Serotonin (5-HT) neuroendocrine challenge tests have been used widely to assess brain 5-HT function in patients with acute major depression (Reference Cowen and Van der KarCowen, 1998). The consensus of these studies is that 5-HT neurotransmission is impaired in unmedicated depressed patients but it is unclear whether this abnormality persists following clinical recovery. The aim of the present study was to use a relatively new probe of 5-HT function, the selective 5-HT reuptake inhibitor citalopram (Reference Joubert, Sanchez and LarssenJoubert et al, 2000), to assess 5-HT neuroendocrine function in patients with acute depression and in those who had made a full clinical recovery from their illness.
METHOD
Subjects
A total of 46 subjects were recruited for the study over an 18-month period from the unit's volunteer database, by advertisement or through out-patient clinics. All subjects gave written informed consent for the study, which was approved by the local ethics committee. The subjects included 16 controls (7 males and 9 females), 14 subjects who met the criteria for DSM-IV (American Psychiatric Association, 1994) major depression (7 males and 7 females) and 16 subjects recovered from at least two episodes of DSM-IV major depression (7 males and 9 females). All subjects were screened using the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID; Reference First, Spitzer and GibbonFirstet al, 1997). Subjects were excluded from the study if they had any history of an Axis I disorder (controls), any significant Axis I disorder apart from depression (acute and recovered groups), a history of a neurological disorder or a major physical illness.
The mean (s.e.) ages of the three groups were similar (controls, 38.6 (3.0) years; acute depression group, 44.5 (3.1) years; recovered depression group, 40.6 (3.1) years), as were their weights (controls, 70.1 (3.1) kg; acute group, 75.9 (3.6) kg; recovered group, 72.2 (3.8) kg). Patients with acute depression were drug-free for at least 3 months and had a mean (s.e.) score on the Hamilton Rating Scale for Depression (HRSD; Reference HamiltonHamilton, 1960) of 20.9 (1.5) and a Beck Depression Inventory (BDI; Reference Beck, Ward and MendelsonBecket al, 1961) score of 25.2 (3.3). Six subjects also met the criteria for melancholic depression. The subjects who were recovered from depression had been euthymic for at least 6 months and medication-free for at least 3 months. Their HRSD score had to be ≤7 (Reference Frank, Prien and JarrettFrank et al, 1991). Their mean (s.e.) score on the HRSD was 1.5 (0.5) and on the BDI was 4.4 (1.3). Two in the recovered group and two in the acutely depressed group had a past history of suicide attempts.
Neuroendocrine testing
All subjects were tested on two separate days in a double-blind, balanced order, placebo-controlled design. The mean (s.e.) gap between the two tests was 10 (2) days, with no significant difference between the groups (data not shown). Subjects fasted after a light breakfast and came to the research unit at 12.00 h. This time was chosen to avoid the sharp morning decline in plasma prolactin and cortisol levels. An indwelling venous canula was inserted and maintained with heparinised saline. Subjects were tested reclining and were not allowed to sleep. After a 30-min rest period for removal of baseline venous samples for prolactin and cortisol estimation, 10 mg citalopram (diluted in 5 ml saline) or 5 ml saline was administered intravenously over 30 min. Blood sampling continued at 15-min intervals for a further 150 min. At 30-min intervals, subjects completed a 100-mm visual analogue rating scale (VAS) for ‘nausea’. All female subjects were tested in the first half of the menstrual cycle.
Biochemical measurements
Following blood collection, plasma was separated by centrifugation and stored at −20°C. Plasma prolactin was measured using a standard immunoradiometric assay (reagents provided by Netria, London, UK). The inter— and intra-assay coefficients of variation of the prolactin assays over the range encompassed by the standard curve were 5% and 1%, respectively. Cortisol was analysed using radioimmunoassay (RIA) (reagents provided by Amersham International, Amersham, and Bioclin, Cardiff, UK) with inter— and intra-assay coefficients of variation over the range encompassed by the standard curve of 10% and 1%, respectively.
Statistical analysis
All data were analysed using SPSS for Windows (version 9.0). Prolactin and cortisol responses to citalopram were analysed as area under the curve (AUC) using the trapezoid method, with subtraction of baseline secretion extrapolated from time zero. Change in AUC (▵AUC) was measured by subtracting the AUC of prolactin and cortisol following placebo challenge from the respective AUC following citalopram challenge. The ▵AUC data were rank transformed in order to approximate satisfactorily to a normal distribution and then subjected to standard parametric analyses (Reference Conover and ImanConover & Iman, 1981) with univariate analysis of variance (ANOVA), where ‘group’ (control, acute depression and recovered depression) was the between-subject factor. Gender, weight and age were added to the ANOVA as covariates. Significant differences on the ANOVA were followed by post hoc unpairedt-tests. In the text, for comprehensibility, the hormonal responses are illustrated by the raw data rather than the rank-transformed results. Correlations also were carried out on rank-transformed ▵AUC data using Pearson's product moment. Baseline levels of prolactin and cortisol were calculated for each group using the average of the baseline values (at time zero) on the placebo and citalopram days and analysed by univariate ANOVA. The VAS scores of nausea were measured as peak change from baseline and also were analysed with an ANOVA.
RESULTS
Citalopram generally was well tolerated except for one female subject with acute depression who vomited after the infusion. This subject was excluded from further analysis. There was no change in VAS nausea ratings after placebo infusion (data not shown). Following citalopram, nausea ratings increased but there was no significant difference in mean (s.e.) peak nausea scores between the groups (F=0.29; d.f.=2.42; P=0.75): controls, 9.7 (5.7) mm; acute group, 9.2 (3.8) mm; recovered group, 14.4 (5.8) mm.
There was no significant difference between mean (s.e.) baseline prolactin levels across the three groups (F=0.60; d.f.=2.42; P=0.55): controls, 174.1 (14.0) mU/l; acute group, 162.0 (16.9) mU/l; recovered group, 151.0 (15.3) mU/l. The ANOVA on the ▵AUC prolactin data showed a significant effect of group (F=3.53; d.f.=2.39, P=0.039).Post hoc analysis showed that, relative to controls, prolactin responses were decreased significantly in both the acute and recovered depression groups, with no significant difference between these two groups (Fig. 1). The covariates of gender (F=0.003; d.f.=1.39; P=0.96), weight (F=1.1; d.f.=1.39; P=0.31) and age (F=0.51; d.f.=1.39;P=0.48) were not significant in the ▵AUC prolactin response.
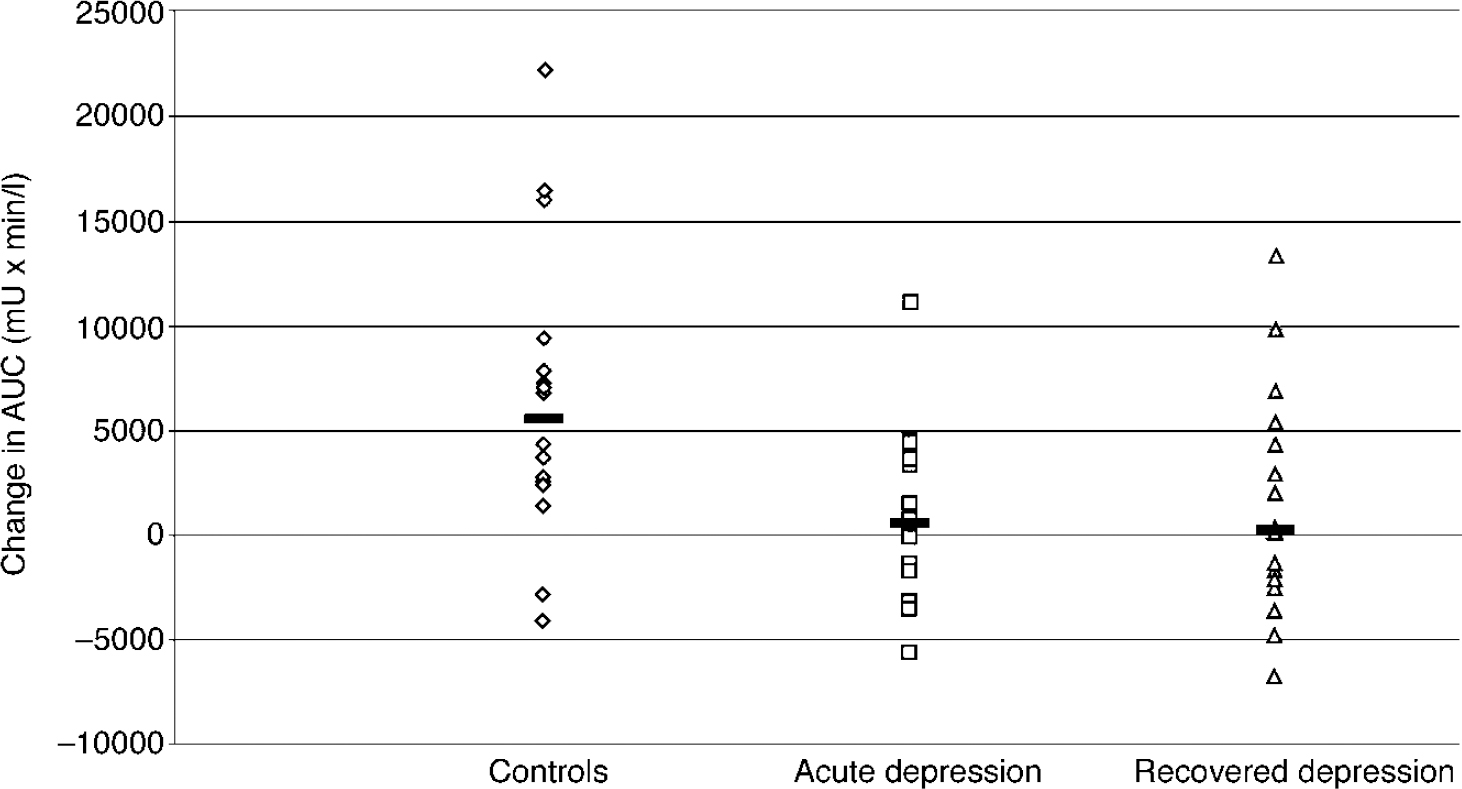
Fig. 1 The ▵AUC prolactin responses of healthy controls (n=16), acutely depressed subjects (n=13) and recovered subjects (n=16) to citalopram (10 mg intravenously). Bars show the median values. The responses of the controls are significantly greater than the responses of those in the acute (P=0.014) and recovered groups (P=0.031) (unpaired test on rank-transformed data).
There was no difference between baseline cortisol levels across the three groups (F=1.618; d.f.=2.42; P=0.210): controls, 16.2 (1.9) μ g/100 ml; acute group, 17.2 (2.03) μg/100 ml; recovered group, 13.2 (0.73) μg/100 ml). The ANOVA of the ▵AUC cortisol data showed a significant effect of group (F=6.03; d.f.=2.39; P=0.005). The post hoc analysis showed that, relative to controls, subjects with acute depression had significantly blunted cortisol responses whereas the recovered subjects did not. In addition, the responses of the recovered subjects were significantly greater than those in the acute group (Fig. 2). The covariates of gender (F=0.47; d.f.=1.39; P=0.50), weight (F=0.01; d.f.=1.35; P=0.95) and age (F=0.29; d.f.=1.39;P=0.60) were not significant in the ▵AUC cortisol response.
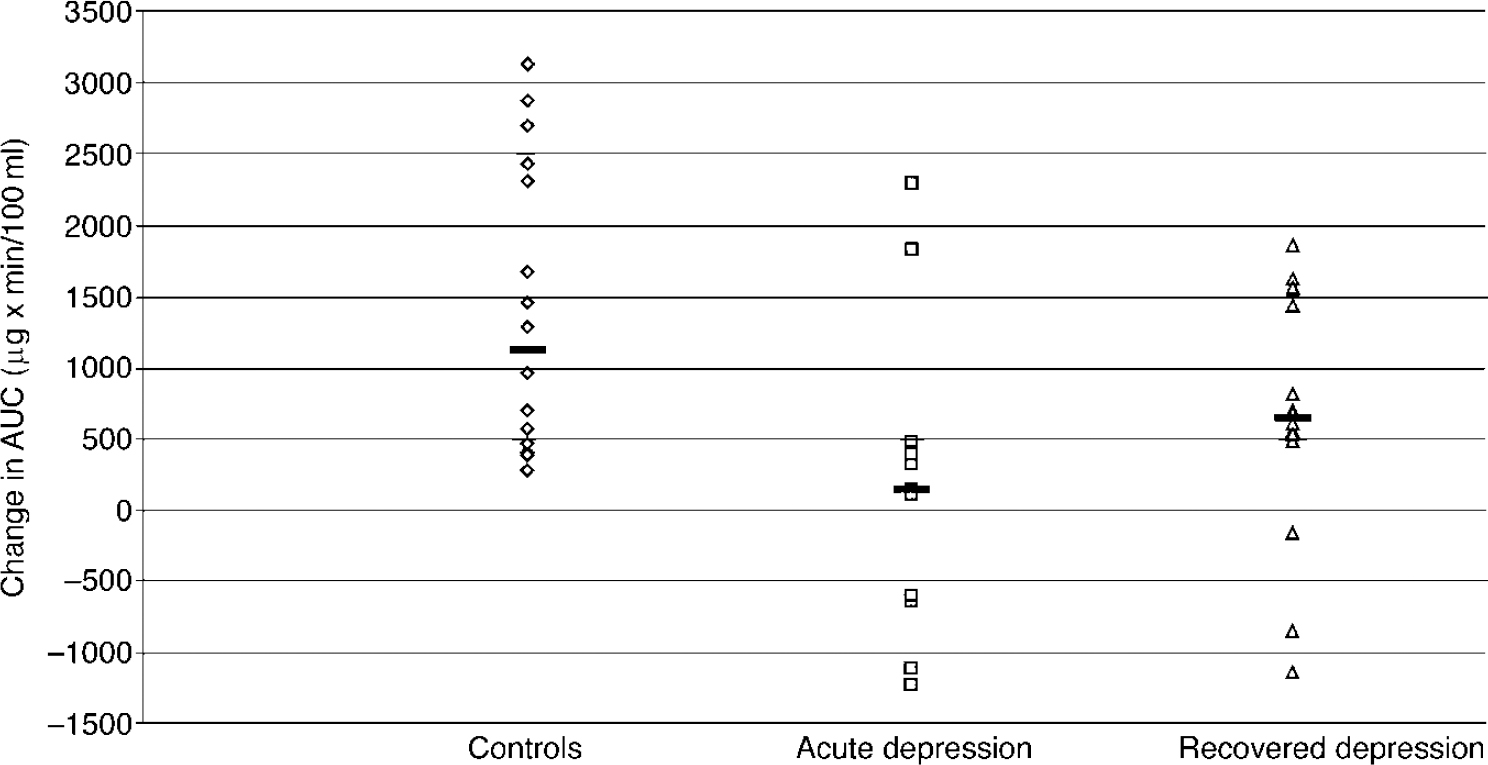
Fig. 2 The ▵AUC cortisol responses of healthy controls (n=16), acutely depressed subjects (n=13) and recovered subjects (n=16) to citalopram (10 mg intravenously). Bars show the median values. The responses of the patients with acute depression are significantly less than those of the healthy controls (P=0.002) or recovered subjects (P=0.038) (unpaired test on rank-transformed data).
There were no significant correlations between HRSD and BDI scores and ▵ AUC cortisol or ▵AUC prolactin responses in either acute or recovered subjects (all P values >0.05). Endocrine responses did not differ in the small number of acutely depressed melancholic subjects (n=6) compared with non-melancholic subjects. In all subjects considered together there were no significant correlations between baseline prolactin and ▵AUC prolactin responses or between baseline cortisol and ▵ AUC cortisol responses. In addition, baseline cortisol did not correlate with ▵AUC prolactin responses (all P values >0.5). However, baseline cortisol and prolactin responses correlated with each other (r=0.30, P=0.047) and the ▵AUC cortisol response correlated with the ▵AUC prolactin response (r=0.46;P=0.002). Finally, there was no correlation between peak nausea score after citalopram and ▵AUC cortisol or ▵AUC prolactin response (allP values >0.05).
DISCUSSION
Our findings indicate that the prolactin and cortisol responses to citalopram are blunted in patients with acute major depression. Following clinical recovery, citalopram-induced prolactin release remains attenuated whereas the cortisol responses return towards normal values.
Citalopram as 5-HT neuroendocrine probe
Citalopram is a highly selective 5-HT reuptake inhibitor that, when administered intravenously, produces dose-related increases in prolactin and cortisol (Reference Seifritz, Baumann and MullerSeifritz et al, 1996; Reference Attenburrow, Mitter and WhaleAttenburrow et al, 2001). Its neuroendocrine profile in this respect is the same as that of other 5-HT reuptake inhibitors such as clomipramine and indalpine (Reference LaakmannLaakmann, 1990). The 5-HT releasing agent d-fenfluramine produces very similar changes (Reference Newman, Shapira and LererNewman et al, 1998). Therefore, the ability of citalopram to increase plasma prolactin and cortisol is likely to be mediated via acute increases in 5-HT neurotransmission in the hypothalamus. The fact that prolactin and cortisol responses to citalopram correlated significantly with each other supports the proposal that they are mediated by a common (probably serotonergic) mechanism.
The most appropriate intravenous dose of citalopram for neuroendocrine challenge has not yet been established clearly. Other studies of citalopram in depressed and healthy subjects have used doses of 20 mg (Reference Seifritz, Baumann and MullerSeifritz et al, 1996; Reference Kapitany, Schindl and SchindlerKapitany et al, 1999) but in healthy volunteers we found that doses of 5 mg and above produce significant increases in prolactin and cortisol relative to placebo (Reference Attenburrow, Mitter and WhaleAttenburrow et al, 2001). We therefore chose a dose of 10 mg as likely to represent a reasonable balance between tolerability and the production of reliable endocrine responses. The identity of the post-synaptic 5-HT receptors that mediate citalopram-induced prolactin and cortisol release has not yet been determined. Our preliminary work suggests that they are unlikely to be of the 5-HT2A/2C receptor subtype (Reference Attenburrow, Mitter and WhaleAttenburrow et al, 2001).
Serotonin neuroendocrine responses in acute depression
Our findings with citalopram are consistent with a large body of data indicating that the endocrine responses to 5-HT challenges, particularly those that act via facilitation of presynaptic 5-HT function, are reliably blunted in patients with acute major depression (Reference Cowen and Van der KarCowen, 1998). In particular, our data are consistent with studies that have found blunted prolactin responses to the 5-HT reuptake inhibitor clomipramine (Reference Anderson, Ware and Da Roza DavisAnderson et al, 1992; Reference Golden, Ekstrom and BrownGolden et al, 1992), as well as one previous investigation using 20 mg of intravenous citalopram (Reference Kapitany, Schindl and SchindlerKapitany et al, 1999). In the latter investigation, citalopram-induced cortisol release was not decreased significantly in patients with depression relative to healthy controls, although a trend in this direction was apparent.
Less-consistent effects in depression have been noted with the prolactin response to the 5-HT releasing agent fenfluramine, where blunted responses may be more apparent in subjects with melancholic depression and in those with impulsive personality traits (Reference Cowen and Van der KarCowen, 1998). This latter observation suggests that features other than current major depression may contribute to blunted endocrine responses to 5-HT challenge, a notion supported by our findings in subjects recovered from depression (see below).
The nature of the impairment in brain 5-HT pathways that is responsible for the blunted endocrine responses to presynaptic 5-HT challenge in acute depression has not been established clearly. However, brain imaging studies have identified decreased numbers of post-synaptic 5-HT1A (Reference Sargent, Kjaer and BenchSargent et al, 2000) and 5-HT2A receptors (Reference Yatham, Liddle and ShiahYathamet al, 2000) in patients with major depression. Perhaps most pertinent to challenge studies with 5-HT reuptake inhibitors are reports of decreased availability of 5-HT reuptake sites in mid-brain and brain-stem regions of patients with depression (Reference Malison, Price and BermanMalison et al, 1998; Reference Willeit, Praschak and NeumeisterWilleit et al, 2000). These abnormalities could contribute to the decreased functional responses to 5-HT challenge identified by neuroendocrine tests.
Serotonin neuroendocrine function in subjects recovered from depression
We found that the prolactin response to citalopram remained blunted in subjects who had recovered from depression and were off medication. Because people who have previous episodes of depression are at high risk of future episodes (Reference Kendler, Kessler and NealeKendler et al, 1993), it is possible that blunted 5-HT-mediated prolactin release may be a trait marker indicating vulnerability to major depression. It is possible also that blunted 5-HT-mediated prolactin release could be a consequence of having experienced an episode of major depression.
To resolve this issue, future investigations will need to study larger numbers of subjects and identify as accurately as possible in each individual the number of previous depressive episodes as well as the length of symptomatic remission. Other studies have suggested that altered 5-HT function may be linked to particular clinical correlates, such as liability to suicidal and aggressive behaviour (Reference Kavoussi, Armstead and CoccaroKavoussi et al, 1997). Again, larger studies will be needed to explore this kind of possibility. However, in our study the small number of subjects who had made suicide attempts suggest that this may not be a prominent factor underlying blunted prolactin responses to citalopram. Recent weight loss also may complicate interpretation of endocrine responses to 5-HT challenge (Reference Cowen and Van der KarCowen, 1998). However, again this is unlikely to be a factor in the blunted prolactin responses in subjects recovered from depression, who did not describe any recent appetite change or weight loss on the clinical rating scales.
Our data are consistent with two of the three studies that have examined prolactin responses to fenfluramine in unmedicated subjects who had recovered from depression (Reference Coccaro, Siever and KlarCoccaro et al, 1987; Reference Shapira, Cohen and NewmanShapira et al, 1993; Reference Flory, Mann and ManuckFlory et al, 1998). However, in two previous studies we have found that recovered patients have normal prolactin responses to the 5-HT precursor L-tryptophan (Reference Upadhyaya, Pennell and CowenUpadhyaya et al, 1991; Reference Smith, Williams and CowenSmith et al, 2000). This suggests that some 5-HT abnormalities in depression are reversible with recovery, whereas others are not. Specifically, the prolactin response to L-tryptophan appears to be mediated via post-synaptic 5-HT1A receptors (Reference Cowen and Van der KarCowen, 1998), whereas the prolactin responses to citalopram are not attenuated by penbutolol, a β-adrenoceptor antagonist with 5-HT1A receptor antagonist properties (further details available from the author upon request).
Unlike citalopram-induced prolactin release, the cortisol responses in euthymic patients did show significant recovery compared with the acute depressed state. This again may reflect differential recovery between distinct 5-HT sub-systems. Another possibility is that blunted cortisol responses to 5-HT challenge are, in fact, partly dependent on the abnormal cortisol regulation often associated with the depressed state. The function of the hypothalamicpituitary-adrenal axis shows extensive recovery with clinical remission (Reference Zobel, Yassouridis and FrieboesZobel et al, 1999) and this could account for the relative normalisation of citalopram-induced cortisol release in recovered patients.
In conclusion, our findings confirm that acute major depression is associated with blunted 5-HT-mediated prolactin release and that this abnormality persists into clinical remission. Further studies will be needed to establish whether this represents a trait marker of vulnerability to depression and, if so, whether its origin is predominantly genetic or environmental.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
• People with a history of recurrent depression have enduring abnormalities in some aspects of brain 5-HT function.
-
• Impaired 5-HT function could be a marker of vulnerability to depressive relapse.
-
• Measures designed to increase brain 5-HT function could decrease the risk of relapse in vulnerable individuals.
LIMITATIONS
-
• Abnormalities in 5-HT neuroendocrine mechanisms do not necessarily reflect changes in 5-HT function in brain regions implicated in the depressive syndrome.
-
• The impaired prolactin response to citalopram in recovered subjects could be a consequence of having been depressed, rather than a marker of vulnerability.
-
• The sample size was too small for men and women to be studied independently.
Acknowledgements
Z. B. is an MRC Clinical Training Fellow and P. J. C. is an MRC Clinical Scientist. We thank Mary-Jane Attenburrow and Birgit Völlm for clinical assistance, Rena Hockney for nursing care and Alison Reed for technical assistance. This study was supported by the Medical Research Council. Citalopram was a gift from Lundbeck Ltd.





eLetters
No eLetters have been published for this article.