In Paper 1 we present the rationale for the development of the Stanley Foundation Bipolar Network (SFBN) and its core methodology (Reference Leverich, Nolen and RushLeverich et al, 2001). In Paper 2 (Reference Kupka, Nolen and AltshulerKupka et al, 2001, this supplement), preliminary results on the demographics of the first 261 patients studied (Reference Suppes, Leverich and KeckSuppes et al, 2001) are summarised and the findings are noted of several published open add-on case series exploring the clinical potential of three of the newer anticonvulsant drugs: lamotrigine (Reference Suppes, Brown and McElroySuppes et al, 1999), gabapentin (Reference Altshuler, Keck and McElroyAltshuler et al, 1999) and topiramate (Reference McElroy, Suppes and KeckMcElroy et al, 2000). By March 2001, more than 500 patients were being followed in the SFBN and its treatment outcome studies.
RATIONALE FOR THE SFBN
The National Institute of Mental Health (NIMH) hosted meetings in 1989 and 1994 to focus on the reasons for and remediation of the fact that studies of bipolar illness had been inadequately funded by the NIMH extramural programme for much of the preceding two decades (Reference Prien and PotterPrien & Potter, 1990; Reference Prien and RushPrien & Rush, 1996). This was particularly evident in comparison with research into the equally prevalent and disabling disorder schizophrenia. Even after these NIMH meetings, little progress was made until recently in ameliorating the situation.
Two of the major reasons cited as underlying the inadequate study of bipolar illness were the erroneous perception that lithium had adequately treated the illness and, more importantly, major methodological arguments about what measures and studies were optimal for this uniquely variable illness and its panoply of presentations. When prominent investigators submitted grant requests on pharmacological treatment of bipolar illness, extramural NIMH review committees would almost invariably reject them because of disagreements about optimal design and assessment methods. Similar problems are now occurring in the NIMH intramural review of work in bipolar illness. Acute studies in bipolar illness are difficult and expensive, especially given the heterogeneity of the illness and its multiple comorbidities.
These problems were further magnified in maintenance trials, and with a general lack of agreement about the best longitudinal measures for trials, NIMH-sponsored maintenance drug studies on bipolar illness virtually ceased following the study of Prien et al (Reference Prien, Caffey and Klett1974), despite the increasing recognition of the inadequacy of existing treatment for a very large proportion of patients with the illness (Reference Goldberg, Harrow and GrossmanGoldberg et al, 1995; Reference Denicoff, Smith-Jackson and DisneyDenicoff et al, 1997a ). One of the few funded studies of which we are aware is the ongoing comparison of lithium v. valproate monotherapy after stabilisation on the combination in patients with rapid-cycling bipolar disorder, undertaken by Calabrese et al (Reference Calabrese, Shelton and Bowden2001). However, in that study only one-quarter of the candidates in the intent-to-treat analysis ever became eligible for randomisation, largely because of the poor response even to this combination of two of the most widely used drugs for bipolar illness. These data continue to reflect the need for new approaches to the illness.
Given this lack of acute and long-term controlled clinical trials, Theodore and Vada Stanley were very generous in asking what they could do to help. The idea of a research network had been generally endorsed at the 1989 and 1994 NIMH bipolar meetings, so we proceeded on this basis. The SFBN was organised so that novel clinical treatments could be explored on a case series basis, and formal trials could be conducted on a randomised basis with either open or blind methodology, using similar core assessments at different levels of rating intensity (Fig. 1). It is hoped that information gleaned at one methodological level could help inform the other (Reference Benson and HartzBenson & Hartz, 2000; Reference Concato, Shah and HorwitzConcato et al, 2000; Reference Leverich, Nolen and RushLeverich et al, 2001).
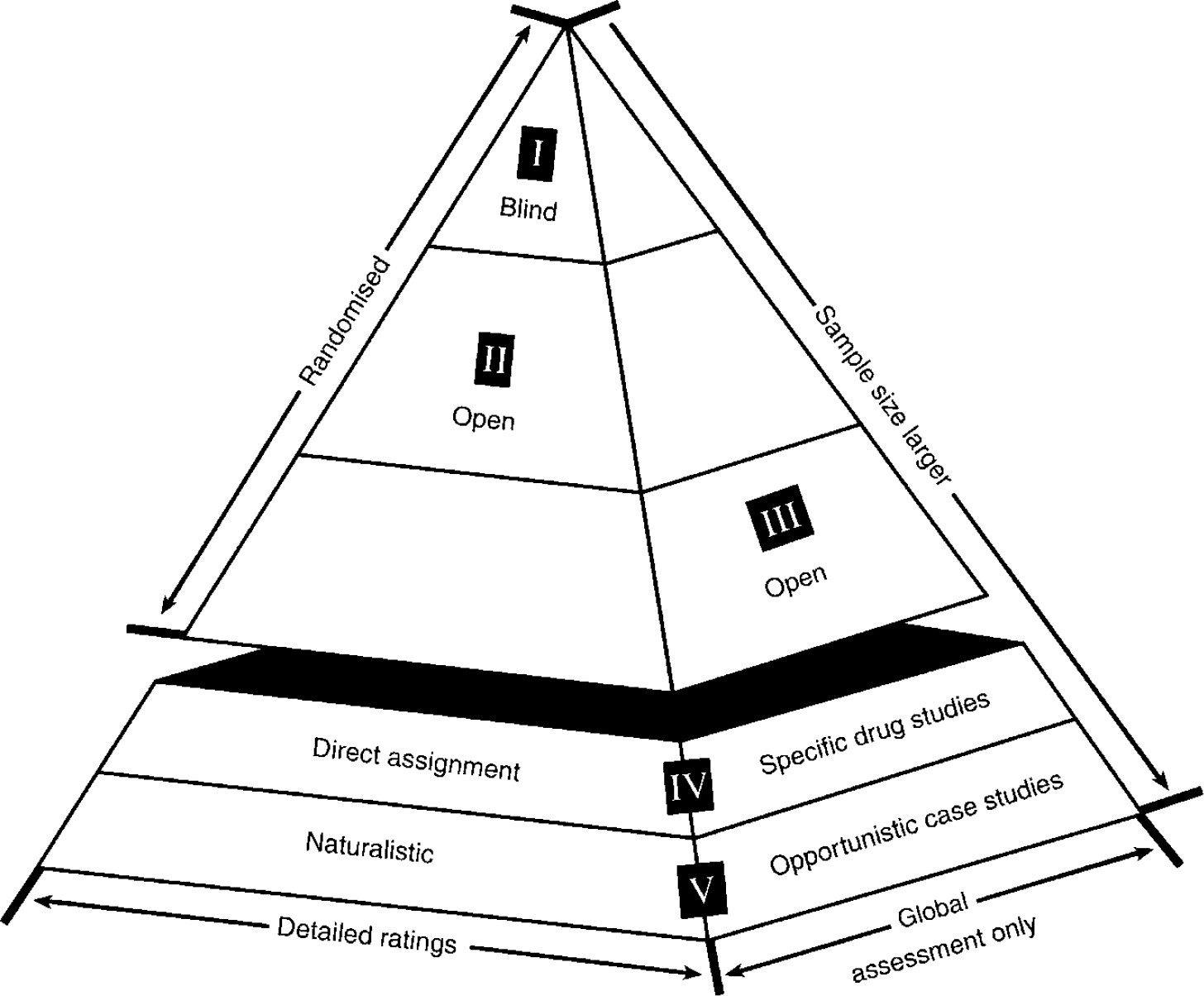
Fig. 1 Pyramidal structure of Stanley Foundation Bipolar Network clinical trials. Core rating instruments are used at all levels, but intensity of observations and degree of study control are increased at levels I and II. Early exploratory studies and clinical case series are conducted so as to inform the design of studies at other levels.
DESIGN AND METHODS OF THE SFBN
The SFBN has enrolled a large number of patients in academic settings (levels I and II: blind, randomised, detailed assessment) and is developing further studies in clinical practice settings (levels III and IV: open, randomised, global assessment and case series). As recommended by the NIMH meetings on bipolar illness, we enrol patients into the naturalistic follow-up phase regardless of the presence of comorbidities (Reference McElroy, Altshuler and SuppesMcElroy et al, 2001); we include patients with non-rapid-cycling forms of the disorder as well as rapid-cycling (≥4 episodes per year) and ultrarapid-cycling (≥4 episodes in 1 month) forms, and other patients who are often excluded from traditional randomised controlled trials. The SFBN continues to study these patients with long-term assessment tools after more formal acute or prophylactic treatment trials are over, to determine from systematic and naturalistic follow-up what treatments are most effective for individuals over time (Reference Leverich, Nolen and RushLeverich et al, 2001).
There are principal investigators at five core or original sites, including Dr Lori Altshuler and Dr Mark Frye (in Los Angeles); Dr John Rush and Dr Trisha Suppes (in Dallas); Dr Paul Keck and Dr Susan McElroy (in Cincinnati); Dr Kirk Denicoff and Ms Gabriele Leverich (in Bethesda); and Dr Willem Nolen and Dr Ralph Kupka (in Utrecht). In 2000, affiliated sites in Freiburg (Dr Jörg Walden) and Munich (Dr Heinz Grunze) were added, sharing common methodology and entering patients in both double-blind and open studies. European sites were included not only because of their shared interest and expertise, but also because of the early availability of some drugs for study that are not yet obtainable in the USA.
The concept of the SFBN is that studies can be conducted at all levels of the pyramidal structure as appropriate to the knowledge of a drug or phase of its development (Fig. 1). At the top of the pyramid are well-controlled studies with relatively small sample sizes where patients are studied in detail longitudinally. Each study requires separate consent and approval by local institutional review boards. At the base are open, randomised and naturalistic case series that can inform and help design studies at the higher levels (Reference Post, Denicoff and LeverichPost et al, 2000a ). Patients also give written consent to participate in this ongoing evaluation and naturalistic treatment. Instead of limiting studies to the traditional randomised controlled trials in academic settings, we decided to employ the same core methodology, but at a reduced level of detail and rating intensity at level III with open randomised studies assessing only global outcomes. Open assigned drug studies are represented at level IV, and naturalistic case series and opportunistic case studies at level V.
Thus, at levels I and II we use intensive clinician and patient daily ratings from the prospective NIMH Life Chart Method (NIMH-LCM; Leverich & Post, Reference Leverich and Post1996, Reference Leverich and Post1998) (Fig. 2(a)). In the SFBN we follow all 500 patients with daily prospective LCM ratings, which gives us a precise measure of type, severity and frequency of mood fluctuations, and the response to treatment both immediately and in the long term. In some clinical setting, it is proposed that at level III and below patients will complete their LCM ratings themselves and then their clinicians will make use of this assessment in their global ratings. These levels are thus designed to minimise the amount of time that clinicians would need to spend on the ratings. A Clinical Global Impressions (CGI) scale as revised for bipolar illness (the CGI-BP; Reference Spearing, Post and LeverichSpearing et al, 1997) is completed at all levels of the SFBN; at level III and below, this global outcome measure may be the only assessment that requires a physician (Fig. 2(b)).
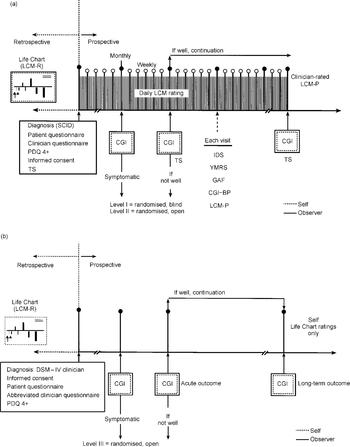
Fig. 2 Assessment of treatment outcomes in Stanley Foundation Bipolar Network randomised trials. Studies involve intense daily longitudinal rating at levels I and II (a), but only global assessments at levels III and below (b), so tests can be performed in clinical practice settings. CGI, Clinical Global Impressions scale; CGI—BP, CGI revised for bipolar disorder; LCM, Life Chart Method (P, prospective; R, retrospective); SCID, Structured Clinical Interview for DSM—IV Axis Disorders; PDQ 4+, Personality Disorders Questionnaire for DSM—IV Axis II Disorders; IDS, Inventory of Depressive Symptomatology; YMRS, Young Mania Rating Scale; GAF, Global Assessment of Functioning; TS, trial summary.
Reliability and validity
The initial data of the NIMH-LCM generated at the NIMH and in the SFBN indicate good reliability and clear validity. The Hamilton Rating Scale for Depression (Reference HamiltonHamilton, 1960) and the Inventory of Depressive Symptomatology (IDS; Rush et al, Reference Rush, Giles and Schlesser1986, Reference Rush, Gullion and Basco1996) were highly correlated (r=0.86 and r=0.78, respectively) with the depression scale of the NIMH-LCM (Denicoff et al, Reference Denicoff, Smith-Jackson and Disney1997b , Reference Denicoff, Leverich and Nolen2000). The Young Mania Rating Scale (Reference Young, Biggs and ZieglerYoung et al, 1978) was also highly correlated with the LCM mania rating (r=0.66) (Reference Denicoff, Leverich and NolenDenicoff et al, 2000), and a robust correlation (r=0.81 and r=0.73) was found in the same two studies (Denicoff et al, Reference Denicoff, Smith-Jackson and Disney1997a , Reference Denicoff, Leverich and Nolen2000) between the LCM average severity of illness (combined depression and mania ratings based on the degree of mood-related functional incapacity) and the Global Assessment of Functioning (GAF) scale (American Psychiatric Association, 1994). This longitudinal rating system (Leverich & Post, Reference Leverich and Post1996, Reference Leverich and Post1998; Reference Leverich, Nolen and RushLeverich et al, 2001) was chosen because the same measure of symptom-driven functional impairment can be used retrospectively when the specific symptoms (required for more detailed cross-sectional rating scales) cannot be easily remembered (Reference Squillace, Post, Savard, Post and BallengerSquillace et al, 1984; Reference Roy-Byrne, Post and UhdeRoy-Byrne et al, 1985; Reference Post, Roy-Byrne and UhdePost et al, 1988). Moreover, in contrast to the GAF, the LCM allows dimensional assessments of mild, moderate and severe mania and depression to be made separately.
Inadequacy of current mood stabilisers
The need for new treatment approaches for patients with bipolar disorder was further highlighted by a recent out-patient clinic study in the Biological Psychiatry Branch of the NIMH. Using most of the core methods noted above, Denicoff et al (Reference Denicoff, Smith-Jackson and Disney1997a ) treated patients with lithium or carbamazepine on a blind randomised basis for 1 year, switched to the other drug the next year, and then gave both drugs in combination in the third year. As illustrated in Fig. 3, counting a responder as someone who was rated as either very much improved (essentially asymptomatic) or much improved (with some residual symptoms) on the CGI scale, response rates to either lithium or carbamazepine monotherapy for 1 year were less than a third, but on the combination of both drugs in the third year, the response rate was over 50% (Reference Denicoff, Smith-Jackson and DisneyDenicoff et al, 1997a ).
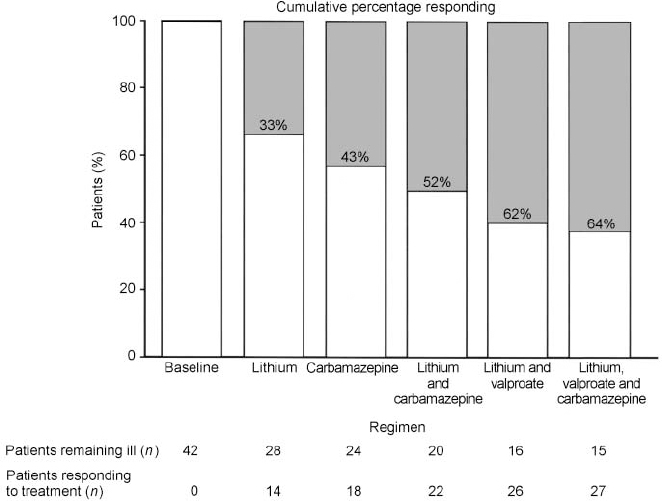
Fig. 3 Cumulative response and failure rates on each regimen. Lithium or carbamazepine was given in the first year on a randomised basis and patients were crossed over to the other drug in the second year. After a third year on the combination of lithium and carbamazepine, inadequate responders (white bars) were offered a fourth and fifth year. Responders (shaded bars) are patients showing much or very much improvement on the Clinical Global Impressions scale.
These data emphasise that these two widely used drugs are very often not effective either alone or in combination, even in a population recruited as out-patients in the Washington, DC area, two-thirds of whom were employed or in higher education. Thus, these data on poor outcome are not based on the highly treatment-resistant group of patients recruited and studied in our in-patient unit (Frye et al, Reference Frye, Ketter and Kimbrell2000a ,Reference Frye, Ketter and Leverich b ). Another discouraging aspect of the former study was that it allowed short-term benzodiazepine/neuroleptic augmentation for mania and antidepressant therapy for breakthrough depression, as needed (Reference Denicoff, Smith-Jackson and DisneyDenicoff et al, 1997a ). Thus, these traditional regimens of lithium or carbamazepine or combination treatment, even with augmentation treatments, still did not yield a high degree of efficacy. Compared with the baseline year, lithium substantially reduced the amount of time in manic phase, as did the combination of carbamazepine and lithium, but none of these treatments was notably effective on average for the overall amount of time spent in depression.
As revealed in Fig. 3, even including data from an additional year on lithium and valproate plus augmentation treatment, and a fifth year on the triple combination (lithium, carbamazepine and valproate plus augmentation), 36% of the original cohort of patients still remained inadequately responsive during any of the 5 years of treatment (Reference Denicoff, Smith-Jackson and BryanDenicoff et al, 1997c ). In particular, treatment of the depressive component of the illness remained more problematic than the manic component, and did not achieve an overall significant decrease in average time depressed in any of the 5 prospective years of treatment compared with the baseline year.
Double-blind comparison of bupropion, sertraline and venlafaxine
Because of these results and similar data from many other clinics in academic centres (reviewed in Reference Gitlin, Swendsen and HellerGitlin et al, 1995; Reference Goldberg, Harrow and GrossmanGoldberg et al, 1995), as well as the paucity of comparative data in bipolar depression, the first decision of the SFBN was to set up a double-blind randomised clinical trial comparing the acute and long-term efficacy of three of the newer antidepressant drugs with different putative mechanisms of action — bupropion (dopaminergic), sertraline (serotonergic) and venlafaxine (serotonergic and noradrenergic) — as adjunctive treatment to previously inadequate mood stabiliser regimens, similar to that designed by Sachs et al (Reference Sachs, Lafer and Stoll1994). Over 125 patients have been randomised in this ongoing study. In the first 64 patients randomised to 95 acute trials in the blind study there was a 14% rate of switch into acute mania (n=6) or hypomania (n=7) in the first 10 weeks of the study. Another 33% have so far switched into mania or hypomania in the intended 1-year continuation phase. Without breaking the blind, a data safety monitoring inquiry yielded evidence that no one drug of the three was under- or overrepresented in either rate of improvement or switch into mania.
Another important goal of the SFBN is evaluation of the profusion of medicines that are potentially either mood stabilisers or unimodal antimanic or antidepressant treatments. We hope to address the question of the optimal sequencing of these drugs for the individual patient as a function of different clinical presentations, subtypes, comorbidities and neurobiological markers.
Thus, the SFBN has a series of embedded randomised trials in which a patient who does not respond to any of the agents in the crossover phases of the first comparative antidepressant trial described above can go on to another randomised trial of, for example, lamotrigine v. tranylcypromine, a monoamine oxidase inhibitor (MAOI). This will be one of the first randomised trials comparing a potential mood stabiliser (lamotrigine) to an MAOI for refractory bipolar depression. Each of these drugs will be used adjunctively to a mood stabiliser.
Exploratory case series on novel anticonvulsants as mood stabiliser
The SFBN also decided to examine open pilot data at level V for each of the anticonvulsants newly approved by the Food and Drug Administration for add-on treatment of epilepsy as potential mood stabilisers. These retrospective case series could then inform more formal comparative trials at higher levels of the Network. These anticonvulsants include lamotrigine (Reference Suppes, Brown and McElroySuppes et al, 1999), gabapentin (Reference Altshuler, Keck and McElroyAltshuler et al, 1999) and topiramate (Reference McElroy, Suppes and KeckMcElroy et al, 2000), which have shown promise in different symptom areas as open adjuncts to previously ineffective treatments. Thus, the SFBN has started to examine the potential effectiveness of a series of new agents in naturalistic treatment, both for breakthrough depression and persistent cycling. Although several of these drugs appear promising, further controlled clinical trials are now needed (Table 1).
Table 1 Response rates in open add-on case series of the Stanley Foundation Bipolar Network
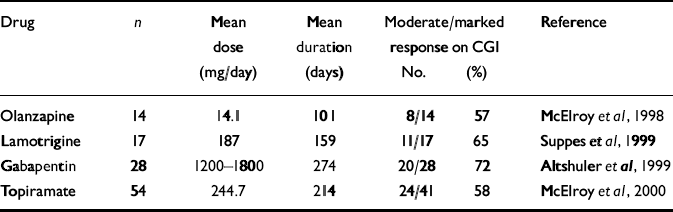
| Drug | n | Mean dose (mg/day) | Mean duration (days) | Moderate/marked response on CGI | Reference | |
|---|---|---|---|---|---|---|
| No. | (%) | |||||
| Olanzapine | 14 | 14.1 | 101 | 8/14 | 57 | Reference McElroy, Frye and DenicoffMcElroy et al, 1998 |
| Lamotrigine | 17 | 187 | 159 | 11/17 | 65 | Reference Suppes, Brown and McElroySuppes et al, 1999 |
| Gabapentin | 28 | 1200-1800 | 274 | 20/28 | 72 | Reference Altshuler, Keck and McElroyAltshuler et al, 1999 |
| Topiramate | 54 | 244.7 | 214 | 24/41 | 58 | Reference McElroy, Suppes and KeckMcElroy et al, 2000 |
Clinical and biological correlates of treatment response
Biological correlates of clinical response are also sought. Consistent data from more than 12 studies (reviewed in Reference Post, Pazzaglia, Ketter, Halbreich and MontgomeryPost et al, 2000b ) indicate that people with bipolar disorder have increased intracellular calcium in their blood elements (platelets, neutrophils or B lymphoblasts), as originally described by Dubovsky et al (Reference Dubovsky, Christiano and Daniell1989). It may be important to know which patients have increased calcium levels and whether this will help identify those likely to respond to a calcium channel blocker or other calcium-active treatment. Hough et al (Reference Hough, Lu and Davis1999) have developed a microassay for intracellular calcium that will allow the Network to address this question. In another example, Frye et al (Reference Frye, Pazzaglia and Luckenbaugh1997) have found that a low baseline level of somatostatin in cerebrospinal fluid is associated with nimodipine response. This is interesting, because nimodipine is one of the few drugs that increase the concentration of somatostatin in the cerebrospinal fluid (Reference Pazzaglia, George and PostPazzaglia et al, 1995).
In addition to these types of neuro-chemical markers, brain imaging and genetic markers are also potentially useful in clinical response prediction. Initial trends from NIMH in-patient data indicate that frontal and paralimbic hypoactivity seems to be more associated with response to nimodipine (Reference Ketter, Kimbrell and GeorgeKetter et al, 1999), gabapentin and lamotrigine (personal communication, T.A. Kimbrell et al, 1997), whereas hyperactivity, particularly in the left insula, seems to be associated with carbamazepine response (Reference Ketter, Kimbrell and GeorgeKetter et al, 1999). Whether such measures will be useful in helping to choose the most appropriate pharmacotherapy or parameters of repeated transcranial magnetic stimulation (rTMS) in the clinic remains to be seen (Reference Kimbrell, Little and DunnKimbrell et al, 1999; Reference Post, Speer, George and BelmakerPost & Speer, 2000; Reference Speer, Kimbrell and WassermanSpeer et al, 2000).
It is likely, however, that single nucleotide polymorphisms (SNPs) will provide information on prediction of individual treatment response and drug tolerability, even prior to their ability to identify new gene markers of illness vulnerability that will become targets for therapeutic intervention. The SFBN focus on detailed aspects of acute and long-term treatment outcome should fit well with the use of SNPs, made available by the sequencing of the human genome.
CONCLUSION
Figure 4 summarises some of the ongoing randomised open or blind clinical trials and naturalistic case series within the SFBN. After the comparative antidepressant trials, the major study in the SFBN is the omega-3 fatty acids trial. Following on the findings of Stoll et al (Reference Stoll, Severus and Freeman1999), we are comparing 6 g of the nearly pure eicosapentaenoic acid (EPA) form of omega-3 fatty acid with placebo in a 4-month double-blind trial to augment existing treatment with mood stabilisers. Ninetyone patients have already been randomised. In contrast with most other SFBN studies, which compare the efficacy of several drugs at a given clinical choice point (at which evidence is unavailable regarding which option is best), this protocol is the first in the Network to use a placebo. Yet it should not be onerous to patients (Reference Post, Denicoff and LeverichPost et al, 2000a ) since it involves a randomised add-on to existing treatment, following which all patients will have the option of an 8-month open treatment trial.
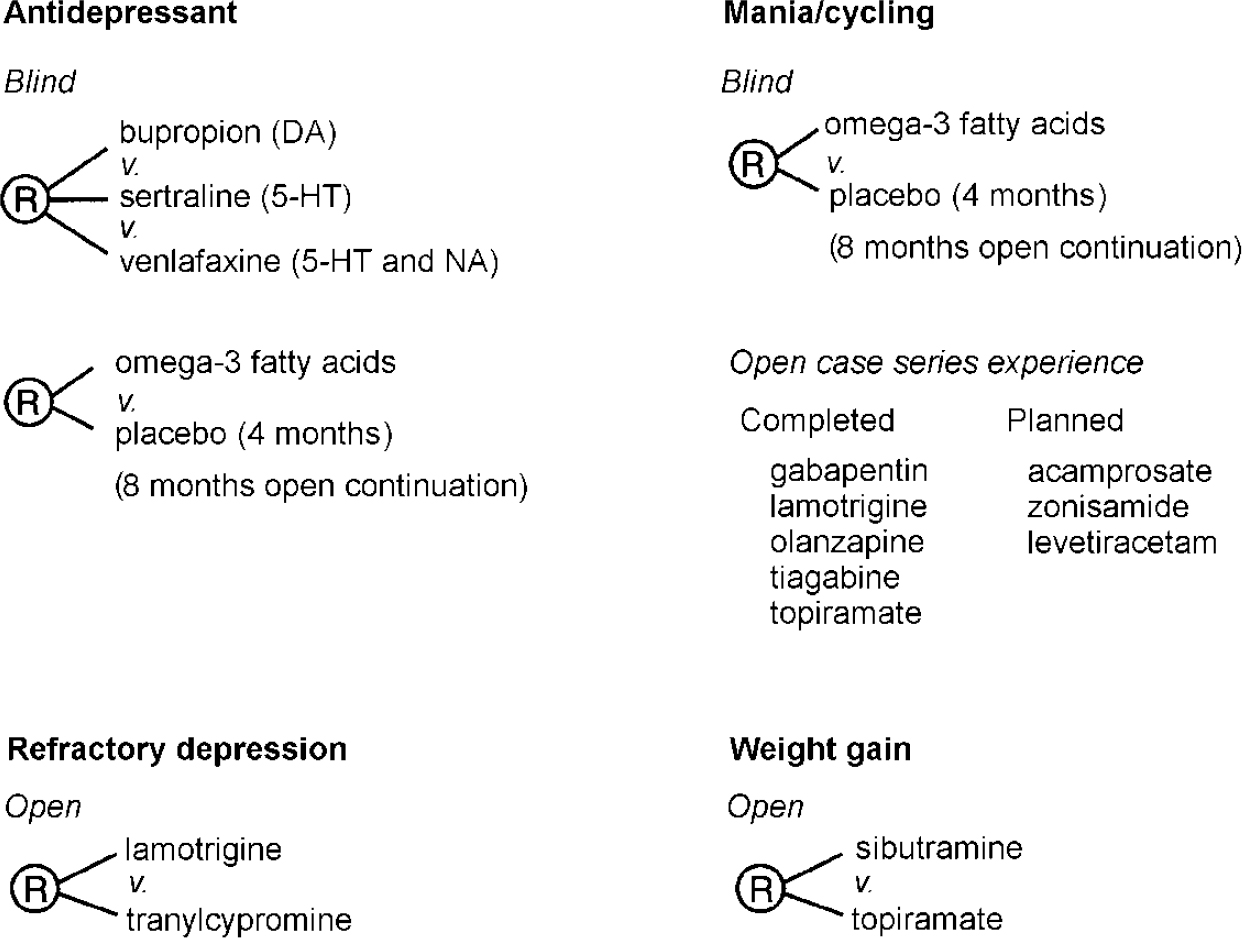
Fig. 4 Network randomised trials as add-on to mood stabilisers. DA, dopaminergic; 5-HT, serotonergic; NA, noradrenergic.
Children and adolescents with very early onset of bipolar disorder are increasingly being recognised, and we hope to develop parallel assessments for this age group, using a ‘Kiddie’ LCM designed to be continuous with the adult version (Reference Leverich and PostLeverich & Post, 1998) (Fig. 5).

Fig. 5 ‘Kiddie’ Life Chart of a 12-year-old child with affective dysfunction from the first year of life.
A newly launched NIMH initiative, the Systematic Treatment Enrichment Program for Bipolar Disorder (STEP-BD), aims to recruit 10 000 patients to participate in a series of open and randomised clinical trials headed by Drs G. Sachs and M. Thase. The SFBN is working in close coordination with STEP-BD to prevent overlap and to ensure maximum exploitation of new therapeutic opportunities.
Given the large number of new putative antimanic, antidepressant and mood-stablising drugs now available for study (Post et al, Reference Post, Frye and Leverich1998a ,Reference Post, Frye and Denicoff b ), we look forward to rapid advances in pharmacotherapy and better matching of individuals to optimal therapies. Now that several drug companies are beginning to study bipolar disorder, one can be hopeful that the proliferation of new information will rapidly improve the therapeutic outcome for patients with this potentially devastating illness.
In summary, the SFBN addresses many of the recommendations of the NIMH conferences on bipolar illness held in 1989 and 1994. Now that we have well-recognised and validated longitudinal outcome measures, such as the prospective NIMH-LCM, and a substantial number of patients in continuous longitudinal treatment and followup, we look forward to advances in the therapeutics for bipolar disorder.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ Currently available treatments leave patients with bipolar illness with substantial residual morbidity.
-
▪ Open studies provide initial preliminary data upon which to draw in the design of more formal randomised clinical trials.
-
▪ A large consortium uses a standard longitudinal rating instrument, the National Institute of Mental Health Life Chart Method, which has been validated and which provides a detailed designation of manic and depressive episodes and their long-term response to treatment.
LIMITATIONS
-
▪ Patients attending academic and speciality clinics may differ from those seen in general practice.
-
▪ Open add-on trials are subject to many methodological confounds and biases.
-
▪ Retrospective psychosocial and course-of-illness data depend, to a large extent, on patient recall, where accuracy is suspect.









eLetters
No eLetters have been published for this article.