The monoamine neurotransmitter serotonin (5-HT) has an important role in both the pathophysiology and treatment of anxiety and panic attacks. Reference Bell and Nutt1,Reference Baldwin, Anderson, Nutt, Bandelow, Bond, Davidson, den Boer, Fineberg, Knapp, Scott and Wittchen2 The strongest evidence that a specific abnormality of the serotonin system predisposes or contributes to anxiety comes from studies of the 5-HT1A receptor. ‘Knockout’ mice bred without 5-HT1A receptors in the cerebral cortex and limbic system show increased anxiety behaviours Reference Parks, Robinson, Sibille, Shenk and Toth3 and patients with panic disorder have been shown by challenge testing to have subsensitivity of these receptors. Reference Lesch, Wiesmann, Hoh, Müller, Disselkamp-Tietze, Osterheider and Schulte4 Furthermore, the azapirone 5-HT1A agonist buspirone is licensed for the treatment of generalised anxiety disorder in the UK. In a systematic review of 36 trials of patients treated for generalised anxiety disorder azapirones, including buspirone, were found to be superior to placebo in short-term studies (4–9 weeks). Reference Chessick, Allen, Thase, Batista Miralha da Cunha, Kapczinski, de Lima and dos Santos Souza5
The development of select radiotracers for the 5-HT1A receptor now allows a direct examination of this receptor system in living patients with panic disorder. Neumeister et al Reference Neumeister, Bain, Nugent, Carson, Bonne, Luckenbaugh, Eckelman, Herscovitch, Charney and Drevets6 reported the first full positron emission tomography (PET) study of 5-HT1A binding in 16 unmedicated patients with panic disorder and 15 healthy controls. Regions of interest examined were limited to the anterior cingulate cortex, posterior cingulate cortex, anterior insula, mesiotemporal cortex, anterior temporopolar cortex and midbrain raphe – all areas of high 5-HT1A binding. Lower 5-HT1A binding occurred in anterior cingulate, posterior cingulate and raphe only in patients with panic disorder. Seven of the 16 patients with panic disorder had comorbid depression, although there was no difference in 5-HT1A binding between the patients with panic disorder with comorbid depression and those without. The radiotracer used for Neumeister et al's study [18F]-FCWAY, gives a reliable signal subcortically, but quantification in cortical areas is limited because of retained fluoride signal in bone. Reference Carson, Wu, Lang, Ma, Der, Herscovitch and Eckelman7
A further recent PET study using [11C]WAY-100635 found reduced 5-HT1A binding in social anxiety disorder. Reference Lanzenberger, Mitterhauser, Spindelegger, Wadsak, Klein, Mien, Holik, Attarbaschi, Mossaheb, Sacher, Geiss-Granadia, Kletter, Kasper and Tauscher8 That study compared 12 unmedicated male patients with social anxiety disorder with 18 healthy controls. Six of these patients had comorbid agoraphobia. Six regions of interest were examined a priori – reduction of 5-HT1A binding was found in the amygdala, anterior cingulate, insula, medial orbitofrontal cortex and raphe. After Bonferroni correction only the differences in the amygdala and anterior cingulate cortex remained significant. There was no significant correlation between state or trait anxiety scores and regional 5-HT1A binding potential in both groups.
In this study, we used the PET tracer [11C]WAY-100635 which allows in vivo quantification of 5-HT1A receptors with exquisite delineation and precision. Reference Pike, McCarron, Lammertsma, Osman, Hume, Sargent, Bench, Cliffe, Fletcher and Grasby9,Reference Gunn, Sargent, Bench, Rabiner, Osman, Pike, Hume, Grasby and Lammertsma10 Specifically, we wished to confirm the findings of Neumeister et al, Reference Neumeister, Bain, Nugent, Carson, Bonne, Luckenbaugh, Eckelman, Herscovitch, Charney and Drevets6 extend the findings to look at more regions of interest and finally examine the effect of SSRI treatment. Our specific hypothesis was that patients with panic disorder would have reductions in 5-HT1A receptor binding in anxiety-associated regions of the brain, and that SSRI treatment would not affect 5-HT1A receptor availability.
Methods
Recruitment of participants
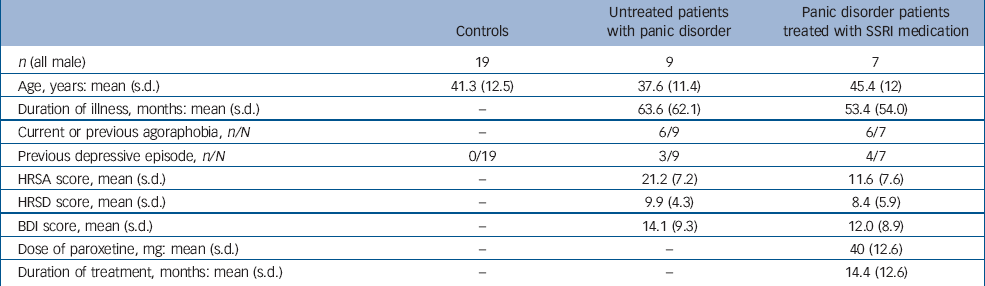
Nine unmedicated male patients with a primary diagnosis of panic disorder with or without agoraphobia (DSM–IV criteria) 11 were recruited for PET scanning from the out-patient clinic of the Psychopharmacology Unit, Bristol Royal Infirmary. Seven patients were naïve to antidepressant therapy and two had taken a tricyclic antidepressant in the past. No patient had been on regular psychotropic medication in the previous 3 months. Exclusion criteria included current or comorbid major depression, serious physical illness, a history of bipolar affective disorder, current or past drug or alcohol dependence, current or previous treatment with mood stabilisers or antipsychotic medication, and current treatment with benzodiazepines. Nineteen healthy age-matched male volunteers were recruited for PET scanning from hospital staff and by advertisement, and acted as the control group. Exclusion criteria for volunteers were a history of psychiatric disorder, including drug or alcohol dependence, or a history of serious physical illness, including neurological disorders.
A further 7 male patients with panic disorder who were established on SSRI treatment were recruited for PET scanning from the same clinic. They had received open-label treatment with paroxetine hydrochloride (Table 1) or sertraline (1 patient: daily dose 50 mg, duration of treatment 24 months) and remained on treatment at the time of the scan. Clinical response was confirmed by a failure to meet DSM–IV criteria for current panic disorder, and an improvement of at least 2 points on the Clinical Global Impression (CGI) scale Reference Guy12 (much improved). Thus, in addition to the 9 untreated patients with panic disorder, we were able to study a second patient group comprising of 7 recovered patients with panic disorder on SSRI treatment.
Table 1 Profile of participants
| Controls | Untreated patients with panic disorder | Panic disorder patients treated with SSRI medication | |
|---|---|---|---|
| n (all male) | 19 | 9 | 7 |
| Age, years: mean (s.d.) | 41.3 (12.5) | 37.6 (11.4) | 45.4 (12) |
| Duration of illness, months: mean (s.d.) | - | 63.6 (62.1) | 53.4 (54.0) |
| Current or previous agoraphobia, n/N | - | 6/9 | 6/7 |
| Previous depressive episode, n/N | 0/19 | 3/9 | 4/7 |
| HRSA score, mean (s.d.) | - | 21.2 (7.2) | 11.6 (7.6) |
| HRSD score, mean (s.d.) | - | 9.9 (4.3) | 8.4 (5.9) |
| BDI score, mean (s.d.) | - | 14.1 (9.3) | 12.0 (8.9) |
| Dose of paroxetine, mg: mean (s.d.) | - | - | 40 (12.6) |
| Duration of treatment, months: mean (s.d.) | - | - | 14.4 (12.6) |
All participants were screened for psychiatric disorders by clinical interview and using the Structured Clinical Interview for DSM–IV (SCID–I). Reference First, Spitzer, Gibbon and Williams13 A screening physical examination was performed and the Hamilton Rating Scale for Depression (HRSD) Reference Hamilton14 was completed. The Beck Depression Inventory (BDI) Reference Beck, Ward, Mendelson, Mock and Erbaugh15 and the Hamilton Rating Scale for Anxiety (HRSA) Reference Hamilton16 were completed 1 h before each scan. All participants gave informed written consent to the study, which was approved by local ethics committees. Permission to administer the radioactive ligand was obtained from the UK Administration of Radioactive Substances Advisory Committee. Agreement to participate in the study was obtained from the general practitioner.
Positron emission tomography scanning was carried out at the Medical Research Council (MRC) Cyclotron Unit, Hammersmith Hospital, London. Patients were accompanied from Bristol by the treating psychiatrist on the day of the scan.
Scanning protocol
Scans were performed on an ECAT 935B PET camera (Control Technology, Inc., Knoxville, Tennessee, USA). This scanner acquires 31 planes of data with an axial field of view (FOV) of 10.5 cm. The scanning method is described in full detail elsewhere. Reference Sargent, Kjaer, Bench, Rabiner, Messa, Meyer, Gunn, Grasby and Cowen17
[Carbonyl-11C]WAY-100635 was prepared at the Cyclotron Unit, MRC Clinical Sciences Centre. A 10 min transmission scan was acquired in two-dimensional mode for correction of tissue attenuation. All participants then received [11C]WAY-100635 injected intravenously over 30 s. Dynamic PET data were acquired in three-dimensional mode for 90 min after injection. The emission data were scatter corrected and reconstructed using a reprojection algorithm.
Kinetic modelling of [11C]WAY-100635
Quantitative tracer kinetic modelling was performed using a reference tissue compartmental model. Cerebellum was used as the reference tissue. The model allows estimation of binding potential and of the relative delivery of radioligand normalised to the cerebellum (R1). Parametric images of binding potential and R1 were calculated as described elsewhere. Reference Gunn, Sargent, Bench, Rabiner, Osman, Pike, Hume, Grasby and Lammertsma10
Regions of interest and statistical analysis
Three groups were defined: untreated patients with panic disorder (n=9), patients treated with SSRI medication (n=7) and controls (n=19). Regions of interest for 21 brain regions were defined using a previously validated database (for the 20 postsynaptic regions) and by hand for the raphe, and regional binding potential values were obtained by applying the regions of interest to the parametric binding-potential images and taking the mean voxel value. Reference Rabiner, Messa, Sargent, Husted-Kjaer, Montgomery, Lawrence, Bench, Gunn, Cowen and Grasby18 Statistical analysis of the regional binding potential and R1 data was performed using a single repeated-measures two-way analysis of variance (ANOVA) with Greenhouse–Geisser correction using SPSS version 10.1 for Windows, one factor being group (controls, untreated panic, treated panic) and the other factor being region. Where significant group or group × region interactions were obtained with ANOVA, post hoc t-tests were performed for binding potential values in individual regions of interest. Analysis was also performed separately for a composite of the 20 postsynaptic regions as a measure of global postsynaptic binding. All statistical tests were two-tailed and reported at P<0.05.
Results
Participants
Characteristics of the scanned participants are shown in Table 1. There were no differences between the three groups in terms of age and no differences in illness characteristics (duration of illness, history of agoraphobia or history of previous depression) between the untreated and treated patient groups. Untreated patients had higher anxiety scores than treated patients on the day of the scan but depression scores were not significantly different. The mean dose and duration of SSRI treatment are consistent with standard clinical treatment for panic disorder. Reference Baldwin, Anderson, Nutt, Bandelow, Bond, Davidson, den Boer, Fineberg, Knapp, Scott and Wittchen2
Region of interest analysis for binding potential
The ANOVA of binding potential values for the three groups found a main effect of group (F=4.15, d.f.=2,32, P=0.025) as expected, a main effect of region (F=81.925, d.f.=20,640, P<0.001) and a region × group interaction (F=2.448, d.f.=40,640, P=0.007).
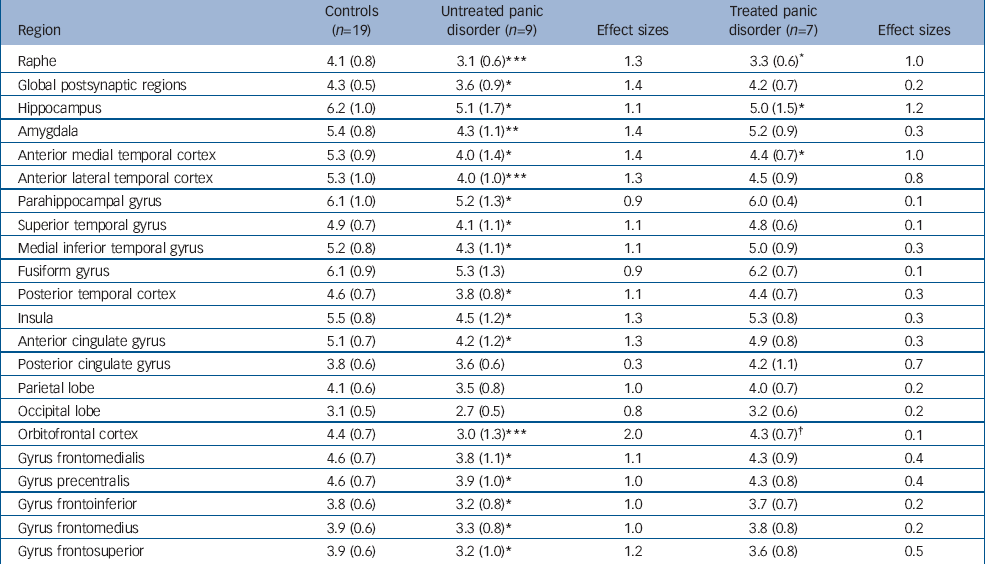
Post hoc unpaired t-tests found binding potential values to be significantly different for untreated patients v. controls in 17 of 21 regions. The areas with the most significant reductions were the raphe, amygdala, orbitofrontal cortex and anterior lateral temporal cortex (P<0.01). Mean binding potential values were lower in the group of untreated patients with panic disorder, relative to the control group, by 24% in the raphe (P<0.005; Fig. 1) and by 16% in all postsynaptic regions combined (P<0.05; Fig. 2).
In contrast, for treated patients v. controls only 3 of the 21 identified regions were significantly different on post hoc t-testing (raphe, hippocampus and anterior medial temporal cortex at P<0.05). No significant difference was seen in global postsynaptic binding for treated patients v. controls.
For treated v. untreated patients, only one region was significantly different (orbitofrontal cortex). No significant difference was seen in global postsynaptic binding for treated patients v. untreated patients.
Region of interest analysis for R1
The ANOVA of R1 values for the three groups found no effect of group (F=1.83, d.f.=2,29, P=0.179), but as expected a main effect of region (F=82.3, d.f.=20,580, P<0.001) and a group × region interaction (F=2.03, d.f.=40,580, P=0.02). Post hoc unpaired t-tests found R1 values to be significantly different between the unmedicated patients and controls in one region of interest only (orbitofrontal cortex: P<0.05).
Table 2 Mean (s.d.) [11C]WAY-100635 binding potential values for brain regions of interest
| Region | Controls (n=19) | Untreated panic disorder (n=9) | Effect sizes | Treated panic disorder (n=7) | Effect sizes |
|---|---|---|---|---|---|
| Raphe | 4.1 (0.8) | 3.1 (0.6)*** | 1.3 | 3.3 (0.6)* | 1.0 |
| Global postsynaptic regions | 4.3 (0.5) | 3.6 (0.9)* | 1.4 | 4.2 (0.7) | 0.2 |
| Hippocampus | 6.2 (1.0) | 5.1 (1.7)* | 1.1 | 5.0 (1.5)* | 1.2 |
| Amygdala | 5.4 (0.8) | 4.3 (1.1)** | 1.4 | 5.2 (0.9) | 0.3 |
| Anterior medial temporal cortex | 5.3 (0.9) | 4.0 (1.4)* | 1.4 | 4.4 (0.7)* | 1.0 |
| Anterior lateral temporal cortex | 5.3 (1.0) | 4.0 (1.0)*** | 1.3 | 4.5 (0.9) | 0.8 |
| Parahippocampal gyrus | 6.1 (1.0) | 5.2 (1.3)* | 0.9 | 6.0 (0.4) | 0.1 |
| Superior temporal gyrus | 4.9 (0.7) | 4.1 (1.1)* | 1.1 | 4.8 (0.6) | 0.1 |
| Medial inferior temporal gyrus | 5.2 (0.8) | 4.3 (1.1)* | 1.1 | 5.0 (0.9) | 0.3 |
| Fusiform gyrus | 6.1 (0.9) | 5.3 (1.3) | 0.9 | 6.2 (0.7) | 0.1 |
| Posterior temporal cortex | 4.6 (0.7) | 3.8 (0.8)* | 1.1 | 4.4 (0.7) | 0.3 |
| Insula | 5.5 (0.8) | 4.5 (1.2)* | 1.3 | 5.3 (0.8) | 0.3 |
| Anterior cingulate gyrus | 5.1 (0.7) | 4.2 (1.2)* | 1.3 | 4.9 (0.8) | 0.3 |
| Posterior cingulate gyrus | 3.8 (0.6) | 3.6 (0.6) | 0.3 | 4.2 (1.1) | 0.7 |
| Parietal lobe | 4.1 (0.6) | 3.5 (0.8) | 1.0 | 4.0 (0.7) | 0.2 |
| Occipital lobe | 3.1 (0.5) | 2.7 (0.5) | 0.8 | 3.2 (0.6) | 0.2 |
| Orbitofrontal cortex | 4.4 (0.7) | 3.0 (1.3)*** | 2.0 | 4.3 (0.7)† | 0.1 |
| Gyrus frontomedialis | 4.6 (0.7) | 3.8 (1.1)* | 1.1 | 4.3 (0.9) | 0.4 |
| Gyrus precentralis | 4.6 (0.7) | 3.9 (1.0)* | 1.0 | 4.3 (0.8) | 0.4 |
| Gyrus frontoinferior | 3.8 (0.6) | 3.2 (0.8)* | 1.0 | 3.7 (0.7) | 0.2 |
| Gyrus frontomedius | 3.9 (0.6) | 3.3 (0.8)* | 1.0 | 3.8 (0.8) | 0.2 |
| Gyrus frontosuperior | 3.9 (0.6) | 3.2 (1.0)* | 1.2 | 3.6 (0.8) | 0.5 |
Correlations with clinical variables
There were no significant correlations of binding potentials against a range of clinical variables, including age, anxiety and depression scores, and treatment dose (data not shown).
Discussion
This PET study measured 5-HT1A receptor density in both untreated and recovered patients with panic disorder. It demonstrates major differences in presynaptic raphe 5-HT1A receptor density between controls and patients in both the untreated and recovered states. This study confirms the findings of Neumeister et al, Reference Neumeister, Bain, Nugent, Carson, Bonne, Luckenbaugh, Eckelman, Herscovitch, Charney and Drevets6 but extends their study to look at more regions of interest and addresses the effect of SSRI treatment in panic disorder. In untreated patients, lower binding was also seen in most postsynaptic regions, most significantly in the ‘anxiety circuit’ of the orbitofrontal cortex, temporal lobe and amygdala. Reference Rosen and Schulkin19,Reference Cannistraro and Rauch20 In treated patients, lower binding was also seen in the raphe, but was much less widespread in postsynaptic regions.
Reduced 5HT1A binding in panic disorder
Recent evidence has specifically implicated 5-HT1A receptors in the control of anxiety and suggested that reduced function of these receptors may contribute to the development of pathological anxiety.
-
(a) It has been shown in animal models that the anxiolytic activity of SSRIs is dependent on increased binding of serotonin to postsynaptic 5-HT1A receptors. Reference Blier and Ward21
-
(b) Some patients with anxiety disorders who have recovered on SSRI treatment can be made symptomatic by acutely depleting tryptophan, the amino acid precursor of serotonin, suggesting a persisting abnormality of postsynaptic serotonin receptors. Reference Bell, Forshall, Adrover, Nash, Hood, Argyropoulos, Rich and Nutt22,Reference Argyropoulos, Hood, Adrover, Bell, Rich, Nash, Rich, Witchel and Nutt23
-
(c) A specific hypofunction of both presynaptic and postsynaptic 5-HT1A receptors in patients with panic disorder has been indicated by challenge testing with the 5-HT1A agonist ipsapirone. Patients showed reduced hypothermic and neuroendocrine effects compared with controls. Reference Lesch, Wiesmann, Hoh, Müller, Disselkamp-Tietze, Osterheider and Schulte4
-
(d) Mice bred with genetic knockout of the 5-HT1A receptor have repeatedly been shown to demonstrate elevated levels of anxiety, and a technique that allowed the ‘switching on’ of the 5-HT1A gene at various stages of development showed that receptors in the forebrain (corresponding to the human cortex and limbic system) had a critical role in the development of anxiety behaviour. Reference Gross, Zhuang, Stark, Ramboz, Oosting, Kirby, Santarelli, Beck and Hen24

Fig. 1 Raphe binding potential values.
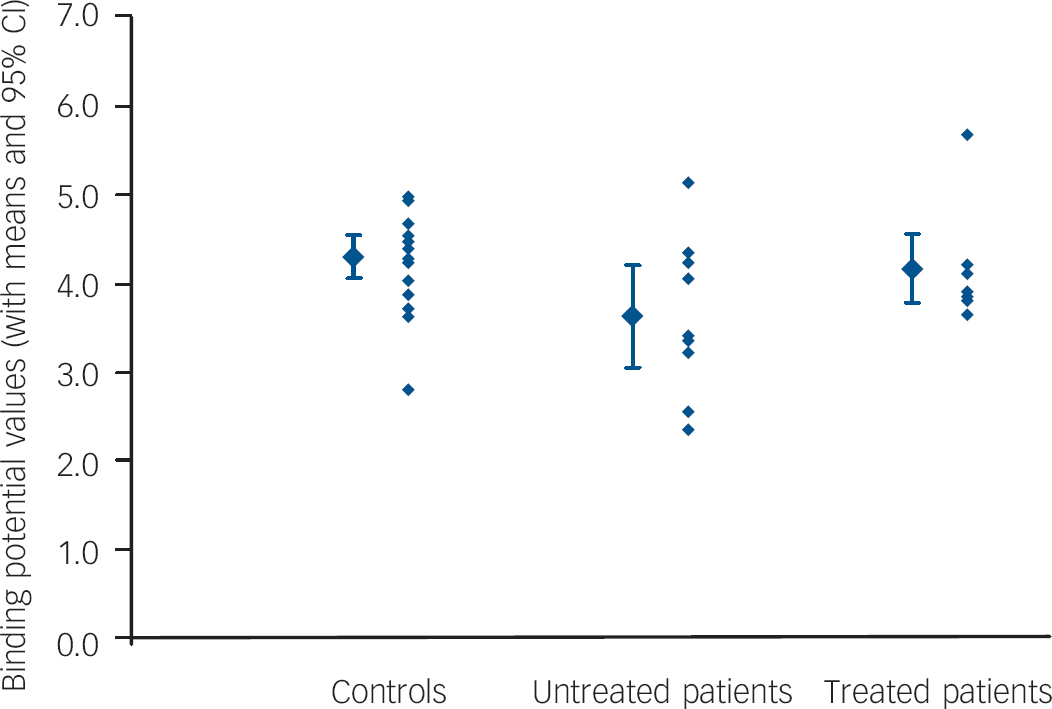
Fig. 2 Global postsynaptic binding potential values.
Our finding of a reduction in postsynaptic 5-HT1A receptor binding in patients with untreated panic disorder is consistent with the reported subsensitivity to pharmacological challenge and with the model of anxiety postulated from studies of 5-HT1A knockout mice. Furthermore, functional brain imaging studies have revealed that circuits involving the amygdala, temporal cortex and orbitofrontal cortex mediate the perception of threat and the experience of anxiety in humans. Reference Gorman, Kent, Sullivan and Coplan25,Reference Malizia, Nutt and Ballenger26 The prominent localisation of reduced 5-HT1A binding in these regions in our study suggests an aetiological role in panic disorder.
Evidence for altered function of gamma-aminobutyric acid and serotonergic neurotransmission in patients with panic disorder is growing, Reference Neumeister, Bain, Nugent, Carson, Bonne, Luckenbaugh, Eckelman, Herscovitch, Charney and Drevets6,Reference Malizia, Cunningham, Bell, Liddle, Jones and Nutt27,Reference Maron, Kuikka, Shlik, Vasar, Vanninen and Tiihonen28 although a primary aetiological role has yet to be established. Although panic may originate in brainstem structures, Reference Bailey, Argyropoulos, Lightman and Nutt29 recent studies have identified an inhibitory role for specific cortical regions, including the orbitofrontal cortex. Reference Kent, Coplan, Mawlawi, Martinez, Browne, Slifstein, Martinez, Abi-Dargham, Laruelle and Gorman30 A deficit of 5-HT1A receptors, as found in our study, may lead to a relative decrease in the ability of the brain to control panic anxiety.
Interesting comparisons can be drawn between our findings and those from PET studies of 5-HT1A binding in patients with depression. Reference Guy12,Reference Drevets, Frank, Price, Kupfer, Greer and Mathis31 These studies also found lower presynaptic and global postsynaptic 5-HT1A binding in patients compared with controls. This similarity is not unexpected as anxiety and depressive disorders have a clinical overlap, both being brought on by stress-related factors and both responding to SSRI medication. Although there were some differences between the disorders in the postsynaptic regions showing the greatest alterations in binding (orbitofrontal and anterior temporal cortices in panic, anterior cingulate in depression), there is a consistent finding of a general reduction in postsynaptic receptors.
Our findings in untreated patients also correspond with those of the previously reported study Reference Neumeister, Bain, Nugent, Carson, Bonne, Luckenbaugh, Eckelman, Herscovitch, Charney and Drevets6 of 5-HT1A receptor binding in patients with untreated panic disorder using [18F]-FCWAY, in which where a reduction in binding was found in the anterior cingulate, posterior cingulate and raphe regions. Using a different radiotracer and quantification method, 5-HT1A receptor binding was reduced in two of these three regions in our study. We also found more widespread reductions of 5-HT1A binding; however, further comparison with the Neumeister et al study is restricted as only six regions of interest were reported in total and receptor quantification with this radioligand is problematic in cortical areas owing to the retention of the fluoride signal in adjacent skull bones. Reference Neumeister, Bain, Nugent, Carson, Bonne, Luckenbaugh, Eckelman, Herscovitch, Charney and Drevets6
This research finding of reduced 5-HT1A binding has therefore now been replicated in both depression and panic disorder, and has important implications for our understanding of the aetiology of these disorders. Animal studies have shown that 5-HT1A binding can be altered by both genetic and environmental factors, including physical and biological stressors, Reference McKittrick, Blanchard, Blanchard, McEwen and Sakai32 and that altered 5-HT1A binding is associated with other abnormalities of serotonin function. Reference Ase, Reader, Hen, Riad and Descarries33 As neuroimaging techniques allow the replication of these findings in human studies, it becomes possible to create a hypothesis of reduced serotonergic neurotransmission via 5-HT1A receptors as a biological pathway that can bring together such diverse factors as genetic predisposition, early life trauma, physical illness and stressful life events in the aetiology of anxiety and affective disorders.
Effect of SSRI treatment on 5-HT1A binding
There was no difference in global postsynaptic binding between recovered patients on SSRI medication and controls, and binding was significantly lower in only two of the forebrain regions and the raphe compared with controls. In animal studies, Welner et al Reference Welner, De Montigny, Desroches, Desjardins and Suranyi-Cadotte34 and Li et al Reference Li, Brownfield, Levy, Battaglia, Cabrera and Van de Kar35 found reduced 5-HT1A binding in the midbrain following chronic fluoxetine administration. However, other animal studies have found no effects of SSRIs on [3H]8-OH-DPAT binding in any brain region. Reference Hensler, Kovachich and Frazer36–Reference Li, Muma, Battaglia and Van de Kar39 The smaller differences from controls in postsynaptic binding in the SSRI-treated group compared with the untreated group may be due to a lack of power to detect a difference between SSRI-treated patients (n=7) and controls. The mean binding potential values for this group were intermediate between those of the unmedicated patients with panic disorder and the healthy controls. Larger numbers of participants may have clarified whether the binding potential values for these patients does indeed lie between the untreated patients and controls.
An alternative explanation is that SSRI treatment of panic disorder may be associated with ‘normalisation’ of 5-HT1A binding. This is probably not a direct effect of SSRI treatment, as treatment was not associated with altered binding in our study of patients with depression, although SSRI treatment in this study was of a shorter duration and at a lower dose. Reference Sargent, Kjaer, Bench, Rabiner, Messa, Meyer, Gunn, Grasby and Cowen17
Our finding in untreated patients with panic disorder of reduced raphe and postsynaptic 5-HT1A binding may be related to the chronic stress associated with, and possibly causal of, anxiety. Stress has been shown in animals to reduce 5-HT1A receptor density and function and, via overstimulation of glucocorticoid receptors, also leads to a loss of dendritic spines in neurons. Reference Duman40 Interestingly, although previous studies had not found a correlation between cortisol levels and 5-HT1A expression in patients with depression, Reference Drevets, Frank, Price, Kupfer, Greer and Mathis31 a strongly negative correlation was recently reported between hippocampal 5-HT1A expression and cortisol levels in social anxiety disorder. Reference Lanzenberger, Wadsak, Spindelegger, Mitterhauser, Klein, Mien, Attarbaschi, Mossaheb, Sacher, Holik, Geiss-Granadia, Kletter and Kasper41 A ‘normalisation’ of postsynaptic 5-HT1A binding with treatment could therefore be explained by a reduction in stress associated with recovery.
Anxiety and depressive disorders are frequently coexisting conditions and have many clinical similarities, including sleep disturbance, fatigue, reduced concentration and irritability. Both respond to treatment with serotonergic antidepressants, which suggests a commonality in underlying pathophysiology. Our imaging data suggest that both disorders share at least one neurochemical pathophysiological feature, namely reduced 5-HT1A binding. Our findings are also consistent with preclinical and clinical studies of anxiety, including the findings that reduced 5-HT1A receptor binding is causal of anxiety-like behaviours in 5-HT1A knockout mice, Reference Gross, Zhuang, Stark, Ramboz, Oosting, Kirby, Santarelli, Beck and Hen24 and that patients with panic disorder have impaired 5-HT1A receptor function in neuroendocrine challenge tests. Reference Lesch, Wiesmann, Hoh, Müller, Disselkamp-Tietze, Osterheider and Schulte4
The numbers of individuals participating in this study are low, but not untypical for PET studies of this kind. None the less, significant reductions in binding potential values were still found in 17 out of 21 brain regions in unmedicated patients with panic disorder compared with healthy controls. On the other hand, reductions in binding potential were only found in 3 out of the 21 regions in the SSRI-treated patients. Less confidence can be placed in the latter finding.
It would be of considerable interest to see whether similar changes in binding potential occur in female patients with panic disorder as the prevalence of this condition is about twice as high in women as in men.
Positron emission tomography is a highly sensitive procedure and it is important to consider sources of bias in the methodology. Our study did not include estimations of brain volume, partly because the magnetic resonance imaging scanner encloses the patient to an extent that most patients with untreated panic disorder would find too frightening. It seems reasonable to proceed without these measurements as there is no evidence from other studies that panic disorder is associated with reduced brain volume. Reference Blier and Ward21 Although the PET scanner is less oppressive than magnetic resonance imaging, our untreated patients were significantly more anxious than controls prior to the scan and subjectively found the scanning procedure more anxiogenic than those in the treated and control groups. This could in theory have contributed to the differences seen, although our evidence suggests that acute physiological alterations of serotonin in humans do not significantly affect cortical WAY-100635 binding, Reference Rabiner, Messa, Sargent, Husted-Kjaer, Montgomery, Lawrence, Bench, Gunn, Cowen and Grasby18 and evidence from animal studies suggests that acute stress does not lead to a significant reduction in 5-HT1A binding. Reference Steciuk, Kram, Kramer and Petty42 Variations in cerebral blood flow of the magnitude expected between anxious patients and controls are unlikely to affect the [11C]-WAY100635 signal. Reference Gunn, Sargent, Bench, Rabiner, Osman, Pike, Hume, Grasby and Lammertsma10 Finally, it has previously been shown that 5-HT1A binding reduces with age, and although the age differences between groups were not significant, our findings of reduced binding were in the group with the younger mean age.
In part because of the cost of PET studies and the logistical problems involved in scanning individuals, participant numbers in PET studies tend to be low and replication of results is therefore acknowledged to be very important in the field. Thus, our study is important as it confirms the findings of Neumeister et al, Reference Neumeister, Bain, Nugent, Carson, Bonne, Luckenbaugh, Eckelman, Herscovitch, Charney and Drevets6 extends these to look at more regions of interest and examines the effect of SSRI treatment.
Clinical implications
Positron emission tomography demonstrates that patients with untreated panic disorder have lower 5-HT1A receptor binding compared with controls. This finding is consistent with predictions from animal models of anxiety. This reduction in binding is especially significant in the orbitofrontal cortex, temporal lobes and amygdala – these regions are known to control anxiety responses and reduced 5-HT1A binding in these areas may be a biological factor that confers vulnerability to anxiety disorders.
Our imaging data shows that reduced 5-HT1A binding is a pathophysiological feature of both panic disorder and depression, and may represent a common neurobiological pathway in the development of these stress-related disorders.
Limitations
The observations of the effects of SSRI treatment would have been more powerful if patients had been scanned before and after treatment. In addition, owing to the use of ionising radiation, only male patients were included in the study.
Acknowledgements
This study was initiated by a grant from SmithKline Beecham, and the scanning costs were covered by a program grant to P.M.G. from the MRC. These data were presented in poster form at the British Association for Psychopharmacology Summer Meeting (20–23 July 2003, Cambridge, UK) and the annual European College of Neuropsychopharmacology Congress (9–13 October 2004, Stockholm, Sweden).







eLetters
No eLetters have been published for this article.