Bipolar disorder is characterised by recurrent episodes of mania and depression, interspersed with periods of apparently complete recovery. The central tenet of recovery has been challenged recently by the high incidence of psychosocial difficulties during remission (Reference ScottScott, 1995) and reports of stable structural abnormalities in the brains of patients with bipolar disorder (Reference Soares and MannSoares & Mann, 1997). Neuropsychological deficits have been reported in previous investigations of patients with bipolar disorder studied in remission (see Reference Martinez-Aran, Vieta and ColomMartinez-Aran et al, 2000, for review), but Ferrier et al (Reference Ferrier, Stanton and Kelly1999) have highlighted the confounding effects of minor affective symptoms on supposedly ‘trait’ performance in bipolar disorder. Neuropsychological testing may give a more specific indication of the functional networks that are abnormal in bipolar disorder and potentially may provide a link to symptomatology. Previous results have guided the choice of tests employed in the present study, with particular emphasis given to ‘executive’ tasks linked to the integrity of the prefrontal cortex. Preliminary evidence supports the hypothesis that these tests are differentially sensitive to damage within human prefrontal cortex (e.g. Reference Bechara, Damasio and DamasioBechara et al, 1994; Reference Dias, Robbins and RobertsDias et al, 1996; Reference Owen, Evans and PetridesOwen et al, 1996). The relationship between neuropsychological effects and clinical variables was also examined in an effort to distinguish a deterioration of function with progression of bipolar disorder (or its treatment) from a trait vulnerability marker under genetic or early environmental control.
METHOD
Subjects
The study was approved by Oxfordshire Psychiatric Research Ethics Committee. Forty-two patients identified from current registers of patients who had been admitted as in-patients and meeting DSM-IV (American Psychiatric Association, 1994) criteria for bipolar I disorder were approached to take part in the study and 32 agreed to participate. Two of the 32 patients tested were subsequently excluded on the basis of their current symptom ratings (see exclusion criteria, below).
Patients were aged 18-60 years and described as euthymic by their consultants. Two subjects had received electroconvulsive therapy (ECT) in the past but not within the preceding year. Four subjects had a prior history of substance misuse (one polydrug, one alcohol, two polydrug and alcohol) but had been abstinent for at least 1 year. Comorbid anxiety disorders were not present in the patient group at the time of testing, as assessed by a truncated version of the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID; Reference First, Spitzer and GibbonsFirst et al, 1995).
Five patients were off medication at the time of testing. The majority of patients were receiving mood-stabilising medication. For most patients (19/30) this was lithium carbonate (dose 400-1200 mg/day, mean 763 mg). Two patients were maintained primarily on carbamazepine, one patient on sodium valproate and one on a combination of the two. These mood-stabilising medications were taken typically in combination with a selective serotonin reuptake inhibitor (SSRI) (nine patients) or a neuroleptic (five patients). Two patients were receiving only SSRI medication. The present series was not, however, an excessively medicated group: 12/30 were on no treatment or on a monotherapy.
Thirty healthy controls were recruited using a psychology subject panel, advertisements and word of mouth. Controls had no prior psychiatric history and had no first-degree relatives with bipolar diagnoses.
Procedure
Neuropsychological testing lasted approximately 2 h. Subjects completed the tests in a fixed order with a break half-way. Four tests from the Cambridge Automated Neuropsychological Test Battery (CANTAB; CeNeS Ltd, Cambridge, UK) were employed (see Reference Elliott, Sahakian and McKayElliott et al, 1996 and Reference Purcell, Maruff and KyriosPurcell et al, 1997, for fuller descriptions), as well as the California Verbal Learning Test (CVLT; Reference Delis, Kramer and KaplanDelis et al, 1987) and a computerised version of the Iowa Gambling Task (Reference Bechara, Damasio and DamasioBechara et al, 1994). With the exception of the CVLT, all tasks were run on a Datalux 486 PC with a 10½-inch touch-screen monitor.
Subject mood was assessed formally using the Hamilton Rating Scale for Depression (HRSD; Reference HamiltonHamilton, 1960) and the Young Mania Rating Scale (Reference Young, Biggs and ZieglerYoung et al, 1978). A cut-off score of 8 was used on both scales to define euthymia, in addition to the prior judgement of the referring clinician. Level of intelligence was assessed in the two groups for the purposes of matching using three measures: years spent in education; the National Adult Reading Test (NART; Reference Nelson and O'ConnellNelson & O'Connell, 1978), which is a measure of premorbid verbal IQ; and the Block Design sub-test of the Wechsler Adult Intelligence Scale — Revised (WAIS—R; Reference WechslerWechsler, 1981).
Neuropsychological tasks
Spatial working memory
Spatial working memory is a self-ordered search task requiring subjects to update the working memory continually to avoid returning to previously searched locations. Dependent variables are ‘between-search errors’ (i.e. returning to boxes that previously have yielded tokens) and a ‘strategy score’ of how organised the search pattern is. Between-search errors are employed in the correlational analysis.
Tower of London
The Tower of London is a problem-solving task demanding forward planning in order to match the arrangements of coloured balls. There are a minimum number of moves in which each problem can be solved (2, 3, 4 and 5) and the subject has a maximum number of moves to solve each problem (5, 7, 9 and 12, respectively). Dependent variables of interest are the average number of moves taken to solve the 4— and 5-move problems and the overall number of problems solved in the minimum number of moves (used in correlational analysis).
Intradimensional—extradimensional shift (ID—ED shift)
The intradimensional—extradimensional shift is a visual discrimination task requiring set learning, reversal learning and an extradimensional set shift (EDS; similar to the Wisconsin Card Sort Test, WCST). The task enables an impairment in attentional set shifting per se to be separated from impairments in concept formation or reversing reward associations, processes that are conflated in the WCST. Dependent variables are summed errors on stages of reversal, errors at EDS stage and summed errors across all stages. Errors at EDS are employed in the correlational analysis.
Rapid visual information processing (RVIP)
Rapid visual information processing is a continuous performance test lasting 7 min, requiring the detection of three-digit target sequences. Dependent variables were the percentage of targets detected, the number of false alarms and the latency to respond. Percentage of targets detected was employed in the correlational analysis.
California Verbal Learning Test (CVLT)
The California Verbal Learning Test is an auditory verbal memory test using a 16-item shopping list that is read to the subject five times. After each trial subjects must repeat back as many items as they can remember (learning score: trials 1-5 summed, used in the correlational analysis). Immediate and delayed (20 min later) free recall also are assessed, followed by item recognition (employing the 16 targets with 28 distractors).
Iowa Gambling Task
The Iowa Gambling Task is a decisionmaking task demanding a series of choices from four decks of cards (A, B, C, D), each characterised by a different reward—punishment profile. Large wins on decks A and B are paired with larger penalties, resulting in net loss. Smaller wins on decks C and D are associated with smaller penalties, and hence net profit. Control subjects typically acquire a bias towards deck C and D within about 40 trials. The main dependent variable on the task is the summed number of choices from the risky decks (A+B).
Statistical procedures
Independent-sample t-tests were performed on key demographic variables (age and measures of intelligence) to determine whether the two groups were adequately matched. Mann—Whitney U tests were applied to the scores from the mood rating scales. Planned comparisons were made for each of the neuropsychological variables with independent-samplet-tests, with the exception of the ID—ED shift data, which were non-parametric and consequently analysed with Mann—Whitney U tests. Partial correlations controlling for score on the HRSD and Young Mania Rating Scale were used to investigate the contribution of mild affective symptoms to neuropsychological performance. Analysis of covariance (ANCOVA) was used to achieve the same controlling procedure in the Ferrier et al (Reference Ferrier, Stanton and Kelly1999) report. However, ANCOVA should be used only where subjects are assigned randomly to two (or more) experimental groups and are found to differ spuriously with regard to a potentially confounding variable (e.g. age). The ANCOVA assumes that the confounding variable would not be present in a sufficiently large sample. This is clearly not the case here, because the groups of subjects are selected on the basis of a psychiatric diagnosis where the confounds (mood scores) are an inherent feature of one group. Keppell & Zedeck (Reference Keppel and Zedeck1990) recommend the use of partial correlations to explore confounding effects in such situations.
With a sample size of 30 per group, this design has a power of 0.80 to detect an effect size of approximately 1.0 with Cronbach's α=0.05. From previous research (Reference Coffman, Bornstein and OlsonCoffman et al, 1990; Reference MoriceMorice, 1990; Reference van Gorp, Altshuler and Thebergevan Gorp et al, 1998) such an effect size was expected for at least the attentional set shifting and verbal memory deficits. As an exploratory exercise, partial correlations were calculated between neuropsychological and psychiatric variables, controlling for age.
RESULTS
Group characteristics for the euthymic bipolar group and controls are displayed in Table 1. The two groups were matched for gender, age, years of education, NART IQ and WAIS Block Design raw score. Despite very low scores on the HRSD and Young scales, the euthymic bipolar group still scored significantly higher than controls on both measures.
Table 1 Group characteristics: mean (s.d.)
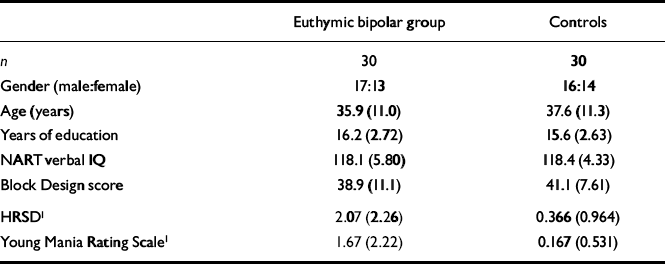
| Euthymic bipolar group | Controls | |
|---|---|---|
| n | 30 | 30 |
| Gender (male:female) | 17:13 | 16:14 |
| Age (years) | 35.9 (11.0) | 37.6 (11.3) |
| Years of education | 16.2 (2.72) | 15.6 (2.63) |
| NART verbal IQ | 118.1 (5.80) | 118.4 (4.33) |
| Block Design score | 38.9 (11.1) | 41.1 (7.61) |
| HRSD1 | 2.07 (2.26) | 0.366 (0.964) |
| Young Mania Rating Scale1 | 1.67 (2.22) | 0.167 (0.531) |
Neuropsychological test data are shown in Table 2. The euthymic bipolar group performed very similarly to matched controls on Tower of London, spatial working memory and the Iowa Gambling Task. Group comparisons revealed three effects: impaired target detection and reduced psychomotor speed on RVIP; increased total errors at ID—ED shift and, more specifically, increased errors at the stage of EDS but not at stages requiring reversal; and impaired immediate recall on the CVLT. However, when controlling for HRSD and Young scores in these analyses the only effect to remain significant was impaired target detection on RVIP.
Table 2 Neuropsychological performance in euthymic patients with bipolar disorder and controls: mean (s.d.)
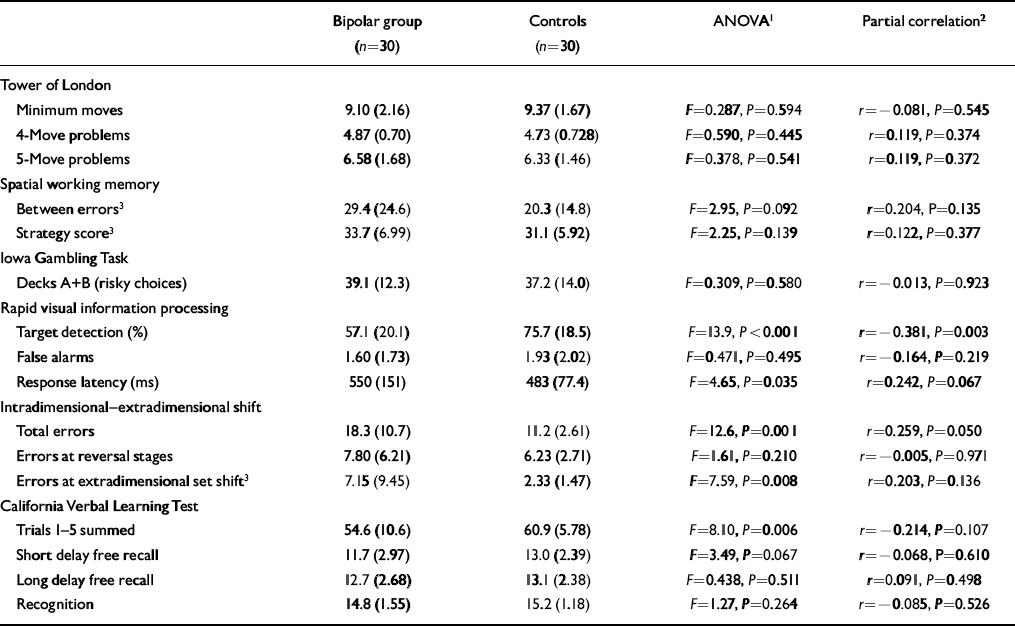
| Bipolar group (n=30) | Controls (n=30) | ANOVA1 | Partial correlation2 | |
|---|---|---|---|---|
| Tower of London | ||||
| Minimum moves | 9.10 (2.16) | 9.37 (1.67) | F=0.287, P=0.594 | r=-0.081, P=0.545 |
| 4-Move problems | 4.87 (0.70) | 4.73 (0.728) | F=0.590, P=0.445 | r=0.119, P=0.374 |
| 5-Move problems | 6.58 (1.68) | 6.33 (1.46) | F=0.378, P=0.541 | r=0.119, P=0.372 |
| Spatial working memory | ||||
| Between errors3 | 29.4 (24.6) | 20.3 (14.8) | F=2.95, P=0.092 | r=0.204, P=0.135 |
| Strategy score3 | 33.7 (6.99) | 31.1 (5.92) | F=2.25, P=0.139 | r=0.122, P=0.377 |
| Iowa Gambling Task | ||||
| Decks A+B (risky choices) | 39.1 (12.3) | 37.2 (14.0) | F=0.309, P=0.580 | r=-0.013, P=0.923 |
| Rapid visual information processing | ||||
| Target detection (%) | 57.1 (20.1) | 75.7 (18.5) | F=13.9, P<0.001 | r=-0.381, P=0.003 |
| False alarms | 1.60 (1.73) | 1.93 (2.02) | F=0.471, P=0.495 | r=-0.164, P=0.219 |
| Response latency (ms) | 550 (151) | 483 (77.4) | F=4.65, P=0.035 | r=0.242, P=0.067 |
| Intradimensional—extradimensional shift | ||||
| Total errors | 18.3 (10.7) | 11.2 (2.61) | F=12.6, P=0.001 | r=0.259, P=0.050 |
| Errors at reversal stages | 7.80 (6.21) | 6.23 (2.71) | F=1.61, P=0.210 | r=-0.005, P=0.971 |
| Errors at extradimensional set shift3 | 7.15 (9.45) | 2.33 (1.47) | F=7.59, P=0.008 | r=0.203, P=0.136 |
| California Verbal Learning Test | ||||
| Trials 1-5 summed | 54.6 (10.6) | 60.9 (5.78) | F=8.10, P=0.006 | r=-0.214, P=0.107 |
| Short delay free recall | 11.7 (2.97) | 13.0 (2.39) | F=3.49, P=0.067 | r=-0.068, P=0.610 |
| Long delay free recall | 12.7 (2.68) | 13.1 (2.38) | F=0.438, P=0.511 | r=0.091, P=0.498 |
| Recognition | 14.8 (1.55) | 15.2 (1.18) | F=1.27, P=0.264 | r=-0.085, P=0.526 |
Target detection on RVIP may be confounded by a tendency to respond impulsively on the task, and signal detection analysis can be applied to data to exclude this possibility. Signal detection analysis derives two independent measures of performance: target sensitivity and response bias (Reference GrierGrier, 1971). One-way analysis of variance (ANOVA) revealed a highly significant difference between the euthymic bipolar group and controls on target sensitivity (F(1,58)=15.4, P<0.001), and no difference on response bias (F(1,58)=1.16, P=0.286).
The RVIP data also may be examined over time: in tasks of sustained attention it is typical to find decreasing performance over the duration of the task. This ‘vigilance decrement’ may be separable from the overall accuracy of responding across the whole task duration. This was examined using a repeated-measures ANOVA with one within-subject factor (seven levels; time on task) and one between-subject factor (bipolar group, controls). Apart from the established main effect of the group, there was a significant main effect of time (F=2.96, P=0.015) and the interaction of time × group approached significance (F=2.04,P=0.076). Post hoc t-tests revealed no significant difference between groups in the first minute of the task but a significant difference (P<0.05) at all subsequent time points. The euthymic bipolar group, therefore, showed a slightly more pronounced vigilance decrement than the controls did on a 7-min task of sustained attention (see Fig. 1).
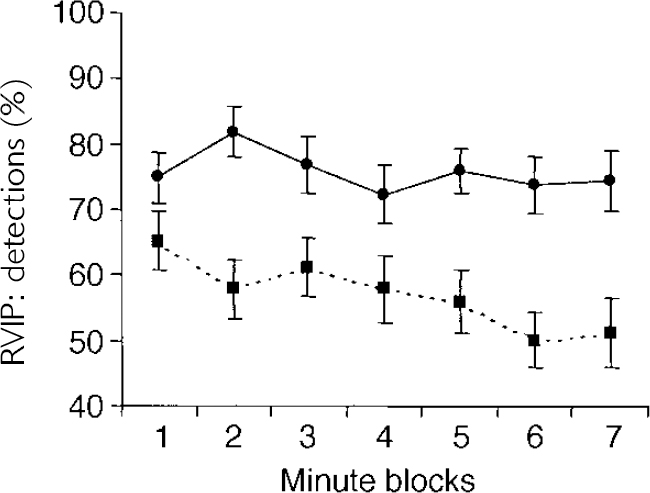
Fig. 1 Performance of patients with bipolar disorder tested in the euthymic state (— — -, n=30) and healthy controls (—, n=30) on the rapid visual information processing (RVIP) task of sustained attention. Performance is grouped into I-min time blocks (mean, s.e.).
Relationship between neuropsychological performance and clinical variables
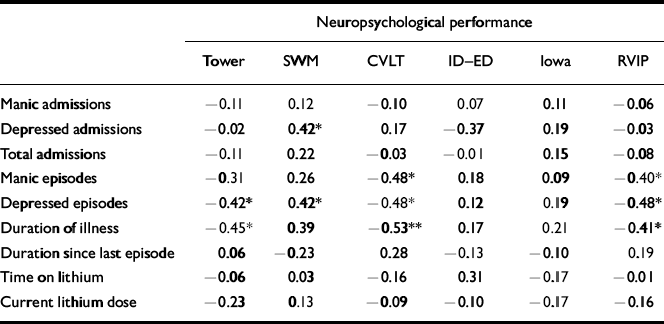
To restrict the large number of correlations that could be computed from these data, the most representative variable from each of the six tasks was included (see Method). Nine clinical variables were examined: number of manic, depressive and total admissions; number of manic and depressive episodes; duration (in months) since first episode; duration (in weeks) since the start of the most recent episode; duration (in months) ever treated with lithium carbonate; and current dosage of lithium carbonate. Partial correlations, controlling for age, are presented in Table 3. It is clear that performance on a number of tests, including RVIP, is correlated with progression of bipolar disorder, as measured by duration since first episode, and number of depressive episodes, in particular.
Table 3 Partial correlations of neuropsychological measures against psychiatric variables, controlling for age
| Neuropsychological performance | ||||||
|---|---|---|---|---|---|---|
| Tower | SWM | CVLT | ID—ED | Iowa | RVIP | |
| Manic admissions | -0.11 | 0.12 | -0.10 | 0.07 | 0.11 | -0.06 |
| Depressed admissions | -0.02 | 0.42* | 0.17 | -0.37 | 0.19 | -0.03 |
| Total admissions | -0.11 | 0.22 | -0.03 | -0.01 | 0.15 | -0.08 |
| Manic episodes | -0.31 | 0.26 | -0.48* | 0.18 | 0.09 | -0.40* |
| Depressed episodes | -0.42* | 0.42* | -0.48* | 0.12 | 0.19 | -0.48* |
| Duration of illness | -0.45* | 0.39 | -0.53** | 0.17 | 0.21 | -0.41* |
| Duration since last episode | 0.06 | -0.23 | 0.28 | -0.13 | -0.10 | 0.19 |
| Time on lithium | -0.06 | 0.03 | -0.16 | 0.31 | -0.17 | -0.01 |
| Current lithium dose | -0.23 | 0.13 | -0.09 | -0.10 | -0.17 | -0.16 |
Post hoc analysis of the RVIP effect in euthymic patients with bipolar disorder
The group difference between the euthymic bipolar group and controls on RVIP accuracy remained highly significant when the four patients with a history of substance abuse and the two patients with a history of ECT were excluded from the analysis (F(1,52)=15.5, P<0.001). The possibility that the RVIP deficit is related to medication status was examined using correlational analyses (see Table 3). The RVIP accuracy was not associated with either current dosage of lithium or the total duration of treatment. When the 11 patients not receiving lithium medication were studied in isolation, the RVIP deficit remained significant (F(1,39)=7.23, P=0.011).
The RVIP accuracy is inversely related to the number of manic episodes, the number of depressed episodes and the duration since the first episode. It is necessary to confirm whether the group difference between euthymic bipolars and controls on the RVIP test is still apparent at an early stage in the evolution of the illness trajectory. To examine this, the eight patients with the shortest illness durations and fewest episodes were identified: less than 40 months had elapsed since their first episode, they had been admitted no more than twice and had experienced no more than four affective episodes. These were compared with eight controls individually matched to the patients with regard to gender, age and IQ scores. Independent-sample t-tests revealed a significant difference between patients and controls on RVIP accuracy (bipolar mean, 61%; control mean, 84%; t=3.24,P=0.006). There were no significant differences on any of the other neuropsychological variables examined in Table 3. These data therefore suggest that RVIP accuracy deteriorates with illness progression but also is impaired in the early stages of bipolar disorder.
DISCUSSION
Of the six neuropsychological tasks employed in the present investigation, only performance on RVIP was impaired in the euthymic bipolar group after controlling for low levels of affective symptoms. The RVIP is a continuous performance task (CPT), assessing mechanisms of sustained attention with a small working memory component (Reference Coull, Frith and FrackowiakCoullet al, 1996). The RVIP task was administered second in the test battery and hence the specificity of the result is unlikely to reflect fatigue or motivational factors. The effect was large and correlated with the chronicity of bipolar disorder. However, the deficit also was present in a subgroup of younger patients selected to be early in their illness course. It is proposed, therefore, that impaired sustained attention may represent a trait marker for bipolar disorder, related to vulnerability to the disorder at a structural and/or neurochemical level.
Many of the patients in the present study were receiving lithium. Although a drug-free or drug-withdrawn cohort would be desirable, it would be hopelessly unrepresentative of clinically severe bipolar disorder. The present series was not an excessively medicated group: 12/30 were on no treatment or on a monotherapy. Previous research has suggested that lithium treatment can adversely affect cognitive functioning, with the most consistent effects in the mnemonic and psychomotor domains (Reference Honig, Arts and PondsHonig et al, 1999). In the current analysis, RVIP performance was not associated significantly with either current lithium dosage or duration of treatment (Table 3), but the small group sizes necessitate a degree of caution when interpreting the correlational analyses. Furthermore, bipolar patients on and off lithium performed similarly on the RVIP task, and the group not receiving lithium remained significantly impaired compared with controls. On these grounds, the persisting RVIP effect in the euthymic phase cannot be attributed to lithium treatment in this study.
The neural substrates of sustained attention are of ongoing research interest. Functional neuroimaging studies in normal subjects have reported right-lateralised activation in the prefrontal cortex during CPT performance (Reference Coull, Frith and FrackowiakCoull et al, 1996; Reference Paus, Zatorre and HoflePaus et al, 1997). Human lesion evidence also supports the view that the right prefrontal cortex is critically involved in sustained attention (Reference Manly, Robertson and RabbittManly & Robertson, 1997). A selective role for noradrenaline in target detection is based on the effects of lesioning the dorsal noradrenaline bundle in rats (Reference Cole and RobbinsCole & Robbins, 1992) and the effects of the α1/α2 agent clonidine on RVIP performance in healthy volunteers (Reference Coull, Middleton and RobbinsCoull et al, 1995). Low doses of clonidine impair RVIP accuracy without affecting impulsive responding (Reference Coull, Middleton and RobbinsCoull et al, 1995), which resembles the pattern of RVIP performance seen in euthymic bipolar patients in the present study. The potential for innovative pharmacological treatment strategies to improve attentional mechanisms has been demonstrated already for attention-deficit hyperactivity disorder (Reference Arnsten, Steere and HuntArnsten et al, 1996).
Sustained attention deficit as a vulnerability factor for psychosis
The failure to sustain attention is an obvious clinical feature of acute mania and, indeed, ‘distractivility’ is a criterion in DSM-IV for the diagnosis of the condition. Measures of sustained attention are abnormal and have even been reported to be correlated with frontal and hippocampal volumes in patients with acute mania (Reference Sax, Strakowski and ZimmermanSaxet al, 1999). Changes in sustained attention from the euthymic to the manic state will provide an interesting test of the idea that neuropsychological measures can directly illuminate psychopathology. Sustained attention in remitted bipolar patients was reported previously by Addington & Addington (Reference Addington and Addington1997) to be intermediate between patients with schizophrenia and controls. The calculated effect size for the bipolar—control comparison was 0.59, representing a medium effect (Reference CohenCohen, 1988). Their CPT paradigm required monitoring for only a single target digit, and may not have placed sufficient demands on the attentional network optimally to reveal the deficit in the bipolar group relative to controls.
In schizophrenia, sustained attention deficit is a robust effect during both symptomatic and asymptomatic phases (Reference Nuechterlein, Dawson, Ventura, Hafner and GattazNuechterlein et al, 1991). Furthermore, groups at high risk of developing the disorder, including first-degree relatives of subjects with schizophrenia (Reference Chen, Liu and ChangChen et al, 1998) and non-clinical subjects with high schizotypy scores (Reference Lenzenweger, Cornblatt and PutnickLenzenweger et al, 1991), also show impairment. Although the present finding in bipolar disorder must be confirmed initially in a larger sample of firstonset patients, further investigation of neuropsychological functioning in groups at high risk of developing bipolar disorder is clearly warranted. In schizophrenia and high-risk groups for schizophrenia there is usually a range of neuropsychological deficits in executive and mnemonic, as well as attentional, domains (Reference Goldberg, Ragland and TorreyGoldberg et al, 1990). A parallel but weaker effect might be expected for bipolar patients, given the difficulty in drawing a definitive boundary between the conditions in either genetic or phenomenological studies (Reference GoldbergGoldberg, 1999). Our findings support the possibility that sustained attention deficit may be a vulnerability marker for bipolar disorder that is shared with other individuals at risk of disorder across the psychotic spectrum.
Highly sensitive state markers in bipolar disorder
Our euthymic patients with bipolar disorder demonstrated impaired performance on the ID-ED shift, (a measure of attentional set shifting) and the CVLT (a verbal memory test). Both effects failed to reach statistical significance after controlling for low levels of affective symptoms. These results replicate but suggest a reinterpretation of previous studies in bipolar patients using the WCST (Reference Coffman, Bornstein and OlsonCoffmanet al, 1990; Reference MoriceMorice, 1990), and the CVLT (Reference van Gorp, Altshuler and Thebergevan Gorpet al, 1998; Reference Krabbendam, Honig and WiersmaKrabbendam et al, 2000): the deficits may represent a depression-related state effect rather than a trait abnormality. These findings are parsimonious in light of research on acute unipolar depression detailing impairments in set shifting (Reference Purcell, Maruff and KyriosPurcell et al, 1997; Reference Merriam, Thase and HaasMerriam et al, 1999) and verbal memory (Reference Austin, Ross and MurrayAustinet al, 1992; Reference Brown, Scott and BenchBrownet al, 1994).
In the present study, euthymic patients with bipolar disorder were not impaired on a number of putative neuropsychological indicators of executive functioning: specifically, Tower of London, spatial working memory (two putative measures of dorsolateral prefrontal cortex function) and the Iowa Gambling Task (which has been reported to be sensitive to damage in more ventral aspects of the prefrontal cortex). Spatial working memory and the Iowa Gambling Task have not been used previously in patients with bipolar disorder. Ferrier et al (Reference Ferrier, Stanton and Kelly1999) included Tower of London in their neuropsychological investigation of euthymic bipolar patients, and similarly found no impairment when controlling for low levels of depression. Performance on the Iowa Gambling Task, unexpectedly, was also normal in schizophrenia (Reference Wilder, Weinberger and GoldbergWilderet al, 1998).
The present findings confirm the importance of controlling for low levels of affective symptoms when investigating remitted patients with mood disorder, and support the assertion that the planning and working memory deficits reported during depression are state-related markers of affective disturbance (Reference Elliott, Sahakian and McKayElliott et al, 1996). Why impairments on some tests (attentional set shifting, verbal memory) appear to develop at lower levels of symptoms than impairments on others (e.g. planning, working memory) remains unclear. Such deficits help to underline the need to improve the treatment of bipolar depression, a major problem accurately described recently as an underestimated challenge (Reference Hlastala, Frank and MallingerHlastala et al, 1997). Further research into the development of neuropsychological impairment with advancing symptoms may provide novel insights into the neural substrates of affective states. One intriguing possibility is that set shifting and memory in bipolar disorder patients are unusually sensitised to low-grade affective symptoms. This phenomenon and a persisting deficit in sustained attention in the euthymic state may help to explain the difficulties in psychological and occupational functioning in patients with bipolar disorder during remission, that served originally to cast doubt on the concept of full recovery between episodes.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
• Patients with bipolar disorder studied during the euthymic state show intact performance on several measures of executive functioning.
-
• A relatively selective deficit exists on a task of sustained attention, potentially linked to vulnerability to bipolar disorder.
-
• Tests of verbal memory and attentional set shifting may be highly sensitive to subtle shifts in mood in patients with bipolar disorder.
LIMITATIONS
-
• A larger number of first-onset patients must be studied to confirm that sustained attention deficit represents a genuine vulnerability marker.
-
• Although lithium medication was unable to explain the profile of effects, many patients were receiving adjunctive medications.
-
• The specificity of this neuropsychological profile to bipolar disorder remains unclear.
Acknowledgements
This work was supported by a Medical Research Council studentship (L. C.). Preliminary results were presented at the 21st International Summer School of Brain Research, Amsterdam, The Netherlands, 23-27 August 1999, and at the Society for Neurosciences Annual Meeting, Miami, Florida, 23-28 October 1999. The authors thank Drs A. Bechara and A. Damasio for use of the lowa Gambling Task.







eLetters
No eLetters have been published for this article.