Tardive dyskinesia is a syndrome of involuntary movements affecting the face and/or the trunk and extremities, which is seen in 20-25% of patients with schizophrenia. Reference Cunningham Owens1 Once it appears it commonly becomes irreversible, and it may sometimes be disabling or in rare cases fatal. Reference Cunningham Owens1 It typically occurs in patients who have received months or years of antipsychotic treatment. As a result it is classified as a late-developing extrapyramidal side-effect of this class of drugs.
Considering tardive dyskinesia simply as a side-effect of antipsychotics may, however, not be entirely justified. This is because essentially similar involuntary movements can also be seen in people with schizophrenia who have not received treatment. According to current estimates, such spontaneous dyskinesias are present in around 4% of patients with first-episode schizophrenia Reference Fenton2 and the frequency may reach 40% in the (nowadays rare) populations of people with chronic schizophrenia who have never been exposed to antipsychotics. Reference Owens, Johnstone and Frith3,Reference McCreadie, Thara, Kamath, Padmavathy, Latha and Mathrubootham4 To account for this finding, it has been proposed that there is an intrinsic vulnerability to involuntary movements in schizophrenia and the role of antipsychotics is one of promotion or acceleration rather than causation. Reference Cunningham Owens1,Reference Whitty, Owoeye and Waddington5 More radically, Crow et al Reference Crow, Owens, Johnstone, Cross and Owen6 have argued that, along with negative symptoms and cognitive impairment, tardive dyskinesia is part of the pattern of deterioration associated with the illness and antipsychotic treatment plays little or no part in its emergence.
Despite its clinical importance, little is known about the biological basis of tardive dyskinesia. The major hypothesis over the years has been that it reflects an increase in dopamine D2 receptor numbers in the basal ganglia. Reference Casey7 This view was originally based on evidence implicating a functional dopamine excess in choreoathetoid movement disorders across a range of neurological disease states, Reference Klawans and Weiner8 and on observations that tardive dyskinesia can be suppressed and exacerbated by dopamine antagonist and agonist drugs respectively. Reference Gerlach9 However, post-mortem Reference Mackay, Iversen, Rossor, Spokes, Bird and Arregui10-Reference Kornhuber, Riederer, Reynolds, Beckmann, Jellinger and Gabriel12 and in vivo studies using radioligand imaging (for references see Alder et al Reference Adler, Malhotra, Elman, Pickar and Breier13 ) have uniformly failed to demonstrate differences in dopamine D2 receptor numbers in people with schizophrenia with and without tardive dyskinesia.
Another avenue of research in tardive dyskinesia has been to try and determine whether it is associated with structural brain changes. An early computed tomography (CT) study found that individuals with schizophrenia and tardive dyskinesia had larger lateral ventricles than those without, Reference Owens, Johnstone, Crow, Frith, Jagoe and Kreel14 but further studies failed to confirm this finding. Reference Hoffman and Casey15 Using MRI, Mion et al Reference Mion, Andreasen, Arndt, Swayze and Cohen16 found a smaller volume of the caudate nucleus, but not other basal ganglia nuclei, in people with schizophrenia with tardive dyskinesia compared with those without. However, this finding has again not been consistently replicated in subsequent studies. Reference Elkashef, Buchanan, Gellad, Munson and Breier17,Reference Waddington, O'Callaghan, Buckley, Madigan, Redmond and Stack18 There are a number of obstacles to detecting brain structural differences between patients with and without tardive dyskinesia. One is that the changes in schizophrenia in comparison to the healthy population are small - for example, the overall reduction of brain volume is about 2%. Reference Wright, Rabe-Hesketh, Woodruff, David, Murray and Bullmore19 Another is that treatment with antipsychotic drugs has been found to cause volume increases in the basal ganglia, Reference Brandt and Bonelli20 which could obscure volume reductions associated with tardive dyskinesia in this region. A final problem is that conventional brain imaging requires the selection of predetermined regions for study, which in the case of tardive dyskinesia has led to a focus on the basal ganglia; other components of the extrapyramidal system, such as the thalamus, substantia nigra, premotor cortex and prefrontal cortex, have not been investigated.
The development of structural imaging methods that map clusters of significant difference throughout the brain between groups of participants, without the necessity of a priori selection of regions of interest, has obvious advantages for answering questions about brain changes associated with tardive dyskinesia. In this study we applied one such technique, voxel-based morphometry (VBM), to a group of people with schizophrenia with and without tardive dyskinesia. We also included a healthy control group in order to be able to determine the relationship of any changes found to the brain volume changes associated with schizophrenia itself.
Method
Participants
The patient sample consisted of 81 adult in- and out-patients with schizophrenia (age range 23-63) drawn from two psychiatric hospitals, Benito Menni CASM and Sant Joan de Déu SSM in Barcelona, Spain. The sample was made up of two subgroups of patients selected on the basis of whether they showed (n = 32) or did not show (n = 49) tardive dyskinesia. All patients met DSM-IV 21 criteria for schizophrenia, based on interview by two psychiatrists plus review of case notes. Patients were excluded if: (a) they were younger than 18 or older than 65 years; (b) they had a history of brain trauma or neurological disease; and (c) they had shown alcohol/substance misuse within the 12 months prior to participation. Patients also needed to have a current IQ in the normal range (i.e. 70+). All patients were right handed. They were all on treatment with antipsychotics: atypical (n = 46), typical (n = 7), combined typical and atypical treatment (n = 27) (detailed drug information was missing for one patient).
A group of 61 healthy controls was also employed. They were selected to be demographically matched to the whole group of patients, and to the patients with and without tardive dyskinesia. They met the same exclusion criteria as the patients. They were recruited from non-medical staff working in the hospital, their relatives and acquaintances, and also from independent sources in the community. They were questioned and excluded if they reported a history of mental illness and/or treatment with psychotropic medication. The study was approved by the local research ethics committee and all patients gave written informed consent after a detailed explanation of the study.
Evaluation of motor disorder
Motor disorder was rated in the patients using a standard examination, which was videotaped. Patients were examined while seated, standing and walking, and ‘activation’ procedures designed to elicit involuntary movements were employed (touching the thumb of each hand to each finger in turn, holding the arms out with the wrists flexed, reciting the months of the year backwards). Ratings on Simpson et al's Reference Simpson, Lee, Zoubok and Gardos22 scale for tardive dyskinesia were made by two raters together (S.S. and E.P.-C.) who had been trained in the assessment of extrapyramidal side-effects. Videos of all patients who showed any evidence of involuntary movements were then reviewed by a third rater (P.J.M.) who had extensive experience in rating tardive dyskinesia; final ratings were made by consensus.
Presence of tardive dyskinesia was defined according to Schooler & Kane's criteria. Reference Schooler and Kane23 These require moderate involuntary movements (rating of ⩾3 on the Simpson scale) in at least one body area, or mild involuntary movements (rating of 2) in at least two different body areas. We additionally required that the patients show positive ratings on the core dyskinetic items on the Simpson scale; any who only scored on the items: increased blinking, tremor of eyelids, tremor of upper lip, tongue tremor, caressing/rubbing face, hair or thighs, restless legs, crossing/uncrossing legs or akathisia were not considered as having tardive dyskinesia. Patients without tardive dyskinesia scored no more than 1 (questionable) on any of the dyskinesia items on the Simpson scale.
Other measures
Symptoms were scored using the Spanish version of the Positive and Negative Syndrome Scale (PANSS). Reference Peralta and Cuesta24 Premorbid IQ was estimated using the Word Accentuation Test (Test de Acentuación de Palabras, TAP), Reference Del Ser, Gonzalez-Montalvo, Martinez-Espinosa, Delgado-Villapalos and Bermejo25 a word reading test that requires pronunciation of Spanish words whose accents have been removed. Current IQ was prorated from four subtests of the Wechsler Adult Intelligence Scale III (WAIS-III) Reference Wechsler26 (vocabulary, similarities, block design, and matrix reasoning).
MRI data acquisition
All participants underwent structural MRI scanning in the same 1.5 Tesla GE Signa scanner (General Electric Medical Systems, Milwaukee, Wisconsin, USA) located at the Sant Joan de Déu Hospital in Barcelona (Spain). High resolution structural T 1 MRI data were acquired with the following acquisition parameters: matrix size 512 × 512; 180 contiguous axial slices; voxel resolution 0.47 × 0.471 mm3; echo (TE), repetition (TR) and inversion (TI) times 3.93 ms, 2000 ms and 710 ms respectively; flip angle 15°.
Structural data were analysed with FSL-VBM, an optimised voxel-based morphometry style analysis Reference Good, Johnsrude, Ashburner, Henson, Friston and Frackowiak27 carried out with FSL tools; Reference Smith, Jenkinson, Woolrich, Beckmann, Behrens and Johansen-Berg28 this yields a measure of difference in local grey matter volume. In a first step, structural images were brain-extracted using BET. Reference Smith29 Next, tissue-type segmentation was carried out and the resulting grey matter partial volume images were then aligned to Montreal Neurological Institute (MNI 152) standard space using the FSL tools FLIRT and FNIRT. The resulting images were averaged to create a study-specific template, to which the native grey matter images were then non-linearly re-registered. The registered partial volume images were then modulated (to correct for local expansion or contraction) by dividing by the Jacobian of the warp field. The modulated segmented images were then smoothed with an isotropic Gaussian kernel with a sigma of 4 mm.
Data analysis
Group comparisons were carried out using a voxel-wise general linear model (GLM) and permutation-based non-parametric testing (for more technical details see www.fmrib.ox.ac.uk/fsl/fslvbm/index.html), correcting for multiple comparisons. These were made with the randomise programme implemented in FSL, using a cluster-based thresholding method with 10 000 iterations and initial cluster-forming threshold Z⩾2.3. The GLM was designed to account for the gender-related variability between participants. Clusters were assessed for significance at P<0.05, fully corrected for multiple comparisons across space. Anatomical locations of the significant clusters were determined by reference to the Harvard-Oxford cortical structural atlas integrated into FSL view (part of FSL) and the AAL atlas of 116 segmented structures within MRIcron software (for more details see www.mccauslandcenter.sc.edu/mricro/mricron/index.html).
Results
Demographic features of the patients and controls
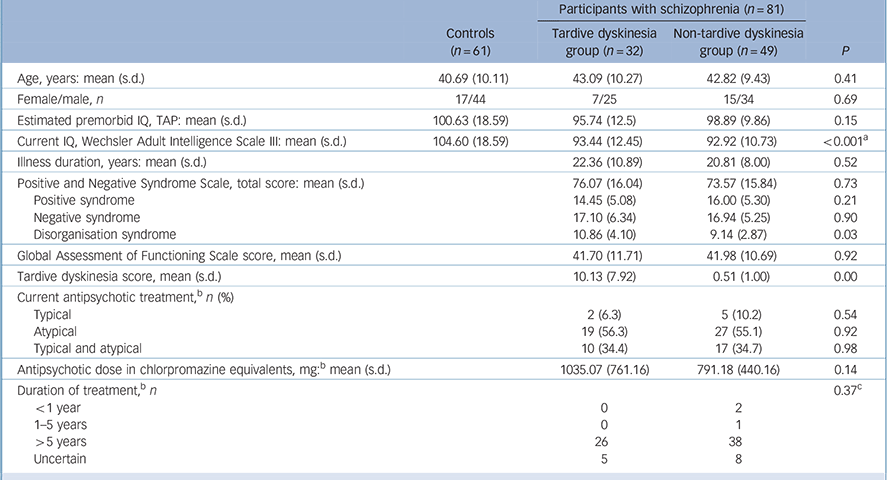
The findings are shown in Table 1. The controls were well matched to the whole group of patients (age: t = −1.34, P = 0.18; gender: χ2 = 0.01, P = 0.92; estimated premorbid IQ: t = 1.52, P = 0.13) and to the patients with and without tardive dyskinesia. The tardive dyskinesia and non-tardive dyskinesia groups were similar in age, but differed in gender distribution (22% v. 31% female), although not significantly. Since frequency of tardive dyskinesia has been found to vary according to gender in some studies, Reference Cunningham Owens1 this minor gender difference was covaried for in the comparison between patients with and without tardive dyskinesia. There were no significant differences between the two patient groups in duration of illness, global severity of illness or antipsychotic dosage in chlorpromazine equivalents (although this was numerically higher in the patients with tardive dyskinesia). They also showed similar levels of overall symptomatology as measured using the PANSS; however, the patients with tardive dyskinesia had significantly higher scores on the PANSS disorganisation factor compared with those without.
Table 1 Demographic and clinical data on the patients
| Participants with schizophrenia (n = 81) | ||||
|---|---|---|---|---|
| Controls (n = 61) |
Tardive dyskinesia group (n = 32) |
Non-tardive dyskinesia group (n = 49) |
P | |
| Age, years: mean (s.d.) | 40.69 (10.11) | 43.09 (10.27) | 42.82 (9.43) | 0.41 |
| Female/male, n | 17/44 | 7/25 | 15/34 | 0.69 |
| Estimated premorbid IQ, TAP: mean (s.d.) | 100.63 (18.59) | 95.74 (12.5) | 98.89 (9.86) | 0.15 |
| Current IQ, Wechsler Adult Intelligence Scale III: mean (s.d.) | 104.60 (18.59) | 93.44 (12.45) | 92.92 (10.73) | <0.001Footnote a |
| Illness duration, years: mean (s.d.) | 22.36 (10.89) | 20.81 (8.00) | 0.52 | |
| Positive and Negative Syndrome Scale, total score: mean (s.d.) | 76.07 (16.04) | 73.57 (15.84) | 0.73 | |
| Positive syndrome | 14.45 (5.08) | 16.00 (5.30) | 0.21 | |
| Negative syndrome | 17.10 (6.34) | 16.94 (5.25) | 0.90 | |
| Disorganisation syndrome | 10.86 (4.10) | 9.14 (2.87) | 0.03 | |
| Global Assessment of Functioning Scale score, mean (s.d.) | 41.70 (11.71) | 41.98 (10.69) | 0.92 | |
| Tardive dyskinesia score, mean (s.d.) | 10.13 (7.92) | 0.51 (1.00) | 0.00 | |
| Current antipsychotic treatment,Footnote b n (%) | ||||
| Typical | 2 (6.3) | 5 (10.2) | 0.54 | |
| Atypical | 19 (56.3) | 27 (55.1) | 0.92 | |
| Typical and atypical | 10 (34.4) | 17 (34.7) | 0.98 | |
| Antipsychotic dose in chlorpromazine equivalents, mg:Footnote b mean (s.d.) | 1035.07 (761.16) | 791.18 (440.16) | 0.14 | |
| Duration of treatment,Footnote b n | 0.37Footnote c | |||
| <1 year | 0 | 2 | ||
| 1-5 years | 0 | 1 | ||
| >5 years | 26 | 38 | ||
| Uncertain | 5 | 8 | ||
TAP, Word Accentuation Test (Test de Acentuación de Palabras).
a. Controls>tardive dyskinesia group; controls>non-tardive dyskinesia group.
b. Missing data for one participant in the tardive dyskinesia group.
c. Analysis carried out excluding participants in ‘uncertain’ category.
Length of antipsychotic treatment in the patients with and without tardive dyskinesia is also shown in Table 1. Most patients had received treatment with both typical and atypical antipsychotics; 1 patient (without tardive dyskinesia) had been treated only with typical antipsychotics, and 5 (all without tardive dyskinesia) had been treated only with atypicals. Most patients had received more than 5 years of antipsychotic treatment; this applied both to conventional antipsychotics (with tardive dyskinesia 80.8%, without tardive dyskinesia 65.9%) and atypicals (with tardive dyskinesia 79.1%, without tardive dyskinesia 80.8%).
Of the patients with tardive dyskinesia, 7 showed only orofacial movements (based on a score of ⩾2 in any facial region), 10 showed only trunk and/or limb movements (based on a score of ⩾2 in any bodily region) and 15 showed both types of movements. The sample also included two patients who had a presentation dominated by multiple tics consistent with tardive Tourette syndrome; however, both these patients also showed typical dyskinetic movements in more than one body area rated at ⩾2. As shown in Table 1, the patients showed on average moderate degrees of motor disorder, although with a wide range (mean Simpson rating scale total score 10.13 (s.d. = 7.92), range 3-48). A breakdown of the scores in the tardive dyskinesia group is shown in Fig. 1.
VBM comparison of the whole group of patients and controls
As this analysis was not a principal objective of the study, the results are only briefly summarised here (see online supplementary material and Fig. DS1 for a more detailed report). There were three large clusters where the patients showed significantly reduced grey matter volume compared with the controls. One of these covered an extensive area of the medial and inferior prefrontal cortex bilaterally, reaching the dorsolateral prefrontal cortex and the pre- and postcentral gyri. This cluster also extended to the bilateral insula, parts of the temporal cortex and the right angular and right inferior and superior parietal cortex. The second cluster was seen bilaterally in the cerebellum. The third was in the middle cingulate gyrus and the supplementary motor area. The patients showed two clusters of significantly greater volume than the controls, in the cerebellar crus and the brain stem.
VBM comparison of patients with and without tardive dyskinesia
This comparison revealed a pattern of largely subcortical volume reduction in the patients with tardive dyskinesia (Fig. 2; a more detailed mapping is shown in online Fig. DS2). Two clusters affected the basal ganglia, particularly the caudate nuclei bilaterally (left side: 1200 voxels, peak at MNI (–16, 16, 4), z-score 4.01; right side: 641 voxels, peak at MNI (14, 12, 2) z-score 4.16). The left-sided cluster additionally extended to both thalami, and the right-sided cluster extended to the right parahippocampus, temporal pole and inferofrontal cortex. A third cluster of volume reduction affected the left parahippocampus, the amygdala, the left temporal pole and marginally the left inferior frontal cortex (323 voxels, peak at MNI (−18, 8, −22), z-score 3.57). A fourth cluster was seen in the right cerebellum (546 voxels, peak at MNI (14, −68, −32), z-score 3.38). There were no clusters where the patients with tardive dyskinesia showed a significantly greater volume than those without tardive dyskinesia.
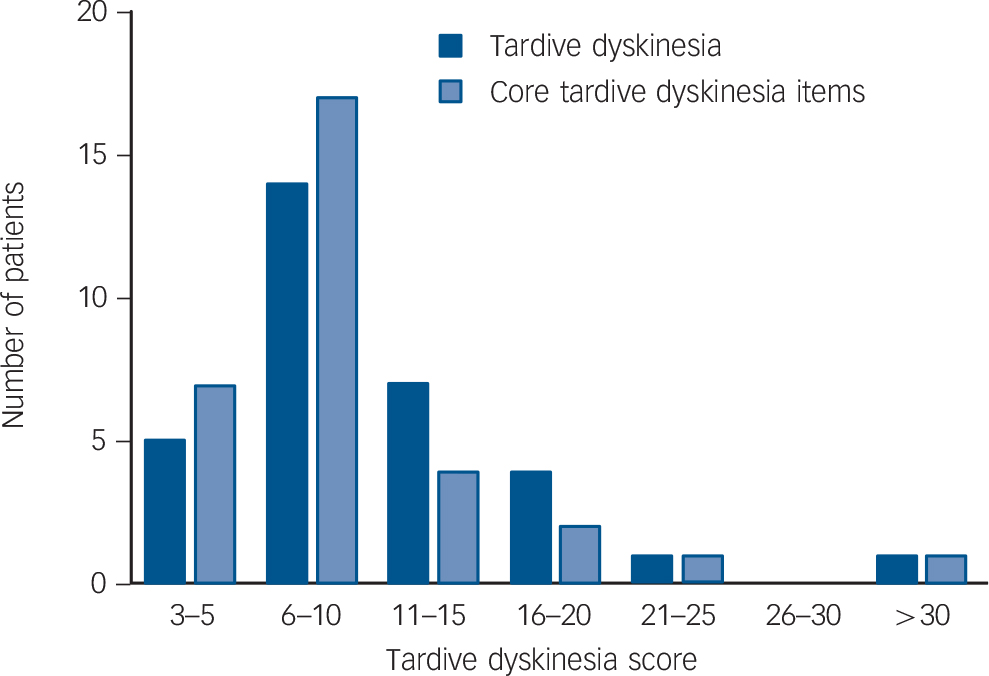
Fig. 1 Breakdown of scores on the Simpson Scale in patients with tardive dyskinesia.
Figure 3 shows the localisation of the volume reductions within the basal ganglia and thalamus. It can be seen that the caudate nucleus was most affected, the putamen to a lesser extent, and the globus pallidus only marginally. In both thalami the area affected was located predominantly medially.
Volume changes in the patients with and without tardive dyskinesia compared with the controls
To determine to what extent the regions of brain volume difference identified between patients with and without tardive dyskinesia were different from the corresponding regions in the controls we created a mask that covered all the voxels where significant differences were found between the two patient groups in the VBM comparison. This mask was then used to generate a region of interest (ROI) for grey matter volume in both patient groups and the healthy controls. The findings are shown in Figure 4(a). The controls had larger grey matter volumes than both the patients with and without tardive dyskinesia (controls: 8336 mm3 (s.d. = 706); patients without tardive dyskinesia 7736 mm3 (s.d. = 734); patients with tardive dyskinesia 7144 mm3 (s.d. = 733)). The differences between the controls and the patients without tardive dyskinesia were significant (t = 4.4, P = 3.0 × 10–5, effect size: 0.8), as were the differences between the controls and the patients with tardive dyskinesia (t = 7.3, P = 1.2 × 10–10, effect size: 1.6). (Significance values for the difference between the patients with and without tardive dyskinesia in this region were not calculated, since this area had already been identified as showing significant differences in the VBM analysis.)
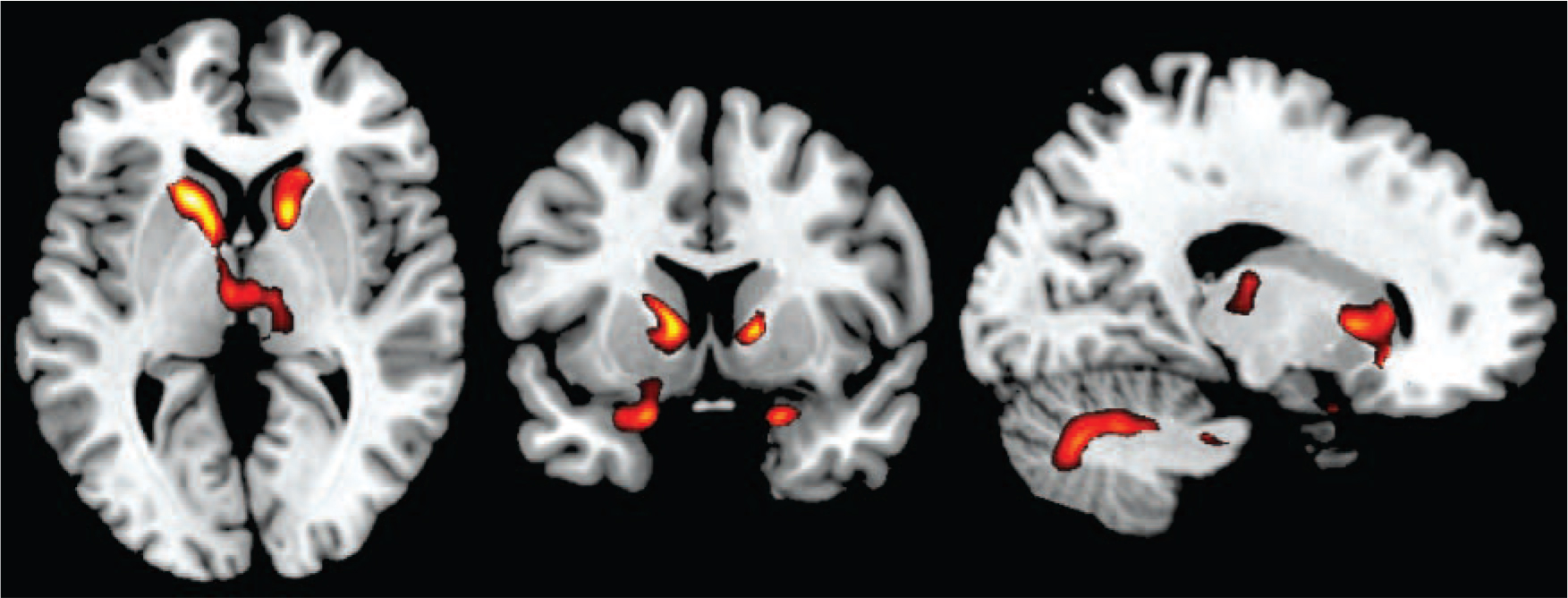
Fig. 2 Voxel-based morphometry findings.
Regions showing significant volume reduction between patients with and without tardive dyskinesia. Threshold set at P = 0.05 corrected for multiple comparisons across space.
We also investigated volume differences between the patients and controls within the basal ganglia. To do this, an additional mask was created based solely on the basal ganglia components of the original mask (i.e. areas of caudate + putamen + globus pallidus contained in the previous mask for differences between patients with and without tardive dyskinesia). These components were identified using the standard atlases provided by the FSL software. Reference Smith, Jenkinson, Woolrich, Beckmann, Behrens and Johansen-Berg28 Within this new mask, the patients without tardive dyskinesia showed similar but slightly larger volumes compared with the controls, but there continued to be a difference between the controls and the patients with tardive dyskinesia (controls: 2880 mm3 (s.d.= 350); patients without tardive dyskinesia: 2920 mm3 (s.d.= 442); patients with tardive dyskinesia 2704 mm3 (s.d. = 290); Fig. 4(b)). The difference between the controls and the patients without tardive dyskinesia was not significant (t = 0.6, P = 0.6, effect size: −0.1) but that between the controls and the patients with tardive dyskinesia was significant (t = 2.3, P = 0.03, effect size: 0.5).
Discussion
Main findings
Using VBM, this study found that compared with people with schizophrenia but without tardive dyskinesia, those with tardive dyskinesia showed reductions in grey matter volume that were predominantly subcortical in distribution and affected particularly the basal ganglia. Within the basal ganglia the changes were localised primarily to the caudate nucleus.

Fig. 3 Three-dimensional view showing the spatial localisation of volume reductions in the basal ganglia and thalamus.
Basal ganglia structures are depicted in red (top panel: caudate nucleus; second panel: putamen; third panel: pallidum; fourth panel: thalamus); regions showing volume reduction within these structures are shown in blue.
Our findings thus support the CT study of Bartels & Themelis Reference Bartels and Themelis30 and the MRI study of Mion et al, Reference Mion, Andreasen, Arndt, Swayze and Cohen16 which also found evidence of caudate nucleus changes, although as noted in the introduction other studies using conventional brain volumetry have had negative findings. To our knowledge there have been no other voxel-based studies of brain or grey matter volume in patients with and without tardive dyskinesia. However, Bai et al Reference Bai, Chou, Lin, Chen, Li and Yang31 examined white matter integrity in 40 people with schizophrenia, 20 with tardive dyskinesia and 20 without, using diffusion tensor imaging (DTI) and voxel-based analysis. They found clusters of decreased fractional anisotropy in two areas that were adjacent to the basal ganglia, the white matter of the left inferior frontal gyrus and temporal lobe, although changes were also seen in other regions.
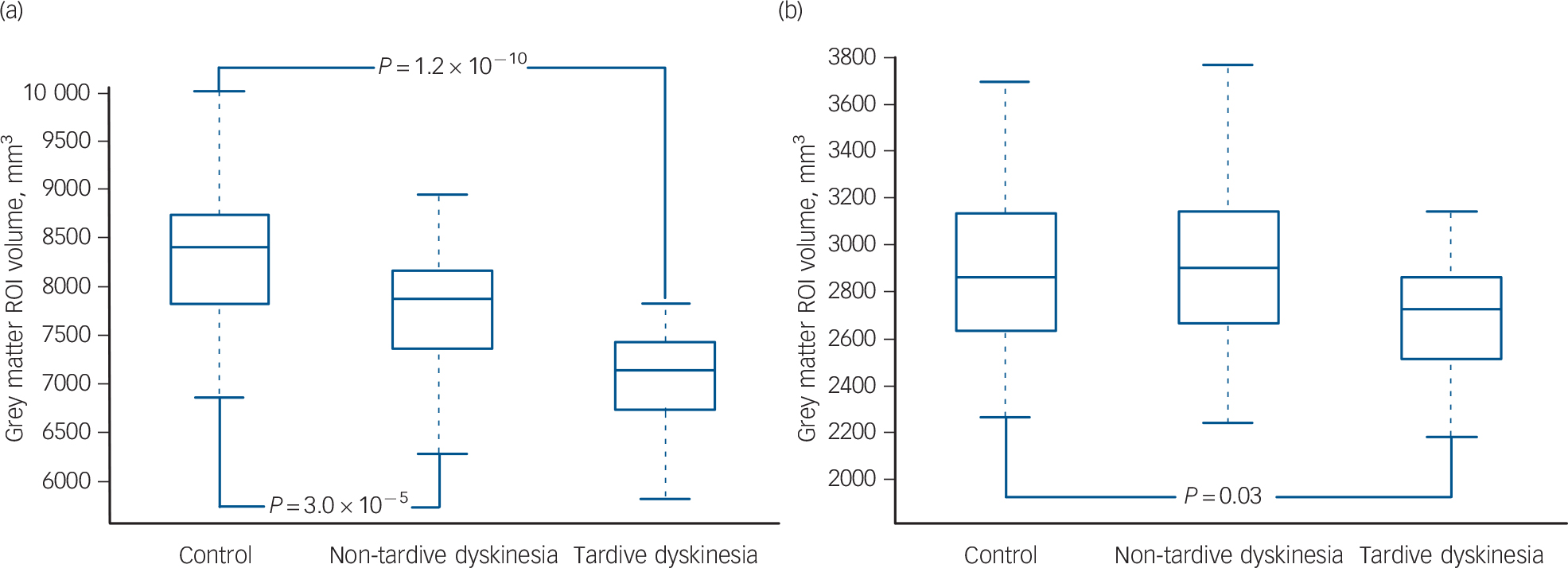
Fig. 4 Box plots of grey matter volumes in a region of interest (ROI) based on areas where the patients with tardive dyskinesia showed significant differences from those without tardive dyskinesia in the voxel-based morphometry analysis.
(a) Using a mask based on all regions where there were significant differences; (b) using a mask based solely on the basal ganglia components of the ROI.
Significance of findings
A finding of brain abnormality involving the basal ganglia is what would be predicted on the basis that tardive dyskinesia is an involuntary movement disorder of the extrapyramidal type. However, the extent to which the volume differences affected the caudate nucleus more than the putamen might be considered surprising. Following the work of Alexander et al, Reference Alexander, DeLong and Strick32 it is widely accepted that the basal ganglia are traversed by a series of cortical-subcortical-cortical ‘loops’, which carry both motor and non-motor information. Although these do not subdivide strictly according to nucleus, Reference Parent and Hazrati33 it is clear that the motor loop passes predominantly through the putamen. Circuits passing through the caudate nucleus are considered to be involved in higher-level control of behaviour, and lesions here tend to reproduce the apathy, perseveration, executive impairment and emotional changes seen after frontal lobe lesions. Reference Alexander, DeLong and Strick32,Reference Cummings34 Nevertheless, it can be noted that the caudate nucleus has been found to be heavily affected in choreiform disorders such as Huntington's disease. Reference Quinn and Schrag35 It may also be relevant that the movements in tardive dyskinesia differ from those seen in other involuntary movement disorders, being more complex, rhythmical and repetitive, particularly when they affect the orofacial region; tics and stereotyped movements are also recognised features of the syndrome. Reference Cunningham Owens1
We also found that the patients with tardive dyskinesia showed volume reductions in the thalamus. Here, the changes were located medially and centrally and therefore almost certainly involved the mediodorsal nucleus. Along with the ventroanterior/ventrolateral (VA-VL) complex, this nucleus forms the final, thalamic relay in the cortical-basal ganglia-cortical loop systems described above. Possibly in keeping with our findings in the basal ganglia, the mediodorsal nucleus receives part of its input from circuits passing through the caudate nucleus, whereas the motor loop passes through the putamen to project to the VA-VL complex, predominantly or exclusively. Reference Alexander, DeLong and Strick32
Analysis of an ROI encompassing the areas where there were differences between the patients with and without tardive dyskinesia found that these regions were smaller in both of the groups of participants than in healthy controls. This suggests that tardive dyskinesia is associated with brain structural changes over and above those occurring in patients with schizophrenia who do not show tardive dyskinesia. However, this pattern did not hold true in the basal ganglia. Here, there was no significant volume difference between the controls and the patients without tardive dyskinesia, although a significant difference between controls and the patients with tardive dyskinesia was still evident. One interpretation of this latter finding is that it reflects a basal ganglia volume increase due to antipsychotic drug treatment. That such treatment-related increases occur is reasonably well-supported by the literature. Reference Brandt and Bonelli20 However, this interpretation also implies that there should be basal ganglia volume reduction in patients with schizophrenia that have not been treated, which has been less consistently found, at least in the small number of studies that have used conventional MRI volume measurement (see references in Chua et al Reference Chua, Cheung, Cheung, Tsang, Chen and Wong36 ). Recently, however, Leung et al Reference Leung, Cheung, Yu, Yip, Sham and Li37 carried out a meta-analysis of six VBM studies of people with first-episode schizophrenia that had never been treated and found evidence for lower grey matter volume in the caudate nucleus bilaterally, as well as in a range of frontal and temporal cortical regions. The volume reductions in the striatum and some other areas were also found to be significantly more extensive than those found in nine studies carried out on treated patients with first-episode schizophrenia.
The patients in our study showed the typical presentation of tardive dyskinesia, i.e. they were chronically ill and in most cases had been on treatment with antipsychotics for several years. It would be interesting to know whether people with schizophrenia with spontaneous dyskinesia also showed brain structural changes. Such patients are not particularly easy to find and so far only two studies have been carried out. McCreadie et al Reference McCreadie, Thara, Padmavati, Srinivasan and Jaipurkar38 studied a sample of 62 people with chronic schizophrenia living in rural India who had never received antipsychotic treatment. Twenty-eight were found to have spontaneous dyskinesia. These patients showed no significant differences in caudate nucleus or lentiform nucleus (putamen and pallidum) volumes on MRI compared with 30 matched patients without dyskinesia. The caudate nucleus was non-significantly smaller in both patient groups than in 31 healthy controls, although the lentiform nucleus was larger (significantly on the left). Mittal et al Reference Mittal, Daley, Shiode, Bearden, O'Neill and Cannon39 rated involuntary movements in 30 mostly untreated patients with a diagnosis of prodromal syndrome (moderate levels of attenuated positive symptoms and/or decline in functioning in the presence of schizotypal personality disorder and/or family history of schizophrenia). They found a significant negative correlation between dyskinesia score and putamen volume, but there was no correlation with caudate nucleus volume. The results were unchanged when six participants who had received some antipsychotic treatment were excluded.
Implications for aetiology
Does a finding that tardive dyskinesia is associated with brain structural alterations have implications for the issue raised at the beginning of this article, of whether tardive dyskinesia is due to drug treatment, disease process or an interaction between the two? The answer has to be that our findings are essentially neutral on this point. One reason for this is that the study was cross-sectional in nature. Thus, for example, we cannot exclude the possibility that participants with schizophrenia who had smaller basal ganglia (and perhaps other brain structures) before they become ill are at greater risk of developing tardive dyskinesia. At first sight, our finding that tardive dyskinesia-associated volume reductions affected the caudate nucleus and putamen, which receive the bulk of the dopaminergic innervation of the basal ganglia, Reference Bjorklund and Dunnett40 but spared the globus pallidus, might be considered to implicate antipsychotic treatment, since these drugs work by blocking dopamine receptors. However, this finding is equally consistent with a factor related to the disease process of schizophrenia, given the putative role of dopamine in this.
In conclusion, this study suggests that tardive dyskinesia is the manifestation of a process or processes that involve brain structural change, and is not just a function of neurochemical changes, for example in postsynaptic D2 receptor numbers, as previously hypothesised. At the same time, this finding should not, by itself, be taken to imply that antipsychotic drugs can cause brain volume reductions, as has recently been claimed in animals Reference Dorph-Petersen, Pierri, Perel, Sun, Sampson and Lewis41 and patients with schizophrenia. Reference Ho, Andreasen, Ziebell, Pierson and Magnotta42
Funding
This work was supported by (a) a Marie Curie Reintegration Grant (MERG-CT-2004-511069 given to E.P.-C.); (b) the Centro de Investigación Biomédica en Red de Salud Mental (CIBERSAM); (c) several grants from the Instituto de Salud Carlos III (Miguel Servet Research Contract to R.S. (CP07/00048) and to E.P.-C. (CP10/00596); Intensification grant to S.S. (10/231); Research Project to E.P.-C. (PI05/2693)) and (d) the Comissionat per a Universitats i Recerca del DIUE from the Catalonian Government (2009SGR211).








eLetters
No eLetters have been published for this article.