Many studies have demonstrated abnormal brain structure and function in the frontal and temporal lobes of patients with schizophrenia (Reference Lawrie and AbukmeilLawrie & Abukmeil, 1998; Reference Wright, Rabe-Hesketh and WoodruffWright et al, 2000). Functional imaging studies show abnormal frontotemporal activations on various tasks (Reference FrithFrith, 1995; Reference Fletcher, McKenna and FristonFletcher et al, 1999), lending support to the hypothesis that the core feature of schizophrenia is a disruption of frontotemporal integration (Reference Friston and FrithFriston & Frith, 1995). A recent negative study does, however, raise doubt as to whether this is a trait marker of the disorder (Reference Spence, Grasby and LiddleSpence et al, 2000). The parietal lobes have been studied less in schizophrenia, but there are suggestions that frontoparietal integration may be similarly abnormal (Reference Spence, Brooks and HirschSpence et al, 1997; Reference Lawrie and AbukmeilLawrie & Abukmeil, 1998; Reference Honey, Bullmore and SharmaHoney et al, 2002). It would be of considerable interest to establish whether functional disconnectivity (see Reference Friston and FrithFriston & Frith, 1995) in schizophrenia is associated with structural abnormalities in specific frontotemporal and frontoparietal white matter tracts, such as the uncinate and arcuate fasciculi and the anterior cingulum (Reference DejerineDejerine, 1895; Reference Petrides and PandyaPetrides & Pandya, 1988). New magnetic resonance imaging (MRI) methodologies, such as diffusion tensor magnetic resonance imaging (DT—MRI), which measures the mobility of water molecules in vivo (Reference Basser, Mattiello and LeBihanBasser et al, 1994), may have the potential to identify structural correlates of impaired functional connectivity in schizophrenia. In this technique, the deviation from pure isotropic diffusion of water molecules along white matter axons is measured and described in terms of the fractional anisotropy. This parameter is thought to provide a useful marker of white matter fibre tract integrity, with high values of fractional anisotropy indicating intact healthy neurons (Reference O'Sullivan, Jones and SummersO'Sullivan et al, 2001).
METHOD
Study participants
Thirty patients with DSM—IV (American Psychiatric Association, 1994) schizophrenia were recruited from the hospital and community services of the Royal Edinburgh Hospital, Scotland. They included equal numbers of men and women, most of whom were on antipsychotic medication. Control subjects were recruited from the community and were well matched to the patients (Table 1). Full ethical approval was obtained from the relevant Ethics Committee and all subjects gave informed consent for all aspects of the study.
Table 1 Demographics (mean (s.d.))

| Average age (years) | Gender ratio (male:female) | Handedness ratio (right:left) | IQ | |
|---|---|---|---|---|
| Schizophrenia (n=30) | 36.4 (11.2) | 15:15 | 27:3 | 106.3 (11.6) |
| Controls (n=30) | 35.7 (12.4) | 15:15 | 26:4 | 110.3 (7.7) |
Procedures
Subjects underwent whole-brain structural MRI and DT-MRI using a GE Signa LX 1.5 T (General Electric, Milwaukee, WI, USA) clinical scanner. The structural MRI imaging consisted of a T1-weighted gradient-echo volumetric sequence followed by a dual-echo fast spin-echo sequence designed to give contiguous axial proton density and T2-weighted magnetic resonance images. In the whole-brain DT—MRI examination, sets of axial diffusion-weighted single-shot echo-planar (DW—EP) images (b min=0 and b max=1000 s/mm2) were collected with diffusion gradients applied sequentially along six non-collinear directions (Reference Basser and PierpaoliBasser & Pierpaoli, 1998). Five acquisitions consisting of a baseline T2-weighted echo-planar image and six DW—EP images — a total of 35 images — were collected per slice position. Other acquisition parameters were 31 contiguous axial slices of 5 mm thickness, a field of view of 240 × 240 mm, an acquisition matrix of 128 × 128 (zero filled to 256 × 256), a repetition time of 10 s and an echo time of 98.8 ms. The set of five component DW—EP images for each gradient direction was averaged to give seven high signal-to-noise ratio images for each slice. Eddy-current-induced artefacts were then corrected in the six averaged DW—EP images (Reference BastinBastin, 1999). Within each voxel the six elements of the apparent diffusion tensor of water (D) were estimated from the signal intensities measured in the DW—EP images (Reference Basser, Mattiello and LeBihanBasser et al, 1994). The directional dependence of water diffusion, or diffusion anisotropy, can be expressed as a scalar quantity, the fractional anisotropy, which varies from zero (diffusion equal in all directions) to unity (diffusion purely unidirectional). Maps of the T2-weighted signal intensity and fractional anisotropy were generated on a voxel-by-voxel basis and converted into Analyze format (Mayo Foundation, Rochester, MN, USA).
Analysis
The image processing methods were based on an optimised voxel-based morphometry technique (Reference Good, Johnsrude and AshburnerGood et al, 2001) and implemented in SPM99 (http://www.fil.ion.ucl.ac.uk/spm/). Both data preprocessing and the statistical parametric map analysis were performed by investigators blinded to subject status (i.e. whether patient or control). The T2-weighted echo-planar images acquired in the DT—MRI examination were segmented to native space using internal spatial normalisation. The white matter segments were then spatially normalised to white matter a priori probability maps to derive white-matter-specific warps. The specific warps were then applied to the raw fractional anisotropy maps and the raw T2-weighted images. The spatially normalised T2-weighted images were segmented into grey matter, white matter and cerebrospinal fluid. The spatially normalised fractional anisotropy images and white matter segments were then smoothed with a 12-mm isotropic full width at half-maximum Gaussian filter. Two random- effects analyses using a measurement of brain volume as a confound were constructed, the first for the fractional anisotropy images and the second for the white matter segments. The small-volume correction tool supplied with SPM99 was used to define a priori hypothesised volumes for the uncinate and arcuate fasciculi and the anterior cingulum. Small-volume correction is a technique where one can perform a region-of-interest analysis in SPM99 (with all the associated benefits of the statistical parametric map method). Based upon the a priori hypothesis, coordinates were selected that represented the midpoints of the structures that we wished to investigate. In this case, coordinates for the three tracts were selected from the Talairach Atlas and converted to Montreal Neurological Institute (MNI) coordinates. The MNI coordinates for the small-volume correction were: 11.0 mm radius spheres centred at [±32, +5, -21] for the uncinate fasciculus, [±31, +11, -23] for the arcuate fasciculus and [±10, +5, -34] for the anterior cingulum. In other words, three spheres of 11.0-mm radius were centred on these coordinates, and mean functional anisotropy values for each voxel within the spheres were compared between the two study groups. The 11.0-mm radius rendered the smallest spheres possible for a valid statistical inference in this particular statistical parametric map analysis.
RESULTS
We found a trend towards significantly reduced functional anisotropy values on comparing patients with schizophrenia with controls within the small-volume correction placed over the left uncinate fasciculus (P=0.06). In addition, we found significantly reduced fractional anisotropy in two voxels in the left arcuate fasciculus (P<0.05). These results are shown graphically in Fig. 1 and separately in Table 2 (left uncinate fasciculus) and Table 3 (left arcuate fasciculus). In Table 2 the coordinates of the voxel showing the maximal point of fractional anisotropy reduction in the schizophrenia group is reported. This voxel lies on the border of the left uncinate and left inferior longitudinal fasciculus and shows a trend towards significance. In Table 3, the coordinates of two voxels showing significantly reduced fractional anisotropy values in the schizophrenia group are reported. Both voxels are within the white matter of the left arcuate fasciculus and both are located anteriorly in the tract.
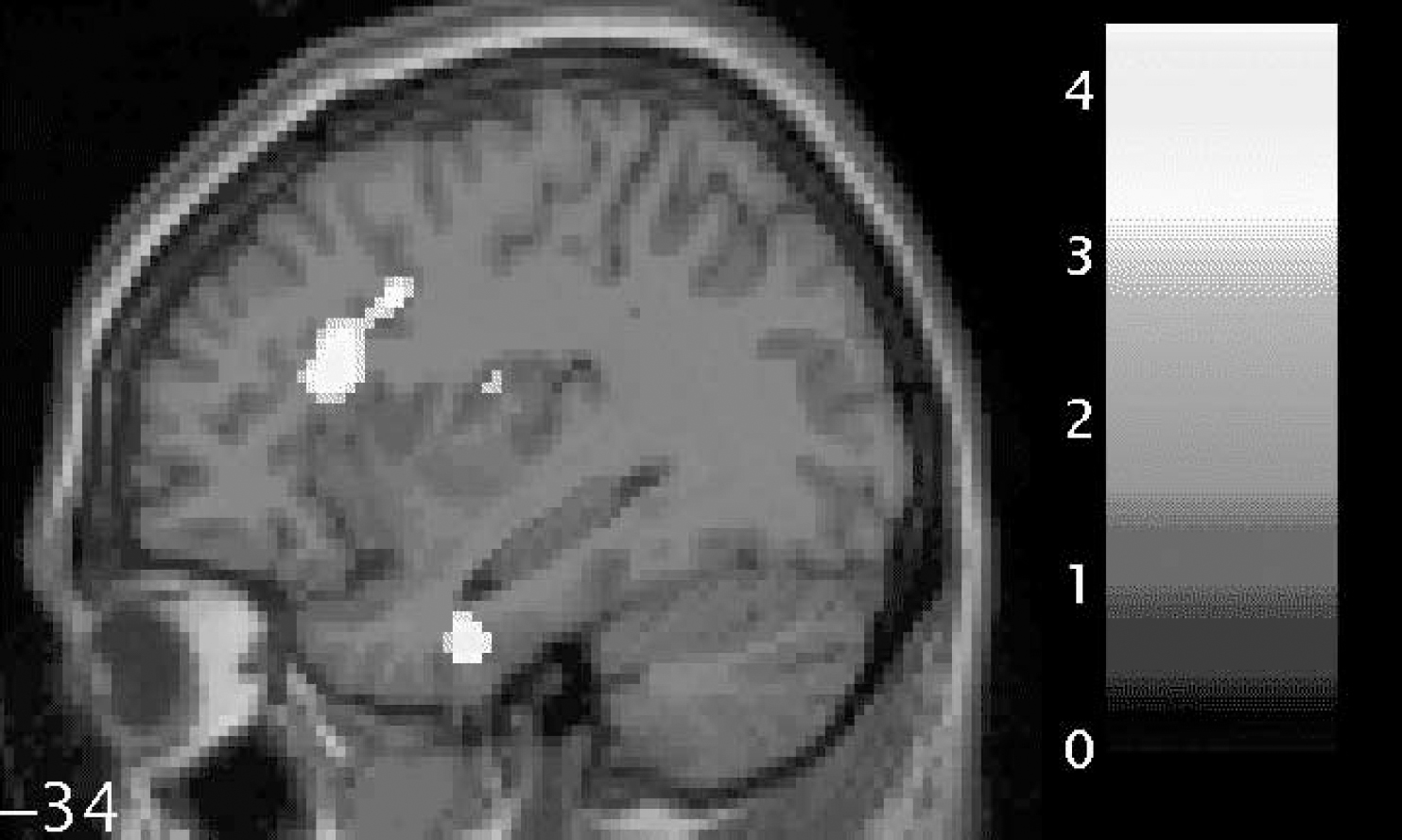
Fig. 1 Statistical parametric map (in sagittal section) showing regions of fractional anisotropy reduction (brightest areas) in the white matter of the left uncinate fasciculus (lower; relatively inferior) and left arcuate fasciculus (upper; relatively superior) for patients with schizophrenia compared with controls, overlaid on the corresponding T1 image (threshold at P=0.001, uncorrected). The bar shows z-values corresponding to the figure. The number beside the slice shows the displacement on the x-axis in millimetres from the origin (Montreal Neurological Institute coordinates). This figure was created using M. Brett's ‘display_slices.m’ matlab script.
Table 2 Left uncinate fasciculus1

| Voxel P (corrected) | Talairach coordinates x,y,z (mm) | Point of maximal change |
|---|---|---|
| 0.06 | -33.66, -4.08, -23.35 | Left temporal lobe, border of uncinate faciculus/inferior longitudinal fasciculus |
Table 3 Left arcuate fasciculus1

| Voxel P (corrected) | Talairach coordinates x,y,z (mm) | Point of maximal change |
|---|---|---|
| <0.05 | -34.65, 20.25, 16.49 | Left frontal lobe, arcuate fasciculus |
| <0.05 | -32.67, 9.27, 29.93 | Left frontal lobe, anterior part of arcuate fasciculus |
No significant differences were detected in the small-volume corrections placed over the right arcuate fasciculus, the right uncinate fasciculus or the right and left anterior cingulum. There were no volumetric differences between the groups in the white matter segments derived from the T2-weighted images.
DISCUSSION
This is a hypothesis-driven study using a novel means of imaging white matter to identify structural disconnectivity in schizophrenia. The results are in keeping with the hypothesis that specific frontotemporal and frontoparietal white matter tracts, namely the uncinate fasciculus and the arcuate fasciculus, are abnormal in schizophrenia. This is consistent with the findings of functional imaging studies, which have demonstrated abnormalities in the functional relationship between frontal and temporal (Reference FrithFrith, 1995; Reference Fletcher, McKenna and FristonFletcher et al, 1999) and frontal and parietal (Reference Spence, Brooks and HirschSpence et al, 1997; Reference Honey, Bullmore and SharmaHoney et al, 2002) lobes in schizophrenia. Furthermore, our finding of structural changes lateralised to the left hemisphere may be consistent with the cerebral asymmetry hypothesis (Reference CrowCrow, 1995), although it should be noted that we did not specifically test this.
Diffusion tensor MRI
Diffusion tensor MRI is a relatively new structural imaging modality that measures the three-dimensional mobility of brain water molecules in vivo (Reference Basser, Mattiello and LeBihanBasser et al, 1994). In this technique, the apparent diffusion tensor of water (D) is calculated for each voxel in an image from sets of diffusion-weighted magnetic resonance images. Owing to the presence of axonal membranes, myelin sheaths and micro-filaments, water molecules diffuse preferentially along axons rather than across them. Thus, within coherent, ordered white matter structures, the mobility of water is greatest in the principal direction of the fibre tract. This directional dependence of water diffusion is termed ‘diffusion anisotropy’ and can be represented by a range of scalar parameters, or diffusion anisotropy indices, derived from D (Reference BasserBasser, 1995). The most common diffusion anisotropy index is the fractional anisotropy, which varies from zero (diffusion equal in all directions) to unity (diffusion purely unidirectional). Extensive in vivo and in vitro experiments on various non-myelinated neuronal fibres, axons with large axoplasmic spaces and neurons in which fast axonal transport has been inhibited indicate that the primary determinant of white matter anisotropic diffusion is the dense packing of axonal membranes, with myelin playing a secondary role (Reference Beaulieu and AllenBeaulieu & Allen, 1994). Any pathological factors that alter the structural organisation and/or reduce the density of axonal membranes might be expected to cause a reduction in diffusion anisotropy values compared with those measured in normal brain. Thus, fractional anisotropy is taken to be a marker of neuronal integrity, with high fractional anisotropy values indicating healthy, intact white matter tracts (Reference O'Sullivan, Jones and SummersO'Sullivan et al, 2001).
Previous DT—MRI studies in schizophrenia
Previous studies have reported reduced fractional anisotropy in both frontal white matter and the splenium of the corpus callosum on exploratory analyses (Reference Buchsbaum, Tang and PeledBuchsbaum et al, 1998; Reference Lim, Hedehus and MoseleyLim et al, 1999; Reference Agartz, Andersson and SkareAgartz et al, 2001; Reference Foong, Symms and BarkerFoong et al, 2002). Some have used statistical parametric mapping methods of analysis and the results are varied (Reference Buchsbaum, Tang and PeledBuchsbaum et al, 1998; Reference Agartz, Andersson and SkareAgartz et al, 2001; Reference Foong, Symms and BarkerFoong et al, 2002). None has used the small-volume correction tool and future studies may benefit from using this technique. A study by Kubicki (Reference Kubicki, Westin and MaierKubicki et al, 2002) is interesting in terms of our own findings and indeed the asymmetry hypothesis (Reference CrowCrow, 1995). Presumably motivated by a similar interest in frontotemporal connections, they looked at diffusion anisotropy in the uncinate fasciculus and found a group-by-side interaction in the patient group, although the fractional anisotropy was reduced on the left side and their smaller number of subjects may have reduced the power to find statistically significant differences.
Structural basis of functional disconnectivity
Although there is evidence for a disturbance of the functional relationship between frontal and temporal and frontal and parietal lobes in schizophrenia, it is not clearly understood whether white matter connections between these regions are structurally abnormal. With DT—MRI, however, it was possible to investigate this hypothesis. Data from both human and primate dissection studies suggested that these association tracts may include the uncinate fasciculus, the anterior cingulum and the superior longitudinal or arcuate fasciculus (Reference DejerineDejerine, 1895; Reference Petrides and PandyaPetrides & Pandya, 1988). In this study we applied current analysis techniques to fractional anisotropy maps obtained from DT—MRI data to test the hypothesis that these specific frontotemporal and frontoparietal white matter tracts would be disrupted in patients with schizophrenia. Such a disruption would manifest itself as a reduction in fractional anisotropy values in patients with schizophrenia compared with controls.
The arcuate fasciculus is a major association tract connecting large parts of the frontal association cortices with parietal and temporal association areas (Reference DejerineDejerine, 1895). It also forms the main connection between Wernicke's and Broca's language areas. Our finding of reduced neuronal integrity in the left arcuate fasciculus supports the notion that schizophrenia is a disorder of large-scale neurocognitive networks rather than specific regions and, as others suggest, pathological changes in this disorder should be sought at the supra-regional rather than regional level (Reference Sigmundsson, Suckling and MaierSigmundsson et al, 2001). Both structural and functional abnormalities of frontoparietal networks have been described in schizophrenia (Reference Schlaepfer, Harris and TienSchlaepfer et al, 1994; Reference Honey, Bullmore and SharmaHoney et al, 2002), and may constitute a basis for the wide range of cognitive functions impaired in the disorder, such as selective attention, language processing and attribution of agency.
The uncinate fasciculus is the largest of the three fibre tracts connecting the frontal and temporal lobes, and dissection studies have demonstrated that the bulk of these fibres connect the orbital and medial prefrontal cortex (including anterior cingulate cortex) to the amygdala, entorhinal cortex and rostral superior temporal gyrus (Reference Petrides and PandyaPetrides & Pandya, 1988; Reference Morris, Pandya and PetridesMorris et al, 1999). These frontal and temporal cortical regions show grey matter volume changes in many structural imaging studies of schizophrenia (Reference Lawrie and AbukmeilLawrie & Abukmeil, 1998; Reference Wright, Rabe-Hesketh and WoodruffWright et al, 2000) and possibly the greatest structural decrements. During early brain development, ingrowing association fibres linking these frontal and temporal cortices could encounter abnormal termination sites and form aberrant connections in the superficial layers. Pruning of aberrant connections during the second and third decades of life could lead to impoverished dendritic arborisation rather than neuronal depopulation (Reference HarrisonHarrison, 1999). Such a mechanism may possibly account for grey and white matter loss and reduced inter-correlations of these volumes (Reference Wible, Shenton and HokamaWible et al, 1995; Reference Woodruff, Wright and ShuriquieWoodruff et al, 1997), as well as our findings of reduced neuronal integrity in the uncinate fasciculus. A recent post-mortem study of the uncinate fasciculus in schizophrenia merits particular consideration (Reference Highley, Walker and EsiriHighley et al, 2002). Right-greater-than-left asymmetry of the uncinate fasciculus was demonstrated in both patients and controls, with no significant differences in asymmetry between the two groups. A possible interpretation, in terms of our own findings, is that the different techniques are examining different aspects of uncinate morphology. The study by Highley et al (Reference Highley, Walker and Esiri2002) may yield information about fibre number and density, whereas our study is detecting differences in neuronal integrity as the uncinate tracts disperse near their termination in the temporal lobe.
Methodological issues
Our findings are unlikely to be artefactual, given that the third tract that we studied — the anterior cingulum — showed no differences in fractional anisotropy. The relatively large numbers of subjects involved provide adequate power and suggest generalisability. The two groups were well matched and the automated methods of analysis optimised power and minimised error. The voxel-based morphometry analysis of white matter volume showed no differences between the two groups, supporting the view that the reductions in diffusion anisotropy in the left uncinate fasciculus and left arcuate fasciculus can be attributed to impaired neuronal integrity rather than to volumetric differences.
Most of our patients were on psychotropic medication at the time of scanning but it does not seem likely that this potential confounder could account for the findings because any effects would not have been localised. Smoothing with a 12-mm Gaussian filter promotes the detection of 12-mm differences in spatial extent but may miss smaller differences. This could explain our failure to find effects on the right side and in the anterior cingulum. The anterior cingulum is a particularly slender tract and therefore more liable to partial volume effects in statistical parametric map analysis of this structure. It may be, of course, that the anterior cingulum is normal in schizophrenia and that the uncinate fasciculus and arcuate fasciculus are the main structures implicated in frontotemporal and frontoparietal disconnectivity.
Our findings of reduced neuronal integrity in the left uncinate fasciculus and left arcuate fasciculus suggest structural disconnectivity in schizophrenia and also, we would argue, suggest that aberrant connectivity is a wider feature of fibre tracts linking the prefrontal to posterior cortices. Future studies might seek to elucidate further the exact relationship between the anatomical findings of dysconnectivity and the clinical symptoms of schizophrenia.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ There appear to be structural abnormalities in frontotemporal and frontoparietal white matter tracts in schizophrenia.
-
▪ Novel imaging techniques, such as diffusion tensor magnetic resonance imaging, can be used to investigate the structural integrity of white matter tracts in psychiatric disorders in vivo.
-
▪ We provide further support for the notion that schizophrenia is a disorder of cortical connectivity.
LIMITATIONS
-
▪ Most of our patients were on psychotropic medication at the time of scanning, and this may be a confounder.
-
▪ Smoothing with a 12-mm Gaussian filter promotes the detection of 12-mm differences in spatial extent but may miss smaller differences.
-
▪ The anterior cingulum is a particularly slender tract and therefore more liable to partial volume effects in statistical parametric map analysis of this structure.
Acknowledgements
All magnetic resonance imaging was performed at the SHEFC Brain Research Centre for Scotland (http://www.dcn.ed.ac.uk/bic), and image analyses were performed in the Wellcome Trust Clinical Research Facility, University of Edinburgh (http://www.wtcrf.ed.ac.uk). We thank all of the subjects who participated in this study, as well as the staff of the Department of Clinical Neuroscience, Western General Hospital and the staff of the Royal Edinburgh Hospital who assisted with various aspects of the study. We also thank David Semple and Andrew McIntosh for assistance with recruitment and interviewing of subjects, and Norma Brearley for careful preparation of the manuscript.







eLetters
No eLetters have been published for this article.