding patients with epilepsy) were performed to strengthen our results regarding patients with bipolar disorder. In the analysis excluding patients with epilepsy prior to the index date, lithium users showed a non-significant trend towards decreased cancer risk (HR = 0.767, 95% CI 0.572–1.028). This supports the finding that the indications for anticonvulsants in our study cohort may affect the hazard ratio. However, this would not affect the conclusion of a positive effect of lithium on overall cancer risk.
Second, cancer may develop over a long period of time and most observational studies, including this study, do not include a sufficient follow-up period. Abnormal symptoms may be present several years before a definite diagnosis. However, the same definition is applied to both comparison groups; therefore, the possible misclassification may only have a limited effect on the risk. We adopted a non-strict definition of diagnosis, and the results demonstrated that lithium users had a lower risk of early abnormal signs. The results could be evidence of a lithium effect. However, there is still a need for more studies with longer follow-up to strengthen our conclusion.
Third, because the purpose of the NHI database is reimbursement, there is no information on risk factors for cancer, such as family history, body mass index, smoking or any laboratory parameters. We used patient comorbidities as surrogates to adjust for patient health status and lifestyle in our study. Although there are other unmeasured confounders, we believe the methodology used in this study is robust. Fourth, the actual amount of drugs taken could not be determined from the database. Therefore, our conclusion is based on reasonable adherence with medication use. Fifth, there is no histopathological information in the LHID data-set, therefore we were not able to investigate this issue. A previous study indicated that the association between lithium and cancer risk may depend on the type of cancer. Reference McCubrey, Davis, Abrams, Montalto, Cervello and Basecke2,Reference Cohen, Chetrit, Cohen, Sirota and Modan6
Finally, we may not have identified all individuals taking psychiatric medications. However, psychiatric agents are not over-the-counter drugs. Moreover, bipolar disorder is one of the chronic psychoses on the Registry for Catastrophic Illness in Taiwan. When patients were approved by the Registry, the disease-specific treatment cost was covered by the NHI programme, and no copayment for the ambulatory visit or for in-patient care was required. For these reasons, we anticipate that the probability of patient-used self-paid psychiatric agents is very low. Furthermore, we believe most drug-exposure information in our study cohort is completely documented in the database, even though the data-set does not contain information on out-of-pocket treatments.
Implications
The present findings imply that lithium use was associated with a lower incidence of overall cancer risk in patients with bipolar disorder. There is a dose–response relationship for lithium use and a higher cumulative dose was associated with significant risk reduction in overall cancer risk. Further studies are needed that focus on the impact on different types of cancer.








eLetters
No eLetters have been published for this article.