Regular meetings between a patient and their clinician are at the heart of community mental healthcare. They are used to communicate the patient's condition, personal situation and ongoing treatment. This routine communication has been subjected to little systematic research, and there is no evidence-based method to structure the communication to enhance long-term treatment outcome. A simple communication checklist completed by patients before seeing their clinician led to improved communication and treatment changes, but its effect on long-term outcome was not assessed (Reference Van Os, Altamura and BobesVan Os et al, 2004). New technologies to support patient–clinician communication are increasingly used in healthcare, although rarely in community psychiatry (Reference Ahmed and BoisvertAhmed & Boisvert, 2006). Hence, we devised a computer-mediated procedure to structure patient–clinician dialogue (DIALOG) to ensure that a range of life domains and treatment aspects were consistently addressed and patients’ perspectives always elicited. The results were fed back immediately and were intended to feed directly into patient–clinician discussions and shape subsequent care.
METHOD
The aim of this study was to investigate whether using the new intervention regularly in routine meetings between clinicians and patients with schizophrenia in the community would be associated with more favourable quality of life, fewer unmet needs for care and higher treatment satisfaction after a 1 year period compared with treatment as usual. The hypothesis was tested in a cluster randomised trial in six European countries (trial number ISRCTN75571732).
Settings
This study was conducted in community psychiatric services in Granada (Spain), Groningen (The Netherlands), London (UK), Lund (Sweden), Mannheim (Germany) and Zurich (Switzerland) covering urban and mixed urban–rural areas. The number of participating teams per country varied between two (Lund) and six (London).
All teams were multidisciplinary and provided comprehensive care programmes for people with severe and enduring mental illnesses. They operated a keyworker system in which every patient has a designated clinician working within a team but with lead responsibility for care coordination and delivery. Referrals were determined by residency in the catchment area and age (18–65 years).
Participants
Eligibility criteria for participating clinicians were a professional qualification in mental health or a minimum of 1 year's professional experience in an out-patient setting, and an active case-load as keyworker. The case-loads of participating clinicians were screened to identify suitable patients meeting the following inclusion criteria: living in the community (not 24 h supported accommodation) and treated as out-patients by community psychiatric teams; at least 3 months of continuous care in the current service; capable of giving informed consent; having sufficient knowledge of the language of the host country; having a primary diagnosis of schizophrenia or related psychotic disorder (ICD–10 F20–F29); aged between 18 and 65 years; having routinely at least one meeting with their keyworker every 2 months with the expectation that they would continue with the service for the next 12 months; and having no severe organic psychiatric illness or primary substance misuse. Patients were first informed about the study by clinicians and then – if they agreed – approached by a researcher for consent. The study was approved by relevant ethics committees in the six countries, and written informed consent was obtained from all clinicians and patients.
Design and process of randomisation
The intervention was evaluated using a cluster randomised controlled trial design. Clinicians were randomly assigned to either the intervention or treatment as usual, with a pre–post design over a 1-year period. Cluster randomisation was used to avoid potential contamination between the interventions in the two groups. Clinicians were randomised by computer-generated random block number allocation sequence to ensure an equal balance across sites. The randomisation procedure was completed separately for each country and team. A researcher not involved in the study generated the random allocation sequence. The process of allocating clinicians to the treatment as usual or intervention groups was by numbered, sealed envelopes. Masking of researchers to the allocation of the patients was attempted for the duration of the study. As masking was expected to be difficult to maintain, interviewers’ awareness of patients’ allocation was documented and assessed at the end of the study. In four countries all eligible patients from participating clinicians were asked to take part in the study. In the remaining two countries where clinicians had considerably higher patient case-loads, a maximum random sample of 12 patients was taken per clinician.
Intervention
Clinicians in the control group continued with standard treatment with their participating patients. Clinicians in the intervention group, in addition to continuing with standard treatment with their participating patients, also implemented the new manualised intervention. In the intervention group clinicians used DIALOG, a computer-mediated procedure to discuss 11 domains with their patients. They asked patients to rate their satisfaction with eight life domains (mental health, physical health, accommodation, job situation, leisure activities, friendships, relationship with family/partner, personal safety) and three treatment domains (practical help, psychological help and medication). Each satisfaction item was rated on a rating scale of 1–7, from ‘couldn't be worse’ to ‘couldn't be better’, and followed by a question on whether the patient wanted any additional or different help in the given domain. If the patient answered yes, the type of the requested additional or different support was recorded. The 11 domains were presented in a fixed order and an explicit response was required for each item before proceeding to the next item.
Patients’ answers to all questions were entered directly onto a hand-held computer or laptop using software specifically developed for the study over a 2-year period. Figure 1 illustrates possible screen displays, taking accommodation as an example (all of the other 10 domains can be displayed in the same way). A single domain could be viewed with the current rating compared with the rating 2 months previously. The domain could be viewed in the context of all the other domains in a summary graph comparing previous and current ratings for all 11 domains (end of Fig. 1). All 11 domains could also be viewed as a list in a summary table showing number of points change since the last meeting (e.g. +2, -3).

Fig. 1 The DIALOG intervention. Example of questions and real-time feedback on the domain ‘accommodation’.
The intervention was applied every 2 months in meetings that had been arranged as part of routine care. The new procedure was designed to alter interactions so that the patient's views on their situation and needs for care were the central point of treatment discussions and the patient's view on what kind of help would improve their current situation was made explicit. Patients and clinicians discussed current and previous ratings, reasons for change and what kind of additional or different support might be helpful. The underlying rationale was that providing patients and clinicians with this information would lead to explicit negotiation about what the patient wanted and what the clinician could do about it. This, in turn, would improve subsequent care and the patient's quality of life.
Each clinician in the intervention group was individually trained to use the software by a researcher and provided with written instructions. They were instructed on how the ratings should be used to facilitate a dialogue with the patients, particularly when there were changes since the last rating, explicit dissatisfaction with life domains or treatment aspects, or the patient wanted additional or different support.
Data collection
Collection of baseline data began in December 2002 and post-intervention data collection ended in May 2005. At both time points clinicians and patients were interviewed by researchers who had no involvement in the patients’ care. Patients were interviewed either at the team office or at home, according to their preference.
Outcomes
Outcome in the two groups was compared in a pre–post design. Primary outcome was subjective quality of life (SQOL) at 12 months controlling for baseline score. Quality of life was measured using the Manchester Short Assessment of Quality of Life (MANSA; Reference Priebe, Huxley and KnightPriebe et al, 1999) whereby patients rate their satisfaction with life in general and different life domains on Likert-type scales ranging from 1 (couldn't be worse) to 7 (couldn't be better), an approach that is consistent with the Quality of Life Interview (Reference LehmanLehman, 1988). The mean score of all 12 satisfaction ratings is taken as the indicator of SQOL.
Secondary outcomes were the number of unmet needs for care and satisfaction with treatment at 12 months, controlling in each case for the baseline score. Need for care was measured on the Camberwell Assessment of Need Short Appraisal Schedule, patient-rated version (CANSAS; Reference Slade, Phelan and ThornicroftSlade et al, 1996) which assesses health and social needs across 22 domains. For each domain it distinguishes between ‘no need’ (rating of 0), ‘met need’ (rating of 1) and ‘unmet need’ (rating of 2). Patients’ satisfaction with treatment was assessed on the Client Satisfaction Questionnaire (CSQ–8; Reference Nguyen, Attkisson and StegnerNguyen et al, 1983), which consists of eight items rated from 1 to 4 (with higher scores indicating greater treatment satisfaction).
Interviewers assessed patients’ symptoms on the 30-item Positive and Negative Syndrome Scale (PANSS; Reference Kay, Fiszbein and OplerKay et al, 1987). The scale assesses positive, negative and general symptoms and is rated on a scale of 1–7 (with higher scores indicating more severe symptoms). Socio-demographic and clinical characteristics of patients were obtained at baseline. Psychiatric diagnosis was obtained through a standardised and computer-based method using operationalised criteria (OPCRIT; Reference McGuffin, Farmer and HarveyMcGuffin et al, 1991). Researchers received training in all rating procedures and achieved good interrater reliability using videotaped interviews for PANSS (Cohen's kappa 0.71) and case vignettes for CANSAS (0.90).
Statistical analysis
R version 2.2.0 (Reference Ihaka and GentlemanIhaka & Gentleman, 1996) was used to compare the intervention and control groups in an intention-to-treat analysis. Descriptive statistics are presented, with frequency and percentage distributions for categorical data and means and standard deviations for continuous data.
In the main analyses patients were excluded only if they gave no information at follow-up. A sensitivity analysis using multiple imputation was also carried out to check the effect of excluding these patients. Each outcome was analysed using a mixed-effects model with baseline score for that variable, treatment allocation and length of follow-up as fixed effects, and centre and keyworker as random effects. Length of follow-up was considered as a potentially confounding covariate that might have introduced post-randomisation variance, and centre and keyworker were included in the model to adjust for the effect of clustering. Results are presented as 95% confidence intervals. Assumptions were checked graphically. Effects in the linear mixed-effects model are reported as partial eta squared, which is the proportion of total variability attributable to a factor.
Sample size
We aimed to obtain complete data for 240 patients in each group. With a significance level of α=0.05, this sample size would allow the detection of an effect size of 0.2 with 59% power, and of an effect size of 0.5 with more than 99% power.
RESULTS
Participant flow
One hundred and thirty-four clinicians consented to take part in the study, of whom 64 were randomised to the intervention group and 70 to the control group. From their case-loads, 507 eligible patients agreed to take part, with 236 patients in the treatment as usual and 271 in the intervention group. The number of patients per clinician ranged from 1 to 12 (mean 3.73).
At 12 months, 451 patients (243 intervention, 208 treatment as usual) were re-interviewed (88.9% follow-up). There were 17 keyworker changes during the study, with only one replacement clinician not agreeing to participate. Patient flow during the trial is shown in Fig. 2.

Fig. 2 Trial CONSORT diagram.1 In two centres a maximum random sample of 12 patients was taken per clinician owing to a high patient case-load.
The baseline to follow-up period ranged between 8 and 20 months (mean 12.4, s.d.=1.68 months). The range reflects late recruitment (16 patients had a follow-up of less than 12 months) and difficulties contacting patients and arranging follow-up interviews. For 283 (62.7%) out of the 451 re-interviewed patients, researchers stated they knew their allocation, making the correct assumption in 275 cases. Masking had been compromised through information that was revealed in previous contacts of researchers with the teams or in their assessments of the patients.
The mean number of meetings with structured communication in the intervention group was 5.21. Four patients had no such meeting, 12 patients had one, 14 had two, 15 had three, 40 had four, 45 had five, 46 six, and 95 had seven meetings. The time of all meetings between keyworkers and patients was documented over a 2-month period (i.e. months 6 and 7 of the 12-month study period), and the total time spent by keyworkers and patients in meetings with each other showed no significant difference between the two groups (intervention group, mean 240, s.d.= 201.9 min; control group, mean 251, s.d.=199.2 min).
An intention-to-treat analysis was conducted with the analysis set including all patients with at least one post-randomisation observation.
Baseline characteristics of participants
The characteristics, both socio-demographic and clinical, of clinicians and patients are shown in Table 1. There were no significant differences in the characteristics of participants in the control and intervention groups.
Table 1 Baseline characteristics of clinicians and patients

| Characteristics | Treatment as usual | Intervention |
|---|---|---|
| Clinician | ||
| Age, years: mean (s.d.) | 43.8 (1.0) | 43.8 (1.2) |
| Gender, n (%) | ||
| Female | 39 (55.7) | 43 (68.3) |
| Male | 31 (44.3) | 20 (31.7) |
| Profession, n (%) | ||
| Psychiatric nurse | 31 (44.4) | 33 (51.4) |
| Social worker | 17 (23.8) | 13 (20.8) |
| Psychiatrist | 8 (11.1) | 6 (9.7) |
| Psychologist | 3 (4.8) | 4 (5.6) |
| Length of service, years: mean (s.d.) | 13.0 (1.04) | 14.1 (1.1) |
| Total case-load: mean (s.d.) | 32.7 (66.4) | 28.4 (50.9) |
| Patient | ||
| Age, years: mean (s.d.) | 41.8 (11.6) | 42.5 (11.3) |
| Gender, n (%) | ||
| Female | 83 (35.2) | 88 (32.5) |
| Male | 153 (64.8) | 183 (67.5) |
| Diagnosis, n (%) | ||
| Undifferentiated schizophrenia | 89 (37.7) | 91 (33.6) |
| Paranoid schizophrenia | 63 (26.7) | 89 (32.8) |
| Catatonic schizophrenia | 4 (1.7) | 1 (0.4) |
| Hebephrenic schizophrenia | 10 (4.2) | 7 (2.6) |
| Schizoaffective manic | 7 (3.0) | 19 (7.0) |
| Schizoaffective depression (moderate) | 9 (3.8) | 9 (3.3) |
| Schizoaffective depression (severe) | 2 (0.8) | 3 (1.1) |
| Schizoaffective bipolar disorder | 9 (3.8) | 15 (5.5) |
| Delusional disorder | 2 (0.8) | 1 (0.4) |
| Other non-organic psychotic disorders | 41 (17.4) | 36 (13.3) |
| Length of illness, years: mean (s.d.) | 15.2 (9.9) | 16.6 (10.5) |
| Hospital admissions, n: mean (s.d.) | 4.5 (6.9) | 5.8 (7.6) |
| MANSA score: mean (s.d.) | 4.7 (8.8) | 5.8 (7.6) |
| CSQ score: mean (s.d.) | 25.7 (4.2) | 25.7 (4.1) |
| CANSAS score: mean (s.d.) | 3.0 (3.1) | 2.7 (2.7) |
| PANSS sub-scale scores: mean (s.d.) | ||
| Positive | 14.6 (5.7) | 15.0 (5.8) |
| Negative | 15.7 (6.0) | 17.2 (6.9) |
| General | 31.8 (9.1) | 32.6 (10.1) |
Outcomes
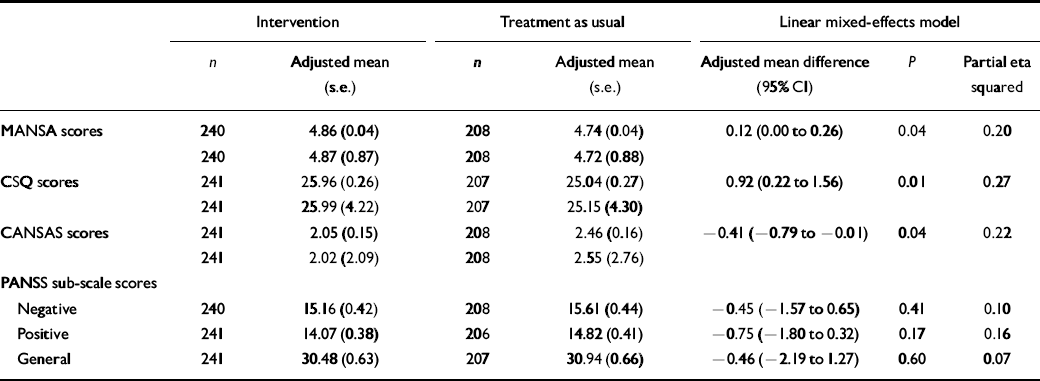
Outcomes are summarised in Table 2. At 12-month follow-up patients in the intervention group had significantly higher SQOL scores, fewer unmet needs and higher treatment satisfaction compared with patients in the control group. The effect sizes based on adjusted means and standard deviations for the three outcomes vary between 0.20 and 0.27.
Table 2 Differences in quality of life, treatment satisfaction and unmet needs between groups at 12-month follow-up
| Intervention | Treatment as usual | Linear mixed-effects model | |||||
|---|---|---|---|---|---|---|---|
| n | Adjusted mean (s.e.) | n | Adjusted mean (s.e.) | Adjusted mean difference (95% CI) | P | Partial eta squared | |
| MANSA scores | 240 | 4.86 (0.04) | 208 | 4.74 (0.04) | 0.12 (0.00 to 0.26) | 0.04 | 0.20 |
| 240 | 4.87 (0.87) | 208 | 4.72 (0.88) | ||||
| CSQ scores | 241 | 25.96 (0.26) | 207 | 25.04 (0.27) | 0.92 (0.22 to 1.56) | 0.01 | 0.27 |
| 241 | 25.99 (4.22) | 207 | 25.15 (4.30) | ||||
| CANSAS scores | 241 | 2.05 (0.15) | 208 | 2.46 (0.16) | -0.41 (-0.79 to -0.01) | 0.04 | 0.22 |
| 241 | 2.02 (2.09) | 208 | 2.55 (2.76) | ||||
| PANSS sub-scale scores | |||||||
| Negative | 240 | 15.16 (0.42) | 208 | 15.61 (0.44) | -0.45 (-1.57 to 0.65) | 0.41 | 0.10 |
| Positive | 241 | 14.07 (0.38) | 206 | 14.82 (0.41) | -0.75 (-1.80 to 0.32) | 0.17 | 0.16 |
| General | 241 | 30.48 (0.63) | 207 | 30.94 (0.66) | -0.46 (-2.19 to 1.27) | 0.60 | 0.07 |
Owing to the floor effect for unmet needs and ceiling effect for quality of life, a substantial improvement was unlikely to be achieved in those patients who already had a positive SQOL and few unmet needs at the beginning of the trial. We therefore conducted a post hoc analysis on the group as a whole, with those patients who at baseline had at least two unmet needs and a SQOL score lower than 5 (i.e. ‘mixed’ or lower). In those 195 patients (106 in the intervention and 89 in the control group), the effect size in relation to SQOL was 0.43 (adjusted mean difference 0.33, P=0.006) and in relation to unmet needs was 0.52 (adjusted mean difference 1.16, P=0.003). As a sensitivity analysis we fitted the same models imputing the missing outcomes using regression, using five sets of imputations. The resulting effect sizes were almost unchanged. The two groups showed no statistically significant difference in any of the psychopathology scores on the PANSS.
DISCUSSION
This study tested the effectiveness of a novel intervention in community care of patients with schizophrenia and related psychotic disorders. This is the first study to change the structure of patient–clinician interaction in community mental healthcare across a range of healthcare systems and to test its effect on long-term outcomes of care. After 12 months, the intervention had a significant positive effect on all three outcomes (i.e. quality of life, unmet needs for care and treatment satisfaction). Previous studies that structured communication between patients and clinicians were based on only a few patients (Reference Ahmed and BoisvertAhmed & Boisvert, 2006) or did not assess its effect on long-term outcome of care (Reference Van Os, Altamura and BobesVan Os et al, 2004). This study using a large sample across different healthcare systems demonstrated the efficacy of computer-mediated communication on outcome over a 1-year period.
This intervention ensured that 11 life and treatment domains were consistently addressed and patients’ views and priorities were always considered (Reference Rosenheck, Stroup and KeefeRosenheck et al, 2005). This is likely to have increased awareness of patients’ views and their changes over time, resulting in care that reduces unmet needs and increases SQOL and treatment satisfaction (Reference Lasalvia, Bonetto and MalchiodiLasalvia et al, 2005). This was achieved although symptom levels did not change. Given the enduring nature of the disorders in our sample, this was as expected and suggests that patients’ quality of life can be improved even when symptoms do not show significant change (Reference Holloway and CarsonHolloway & Carson, 1998; Reference Trieman, Leff and GloverTrieman et al, 1999).
Limitations and strengths
The study should be considered in the light of its limitations. Participating teams and clinicians might not have been representative of the given mental healthcare systems. The novel intervention was not consistently administered, as evidenced by the variation in the number of structured communications for individual patients (although with a mean of approximately 5 per patient), which reflects the pragmatic nature of the trial. Finally, masking of interviewers could not be maintained for the majority of patients, and exclusively subjective measures were used as outcome criteria.
The strengths of the study are that the intervention was tested under routine conditions and in six European healthcare settings, with high follow-up rates of 90% in this often difficult to reach and mobile population. The intervention requires little additional investment and minimal training of clinicians. It did not significantly increase the time spent by keyworkers and patients in meetings with each other, and was viewed favourably by both patients and keyworkers (see online supplement to this paper). It can be applied without reconfiguration of services and would be easy to implement widely. We found a positive effect in a sample with predominantly long-term problems – the mean length of illness was more than 15 years – and the scope to achieve substantial improvements of SQOL in such samples over a 1-year period is usually regarded as somewhat limited.
Intervening in patient–clinician communication
So far, there is a paucity of evidence-based interventions that can be used in routine meetings between clinicians and people with schizophrenia to enhance quality of life (Reference Marshall, Lockwood and GreenMarshall et al, 2004; Reference Slade, McCrone and KuipersSlade et al, 2006). The intervention tested in this study targets patient–clinician communication as the central component of care delivery and structures it in a patient-centred manner. There is evidence that the quality of patient–clinician communication plays a role in treatment outcome. In primary care consultations, a positive patient-centred approach was associated with higher patient satisfaction, less symptom burden and fewer referrals to other services (Reference Little, Everitt and WilliamsonLittle et al, 2001). In mental healthcare, a simple communication checklist completed by patients before seeing their doctor, where they indicated which of 20 common needs they wanted to discuss, led to improved patient–doctor communication and changes in treatment (Reference Van Os, Altamura and BobesVan Os et al, 2004).
The use of computers was also found to facilitate communication between clinicians and people with schizophrenia. Specifically, patients’ responses to structured questions concerning treatment goals and expectations were visually presented and reviewed on a computer screen. This improved discussion of treatment and the identification of realistic goals for therapy (Reference Ahmed and BoisvertAhmed & Boisvert, 2006). The authors proposed that using both visual and auditory techniques may facilitate communication by improving patient attention, information assimilation and reducing interference from psychiatric symptoms such as delusions.
The current intervention is simple, nonintrusive and inexpensive. Although the effect sizes in this study were small, they may be judged significant for the practice of community psychiatry, and the findings should justify wider use of the intervention. It is worth noting that effect sizes were higher in those patients who had more unmet needs and lower quality of life at baseline, which is in line with the results of Van Os et al (Reference Van Os, Altamura and Bobes2004). In these patients medium effect sizes of 0.43 and 0.52 were achieved through the DIALOG intervention. These do not indicate a dramatic change in the living situation of patients on a group level but suggest a real difference for at least some of the patients. It remains unclear to what extent this effect is due to: (a) the mere structuring of the meeting which ensures that important areas are always covered; (b) the focus on patient views of outcome in the meeting; and (c) the specific computer-mediated option of comparing current ratings with previous ratings across different life domains.
If used in routine settings the intervention might facilitate the generation of regular outcome data. As the procedure involves the assessment of central outcome criteria in community psychiatry (i.e. satisfaction with life domains and with treatment), these scores may feed into processes of quality management and service evaluation (Reference McCabe and PriebeMcCabe & Priebe, 2002; Reference Priebe, McCabe and BullenkampPriebe et al, 2002). Gathering outcome data from a procedure that is meaningful to patients and clinicians and beneficial for the individual patient is more likely to be successful than conventional methods of routine outcome measurement in which outcomes are rated by patients outside clinical consultations and the results later made available to clinicians (Reference Gilbody, House and SheldonGilbody et al, 2001; Reference Slade, McCrone and KuipersSlade et al, 2006). The latter approach makes it difficult to determine whether the process of outcomes management had an impact on what clinicians and patients did in clinical consultations. Incorporating the assessment and feedback of outcomes into routine clinical encounters makes it more likely to have a direct impact on what happens in practice when clinicians and patients interact.
In conclusion, a simple computer-mediated procedure to structure routine communication between patient and clinician can have a significant positive effect on treatment outcome over a 1-year period in patients with schizophrenia in the community. Future studies should test the feasibility and effectiveness of similar procedures for improving patient–clinician communication with other patient groups and in other out-patient settings. Moreover, qualitative and experimental research might help to develop interventions that are more effective than DIALOG in influencing both the therapeutic communication and outcome, and identify the mediating processes between better communication and more favourable outcome.
Acknowledgements
The Towards More Effective European Community Care for Patients with Severe Psychosis (MECCA) group includes: Marta Ribes Leyva, Maria F. Soriano Peña, Beatriz Arroyo de Domingo (Granada); Kerstin Wolters, Aukelien Mulder, Jappie Tiersma (Groningen); Rakhee Haque (London); Tommy Björkman (Lund); Marita Reichenbacher, Anette Axt (Mannheim); Patric Meyer, Minka Burgi (Zurich). We thank the clinicians and patients who took part in the Mediating Community Care study, Dr Michael Dewey for statistical advice and Dr Patrick Healey and Greg Mills for software development and technical support. The study was funded by the Research Directorate of the European Commission within the Framework Programme 5 (QLG5-CT-2002-01938).







eLetters
No eLetters have been published for this article.