Antipsychotic agents are the psychotropic drugs most frequently prescribed in individuals with intellectual disability (referred to as learning disability by UK health services). Reference Branford1,Reference Holden and Gitlesen2 Usual indications are comorbid functional psychosis or, more frequently, challenging behaviour. About 25–30% of all individuals with intellectual disability using services regularly receive antipsychotics Reference Branford1,Reference Holden and Gitlesen2 rising to 48% of those with challenging behaviour. Reference Kiernan, Reeves and Alborz3 Recent concerns have focused on the inadequate definition of challenging behaviour and the relative absence of controlled evidence for efficacy. Reference Brylewski and Duggan4–Reference Tyrer, Oliver-Africano, Ahmed, Bouras, Cooray and Deb6 There is also an important risk of harm because antipsychotics can induce hyperglycaemia, hyperlipidaemia, obesity and hyperprolactinaemia. Reference Henderson and Doraiswamy7 People with intellectual disability are less likely to identify relevant medical symptoms accurately and are less amenable to routine blood testing. Hence such adverse effects carry a particular and enhanced threat to their health.
Although the metabolic and endocrine complications of antipsychotic treatment have been extensively investigated for people with severe mental illness, objective data in the intellectual disability population is limited to one study of 41 participants on metabolic side-effects, Reference McKee, Bodfish, Mahorney, Heeth and Ball8 one study on hyperprolactinaemia Reference Hellings, Zarcone, Valdovinos, Reese, Gaughan and Schroeder9 and two other studies, which reported both weight and prolactin levels. Reference Turgay, Binder, Snyder and Fisman10,Reference Handen and Hardan11 This lack of data is confirmed, within a clinical setting, by a recent audit showing that only 3% of individuals with intellectual disability starting an atypical antipsychotic and 1% during follow-up had undergone monitoring of blood glucose, lipids and body weight. Reference Devapriam, Anand, Raju and Bhaumik12 In this paper we describe the first study on the metabolic and endocrine profiles of a representative intellectual disability cohort compared with a general population control group, and between participants with intellectual disability on antipsychotics and those who were antipsychotic naive.
Method
The Oxford Learning Disabilities Study was a cross-sectional observational study, approved by the regional ethics committee: all individuals aged 18–70 years on regular antipsychotic treatment or antipsychotic naive under the care of the Oxford-based psychiatrists of the Ridgeway Partnership (Oxfordshire Learning Disability National Health Service (NHS) Trust) were eligible. The Oxford Biobank cohort Reference Tan, Neville, Liverani, Humphreys, Currie and Dennis13 (OBB, 1396 participants) served as a control group. This is a randomly selected, locally derived general population sample from Oxfordshire with no known medical history, hence antipsychotic naive, on which anonymised biochemical, anthropometric, lifestyle and genetic data have been collected. The unselected general population sample of over 9000 participants of the Health Survey for England (HSE) 2006 14 was used as a control for data not available from the OBB (prevalence of diabetes, HbA1c).
Capacity was assessed as outlined in the Mental Capacity Act 2005, Code of Practice. 15 Written informed consent was given by individuals who were capacitous. Those assenting (but formally incapacitous) were only entered into the study after unanimous agreement was obtained that inclusion in the study was in the individual’s best interest from key people in the person with intellectual disability’s circle of care (which included one or more carer and/or relative). Participants were visited at home by a study nurse, who took consent, completed a questionnaire on family, medical and drug history and lifestyle variables (diet, alcohol, smoking, exercise levels), and measured height and weight for body mass index (BMI), waist circumference and skinfold thickness for body fat distribution and blood pressure. A fasting blood sample was taken for glucose, glycated haemoglobin (HbA1c), lipids, insulin, prolactin, oestradiol, testosterone, gonadotrophins, free thyroxine (T4), thyroid-stimulating hormone (TSH), liver function tests, creatinine, and a full blood count. Glucose was measured by hexokinase, HbA1c by high-performance liquid chromatography (HPLC), triglycerides by standard glycerol-phosphate method, cholesterol by cholesterol esterase with direct measurement of high-density lipoprotein (HDL) cholesterol and low-density lipoprotein (LDL) cholesterol calculated by the Friedewald’s formula. Reference Friedewald, Levy and Fredrickson16 Hormones were measured by immunoassay on a multianalyser (ADVIA Centaur, Bayer Diagnostics). The insulin resistance index (HOMA-IR) was calculated according to the Homeostasis Model Assessment computer model (http://www.dtu.ox.ac.uk/homacalculator/index.php).
Diagnoses were made on review of case notes and based on DSM-IV criteria. 17 The daily chlorpromazine equivalent dose was calculated for comparison with published data for phenothiazines, thioxanthenes, haloperidol, sulpiride, risperidone, olanzapine, quetiapine and aripiprazole. Reference Woods18,Reference Taylor, Paton and Kervin19
Statistical analyses
Means (standard deviations) or medians (ranges) of continuous variables were compared by independent samples t-tests or Mann–Whitney’s test and rates by Fisher’s exact test or chi-squared. A logistic regression model adjusted for age, gender, BMI, waist and exercise levels was used to compare the risk of a raised fasting plasma glucose (FPG ≥5.6 mmol/l) between the antipsychotic-treated and the antipsychotic-naive group and between the participants with intellectual disability (intellectual disability group) and the OBB participants (OBB control group). The study had 80% power for a difference of 0.5 mmol/l in FPG and 0.5% in HbA1c. Analyses were carried out on SPSS (v.14) and STATA (v.10) for Windows.
The metabolic and anthropometric data from participants with diabetes on hypoglycaemic therapy were not included in the comparisons of the antipsychotic-treated v. antipsychotic-naive groups. All participants with diabetes were included in the prolactin-related analyses.
Results
Of 349 potentially eligible individuals identified, 213 (61.0%) participated in the study. A total of 6% were excluded for psychiatric reasons, 1% for medical ones and 31% either refused or the carers refused. Eleven people were subsequently excluded because miscellaneous conditions confounded interpretation of the results (Fig. 1).
We report data on 202 participants with intellectual disability (mean age 42.1 (s.d. = 12.8), 52% men, 48% women), 62% of whom were capacitous. Of these 94% were of European ethnicity, 14% were smokers and 22% reported some alcohol intake. Almost all were living in sheltered accommodation. A total of 83% agreed to a blood sample and 93% to physical measurements. Skinfold measurements were resisted by many participants and the data were of insufficient quality for analysis.
A total of 48% of the participants had mild, 30% moderate, 17% severe and 5% profound DSM-IV mental retardation. In 76 (38%) of the participants the intellectual disability was associated with a known condition, most frequently autistic disorder and Down syndrome (27% and 22% of those with a known diagnosis). The rest had a wide range of disorders, including chromosomal abnormalities; 36% had epilepsy. Participants with mild intellectual disability were overrepresented as some carers were reluctant to enter more severely ill people in the study because of a fear they might become too distressed.
Psychiatric diagnoses by degree of intellectual disability are shown in Table 1; 64% of the total study participants had a concomitant psychiatric disorder, most frequently depression (18%), schizophrenia and other psychotic disorders (17%), autistic disorder (10%), bipolar mood disorder (7%), anxiety (6%), attention-deficit hyperactivity disorder (1%) and other conditions.
Of the total sample 138 (68%) were on antipsychotics and 64 (32%) were antipsychotic naive. Eighty participants (58%) in the antipsychotic-treated group had challenging behaviour, which was the commonest reason for the prescription. There were more men (59%) in the antipsychotic-treated and more women (61%) in the antipsychotic-naive group. In the whole study group, 27% of the participants had a diagnosis of challenging behaviour only, in the absence of psychiatric disorders.
Of the 202 participants, 97% were on one or more psychotropic agents, with a mean of 2.0 drugs per participant (1.4 excluding anti-epileptics). In total 68% were on antipsychotics, 42% on antidepressants, 39% on anti-epileptics, 25% on benzodiazepines (generally ‘as required’ rather than regularly), 2% on non-benzodiazepine hypnotics and 1% on lithium.
Of the 138 antipsychotic-treated participants, 48% were on risperidone, 18% on olanzapine, 10% on thioxanthenes, 9% on chlorpromazine or other first-generation phenothiazines, 9% on quetiapine, 7% on amisulpride or sulpiride, 4% on haloperidol, 2% on aripiprazole and 1% on clozapine. The
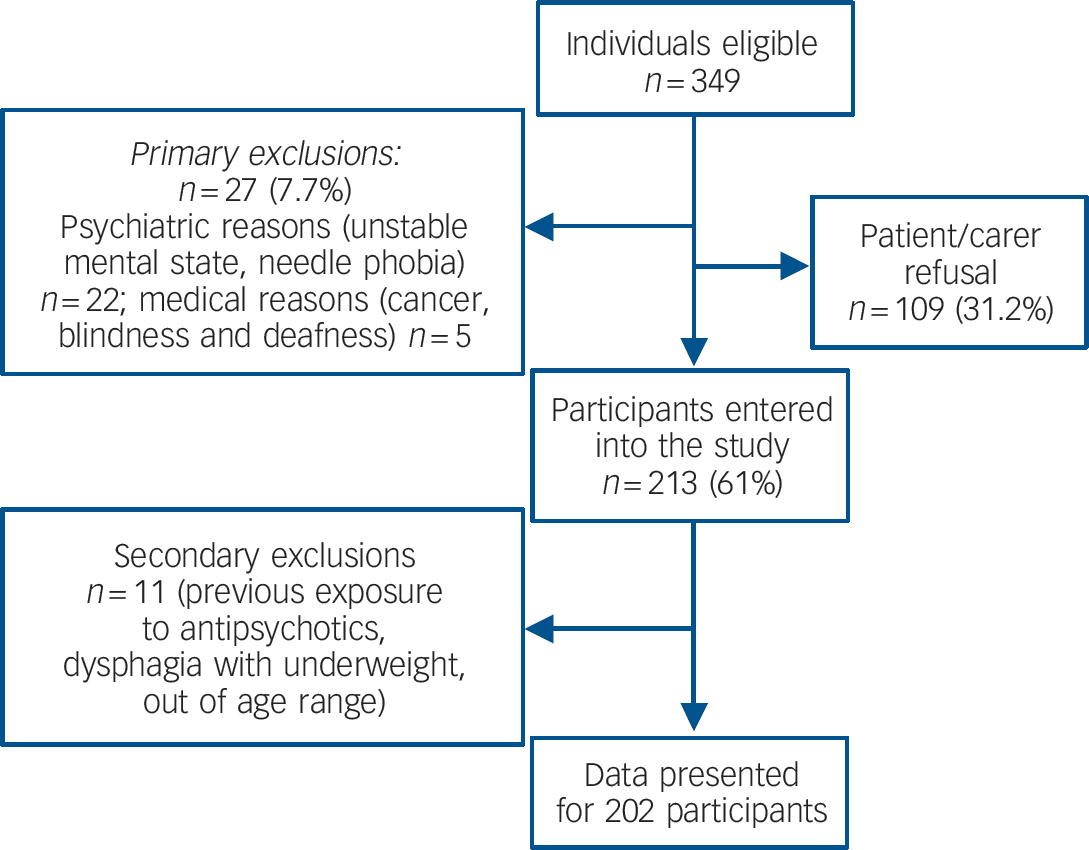
Fig. 1 Flow chart of participants.
Table 1 Psychiatric diagnoses by degree of intellectual disability

| % | |||
|---|---|---|---|
| Mild intellectual disability (n = 97) | Moderate intellectual disability (n = 61) | Severe or profound intellectual disability (n = 44) | |
| Schizophrenia/schizoaffective/psychotic disorders | 28 | 7 | 4 |
| Depression | 24 | 16 | 7 |
| Bipolar mood disorder | 7 | 8 | 2 |
| Anxiety disorders | 7 | 2 | 3 |
| Autistic disorder | 4 | 15 | 18 |
| Dementia | 7 | – | – |
| Obsessive–compulsive disorder | 3 | – | – |
| Attention-deficit hyperactivity disorder | 2 | 1 | – |
| Panic disorder | 2 | – | – |
| Personality disorders | 2 | – | – |
| Asperger syndrome | 1 | – | – |
Table 2 Type and doses of antipsychotics used by intellectual disability group
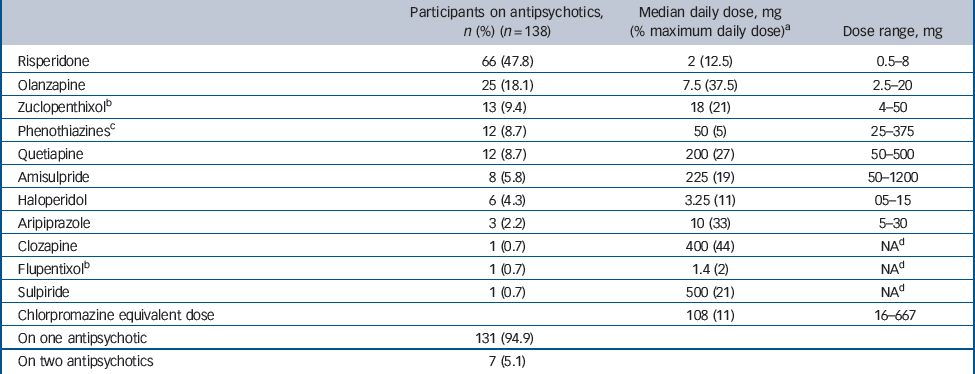
| Participants on antipsychotics, n (%) (n = 138) | Median daily dose, mg (% maximum daily dose)a | Dose range, mg | |
|---|---|---|---|
| Risperidone | 66 (47.8) | 2 (12.5) | 0.5–8 |
| Olanzapine | 25 (18.1) | 7.5 (37.5) | 2.5–20 |
| Zuclopenthixolb | 13 (9.4) | 18 (21) | 4–50 |
| Phenothiazinesc | 12 (8.7) | 50 (5) | 25–375 |
| Quetiapine | 12 (8.7) | 200 (27) | 50–500 |
| Amisulpride | 8 (5.8) | 225 (19) | 50–1200 |
| Haloperidol | 6 (4.3) | 3.25 (11) | 05–15 |
| Aripiprazole | 3 (2.2) | 10 (33) | 5–30 |
| Clozapine | 1 (0.7) | 400 (44) | NAd |
| Flupentixolb | 1 (0.7) | 1.4 (2) | NAd |
| Sulpiride | 1 (0.7) | 500 (21) | NAd |
| Chlorpromazine equivalent dose | 108 (11) | 16–667 | |
| On one antipsychotic | 131 (94.9) | ||
| On two antipsychotics | 7 (5.1) |
a Maximum daily dose as recommended for psychoses in the British National Formulary 20 (risperidone: 16 mg, olanzapine 20 mg, zuclopenthixol 86 mg, chlorpromazine 1 g, quetiapine 750 mg, amisulpride 1200 mg, haloperidol 30 mg, aripiprazole 30 mg, clozapine 900 mg, flupentixol 57 mg, sulpiride 2400 mg).
b Two- or four-weekly dose divided by number of days.
c Chlorpromazine, trifluoperazine: dose expressed as chlorpromazine equivalent.
d NA, not applicable: only one participant on each of these agents.
majority, 95%, were on one antipsychotic alone, 5% on two. Median duration of antipsychotic use was 8 years (range 0.5–47), the median daily chlorpromazine equivalent dose was 108 mg (range 16–667, Table 2).
Anthropometric and metabolic indices
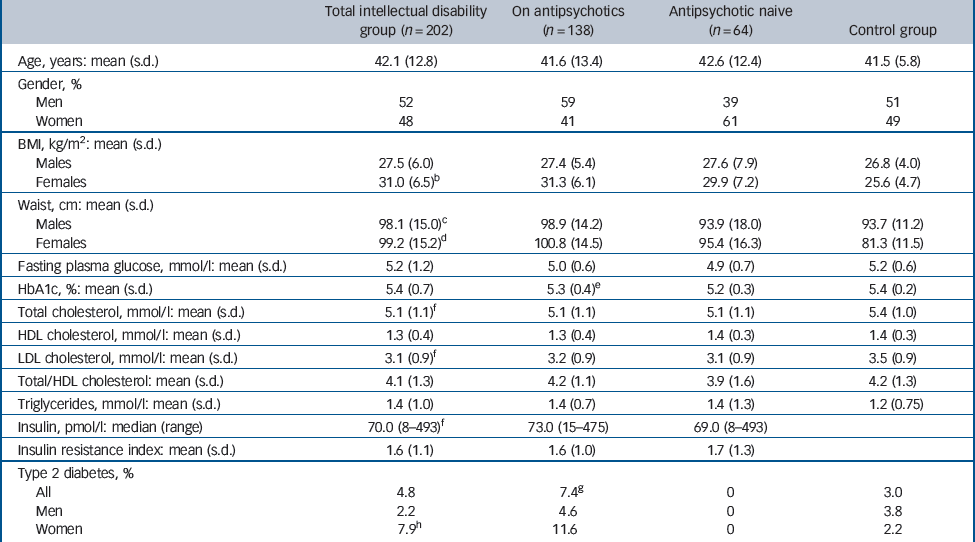
In the comparison between the intellectual disability and the OBB control group, the mean BMI was significantly higher in women in the intellectual disability group (31.0 (s.d. = 6.5) v. 25.6 (s.d. = 4.7), P<0001), but it was similar between the men (27.5 (s.d. = 6.0) v. 26.8 (s.d. = 4.0), P = 0.30, Table 3). Waist circumference was higher in the intellectual disability group (98.1 (s.d. = 15.0) v. 93.7 (s.d. = 11.2) cm, P<0.01 for men and 99.2 (s.d. = 15.2) v. 81.3 (s.d. = 11.5) cm, P<0.001 for women). Prevalence of overweight and obesity was higher in the women in the intellectual disability group than those in the OBB control group (81.2% v. 45.9%, P<0.001), whereas there was no statistically significant difference between the men (64.0% v. 64.8%, P = 0.878). The 81.2% prevalence of overweight and obesity in the intellectual disability group women was also higher than in the HSE control group women of a similar age, where it was 54%, whereas the 64% prevalence for the intellectual disability group men was similar to the 68% rate reported in the HSE control group men (P = 0.385).
Although both the men and the women in the intellectual disability group were more sedentary than their general population counterparts (P<0.001 for both), as assessed by a standardised questionnaire exploring weekly routine physical activity, the women in the intellectual disability group were also more sedentary than the men in this group (P<0.001). This could partly explain the high BMI of the women, particularly as there was no difference in the prevalence of syndromes associated with obesity, such as Down syndrome, between women and men.
When comparing the antipsychotic-treated and the antipsychotic-naive groups, BMI did not differ between the groups either in men (27.4 (s.d. = 5.4) v. 27.6 (s.d. = 7.9), P = 0.895) or in women (31.3 (s.d. = 6.1) v. 29.9 (s.d. = 7.2), P = 0.374). Waist circumference was also similar between the two groups (98.9 (s.d. = 14.2) v. 93.9 (s.d. = 18.0) cm, P = 0.266 for the men, and 100.8 (s.d. = 14.5) v. 95.4 (s.d. = 16.3) cm, P = 0.395 for the women), as was the prevalence of overweight and obesity (84% v. 79%, P = 0.523 in women and 65% v. 59%, P = 0.592 in men).
Table 3 Clinical and biochemical characteristics of intellectual disability and control groupsa
| Total intellectual disability group (n = 202) | On antipsychotics (n = 138) | Antipsychotic naive (n = 64) | Control group | |
|---|---|---|---|---|
| Age, years: mean (s.d.) | 42.1 (12.8) | 41.6 (13.4) | 42.6 (12.4) | 41.5 (5.8) |
| Gender, % | ||||
| Men | 52 | 59 | 39 | 51 |
| Women | 48 | 41 | 61 | 49 |
| BMI, kg/m2: mean (s.d.) | ||||
| Males | 27.5 (6.0) | 27.4 (5.4) | 27.6 (7.9) | 26.8 (4.0) |
| Females | 31.0 (6.5)b | 31.3 (6.1) | 29.9 (7.2) | 25.6 (4.7) |
| Waist, cm: mean (s.d.) | ||||
| Males | 98.1 (15.0)c | 98.9 (14.2) | 93.9 (18.0) | 93.7 (11.2) |
| Females | 99.2 (15.2)d | 100.8 (14.5) | 95.4 (16.3) | 81.3 (11.5) |
| Fasting plasma glucose, mmol/l: mean (s.d.) | 5.2 (1.2) | 5.0 (0.6) | 4.9 (0.7) | 5.2 (0.6) |
| HbA1c, %: mean (s.d.) | 5.4 (0.7) | 5.3 (0.4)e | 5.2 (0.3) | 5.4 (0.2) |
| Total cholesterol, mmol/l: mean (s.d.) | 5.1 (1.1)f | 5.1 (1.1) | 5.1 (1.1) | 5.4 (1.0) |
| HDL cholesterol, mmol/l: mean (s.d.) | 1.3 (0.4) | 1.3 (0.4) | 1.4 (0.3) | 1.4 (0.3) |
| LDL cholesterol, mmol/l: mean (s.d.) | 3.1 (0.9)f | 3.2 (0.9) | 3.1 (0.9) | 3.5 (0.9) |
| Total/HDL cholesterol: mean (s.d.) | 4.1 (1.3) | 4.2 (1.1) | 3.9 (1.6) | 4.2 (1.3) |
| Triglycerides, mmol/l: mean (s.d.) | 1.4 (1.0) | 1.4 (0.7) | 1.4 (1.3) | 1.2 (0.75) |
| Insulin, pmol/l: median (range) | 70.0 (8–493)f | 73.0 (15–475) | 69.0 (8–493) | |
| Insulin resistance index: mean (s.d.) | 1.6 (1.1) | 1.6 (1.0) | 1.7 (1.3) | |
| Type 2 diabetes, % | ||||
| All | 4.8 | 7.4g | 0 | 3.0 |
| Men | 2.2 | 4.6 | 0 | 3.8 |
| Women | 7.9h | 11.6 | 0 | 2.2 |
BMI, body mass index; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
a Comparisons are between individuals in the antipsychotic-treated v. antipsychotic-naive intellectual disability group and total intellectual disability group v. general population group. Control data are from the Oxford Biobank (OBB) cohort (n = 1396) except for HbA1c and type 2 diabetes prevalence data, which have been obtained from the Health Survey for England (HSE) age- and gender-matched population (n = 9352).
b P < 0.001 (intellectual disability v. OBB women).
c P < 0.01 (intellectual disability v. OBB men).
d P < 0.001 (intellectual disability v. OBB women).
e P < 0.01 (antipsychotic-treated v. antipsychotic-naive participants).
f P < 0.001 (intellectual disability v. OBB participants).
g P = 0.052 (antipsychotic-treated v. antipsychotic-naive participants).
h P = 0.0106 (intellectual disability v. HSE women).
The intellectual disability group had a significantly lower total cholesterol (5.1 (s.d. = 1.1) v. 5.4 (s.d. = 1.0), P<0.001) and LDL cholesterol (3.1 (s.d. = 09) v. 3.5 (s.d. = 0.9), P<0.001) compared with those in the OBB control group, while FPG, triglycerides and HDL cholesterol were similar. A logistic regression model adjusted for age, gender, BMI, waist and exercise levels, which excluded the participants who had diabetes at entry to the study, showed our intellectual disability group to be less likely to have an FPG level in the hyperglycaemic range (>5.5 mmol/l) than those in the OBB control group (adjusted odds ratio (OR) = 0.44, P<0.05, 95% CI 0.25–0.75). The mean HbA1c of 5.4% (s.d. = 0.7) (International Federation of Clinical Chemistry (IFCC) 36 mmol/mol (s.d. = 7)) found in the whole study group was virtually identical to the HbA1c of 5.4% (s.d. = 0.2%, IFCC 36 mmol/mol (s.d. = 2)) of the general population sample of similar age in the HSE control group.
In the comparison between the antipsychotic-treated and the antipsychotic-naive group mean FPG levels were not different (5.0 (s.d. = 0.6) v. 4.9 (s.d. = 0.7) mmol/l, P = 0.559), nor were total, HDL cholesterol, LDL cholesterol, total/HDL cholesterol ratio, triglycerides, insulin or the insulin resistance index. There was no difference in the risk of a raised (>5.5 mmol/l) FPG between the two groups. The HbA1c levels were significantly higher in the antipsychotic-treated group but the absolute difference was too small to be considered clinically significant (5.3% (s.d. = 0.4) v. 5.2% (s.d. = 0.3), P<001, 95% CI of the difference 0.044–0.290, IFCC 34 (s.d. = 6) v. 33 (s.d. = 4) mmol/mol).
Type 2 diabetes
At study entry, there were seven participants known to have developed type 2 diabetes after exposure to antipsychotic drugs. Another participant on antipsychotics was diagnosed as having diabetes during the study. Prevalence of type 2 diabetes was 7.4% in the antipsychotic-treated group and nil in the antipsychotic-naive group, an apparent difference albeit of borderline statistical significance (P = 0.052).
For the comparison of intellectual disability v. age- and gender-matched general population participants, prevalence of type 2 diabetes was defined similarly to the HSE control group as the percentage of participants with previously known type 2 diabetes out of the whole of the study group. Diabetes prevalence was similar in the men (2.2% v. 3.8%, P = 0.584) but was higher in the women in the intellectual disability group than in women in the HSE control group (7.9% v. 2.2%, P = 0.0106) of similar age.
Of the eight participants with type 2 diabetes diagnosed after initiation of antipsychotic treatment, three were currently on olanzapine and one each on risperidone, amisulpride, zuclopenthixol, haloperidol and trifluoperazine; all but one had been exposed to various antipsychotics over the years. Mean antipsychotic treatment duration before diagnosis of diabetes was 9.7 years (s.d. = 7.7, range 2–20). Mean age at diagnosis of diabetes was 42.7 years (s.d. = 8.8). Of the participants with diabetes, one had a history of developing
Table 4 Prolactin levels, hyperprolactinaemia and hypogonadism
| Antipsychotic naive (n = 64) | On antipsychotics (n = 138) | Risperidone (n = 66) | Amisulpride/sulpiride (n = 9) | Other antipsychotic (n = 63) | |
|---|---|---|---|---|---|
| Prolactin mU/l, median (range) | |||||
| Males | 219 (111–549) | 365 (120–1476) | 500 (180–1476) | 906 (423–1325) | 235 (120–662) |
| Females | 228 (88–2075) | 533 (103–5959) | 1078 (192–5959) | 1835 (1358–2235) | 280 (144–969) |
| Hyperprolactinaemia,a % | |||||
| Males | 15 | 44 | 70 | 100 | 7 |
| Females | 10 | 47 | 72 | 100 | 9 |
| Hyperprolactinaemic hypogonadism,b % | |||||
| Males | 0 | 3 | 4 | 0 | 0 |
| Females | 0 | 77 | 75 | 80 | 0 |
a Prolactin >375 mU/l in men and >620 mU/l in women.
b Hyperprolactinaemic hypogonadism is expressed as the percentage of participants who were hyperprolactinaemic that developed hypogonadism as a complication of hyperprolactinaemia. Participants in whom the diagnosis of hypogonadism is impossible, namely those on exogenous sex steroids, have been excluded from this analysis.
uncontrolled diabetes when quetiapine was added to his haloperidol treatment and another one experienced near normalisation of glucose indices when her treatment was changed from olanzapine to risperidone.
Risk factors for diabetes
A family history was obtained for 68% of participants. This was positive for diabetes in at least one first- or second-degree relative in 86% of those with diabetes and in 41% of those participants without diabetes (P<0.05). South Asian or African–Caribbean ethnicity was 11% in those with and 4.8% in those without diabetes (P = 0.377). Participants with diabetes tended to be older (46.3 (s.d. = 7.0) v. 41.9 (s.d. = 13.1) years, P = 0.319) and more obese (BMI 30.8 (s.d. = 4.8) v. 28.8 (s.d. = 6.3), P = 0.355) than those without diabetes.
Prolactin, gonadal function and bone mineral density
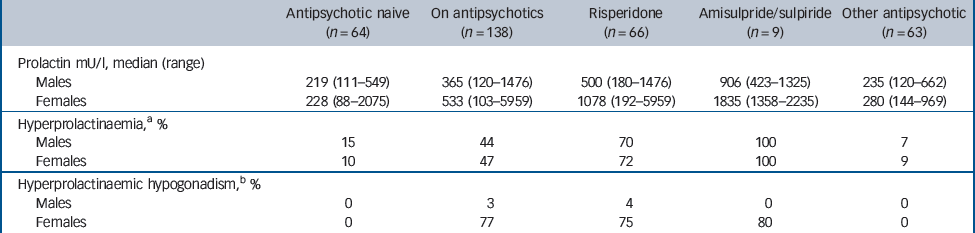
Hyperprolactinaemia (prolactin>375 mU/l in men and>620 mU/l in women) was present in 45% of participants on antipsychotics and in 12.5% of those who were antipsychotic naive (Table 4). Prevalence was much higher in individuals on risperidone and amisulpride/sulpiride, 70% and 100% respectively. Most participants with hyperprolactinaemia not explained by risperidone, amisulpride or sulpiride, whether in the antipsychotic or antipsychotic-naive group, were taking other drugs that can cause prolactin elevation such as antidepressants (e.g. citalopram), peripheral dopamine antagonists or proton pump inhibitors.
Of the 21 women with antipsychotic-induced hyperprolactinaemia, 8 premenopausal women were taking exogenous sex hormones. Of the remaining 13 women, 10 were hypogonadal, as defined by a history of amenorrhoea or by gonadotrophins below the menopausal range in pre- and postmenopausal women respectively. Of 27 men with hyperprolactinaemia, only 1 was hypogonadal, as defined by a testosterone level below the normal range. The prolactin levels in women who were hypogonadal ranged between 1083 and 5959 mU/l and in the man who was hypogonadal it was 1105 mU/l. Eight of nine patients with hyperprolactinaemic hypogonadism who underwent a dual-energy x-ray absorptiometry scan had decreased bone mineral density (T score ≤1.0).
Discussion
Main findings
This is the first study to compare metabolic and endocrine parameters in a representative community-dwelling intellectual disability population with a locally recruited population of controls and between individuals naive to antipsychotic drugs and those taking them for psychosis or challenging behaviour. The study was widely inclusive, being open to all patients aged 18–70 in a single NHS trust under psychiatric care, thus it is very likely to have mirrored current routine clinical practice. It is extremely difficult to collect data of this sort in an intellectual disability population so the 61% participation rate is a considerable achievement. Failure to participate was primarily for arbitrary reasons relating to carer agreement rather than any significant clinical bias. In the antipsychotic-treated group, monotherapy was the rule (95% of people) and doses were generally low, both compared with schizophrenia Reference Uchida, Suzuki, Mamo, Mulstant, Tanabe and Inagaki21,Reference Lieberman, Stroup, McEvoy, Swartz, Rosenheck and Perkins22 and with a previous study in people with intellectual disability, in which the mean chlorpromazine equivalent dose was 372 mg (range 20–4067), Reference Ahmed, Fraser, Kerr, Kiernan, Emerson and Robertson23 more than three times the median 108 mg (range 16–667) found in our study. Almost half of the participants were on risperidone, which is in line with the limited evidence for benefit in the treatment of challenging behaviour. Reference Gagiano, Read, Thorpe, Eerdekens and Van Hove24,Reference Deb, Sohanpal, Soni, Lenotre and Unwin25
Comparisons with the general population showed that glucose and lipid parameters were on average the same or even more favourable in the intellectual disability group. This contrasts with findings in individuals with severe mental illness, in which metabolic abnormalities are highly prevalent. Reference McEvoy, Meyer, Goff, Nasrallah, Davis and Sullivan26 Moreover, there was a paucity of other metabolic and cardiovascular risk factors such as alcohol excess, smoking or an atherogenic diet, which are common in the general population, and even more so in populations with severe mental illness. Nevertheless, in women, not men, prevalence of overweight/obesity and of type 2 diabetes were markedly higher in the intellectual disability group. Whereas the favourable lipid profile of our intellectual disability group could be explained by a healthy diet provided in sheltered accommodation and by the other positive lifestyle factors, the highly sedentary lifestyle led by the women in this group could partly explain their high prevalence of obesity, which could be the main determinant for their high rates of diabetes. A higher prevalence of obesity in women with intellectual disability compared with men with intellectual disability and with women in the general population has been previously reported. Reference Robertson, Emerson, Gregory, Hatton, Turner and Kessissoglous27,Reference Emerson28 Moreover, although people with intellectual disability have lower levels of physical activity than participants from the general population, the risk of inactivity is higher in women with intellectual disability than men. Reference Emerson28
The metabolic profile of participants in the intellectual disability group on antipsychotics was essentially identical to that of those in the group who were antipsychotic naive. It is reassuring that indices of obesity and of glucose and lipid metabolism were not significantly different, statistically or clinically, between the antipsychotic-treated and the antipsychotic-naive individuals. The absence of any effect could be due, in part, to the relatively small number of people taking antipsychotics with the highest potential metabolic impact, such as olanzapine and clozapine, and to the generally low doses. All the cases of diabetes occurred in individuals on antipsychotics, but the study was underpowered to estimate differences in prevalence rates with confidence. Although antipsychotics, on average, did not increase metabolic risk, the trend towards a higher prevalence of type 2 diabetes in the antipsychotic-treated group may suggest that a subgroup of individuals are particularly vulnerable to the hyperglycaemic potential of these drugs. This accords with the relatively young age at diagnosis of diabetes and the higher prevalence of a positive family history compared with those without diabetes. However, these individuals did not represent the tip of a metabolic iceberg, given the normal findings in participants without diabetes.
A total of 45% of antipsychotic-treated participants (70% of those on risperidone, 100% of those on amisulpride or sulpiride) had hyperprolactinaemia, with secondary hypogonadism in 77% of affected women but only 4% of affected men. A raised prolactin was equally frequent in antipsychotic-treated participants of either gender but, as reported by previous studies, Reference Kuruvilla, Peedicayil, Srikrishna, Kuruvilla and Kanagasabapathy29,Reference Bushe, Yeomans, Floyd and Smith30 prolactin elevation was more severe in women than men, thus explaining the gender differential in the prevalence of hypogonadism. Clinically significant hyperprolactinaemia, causing secondary hypogonadism, was exclusively seen in participants on risperidone, amisulpride or sulpiride even at low doses. As in earlier studies, Reference O'Keane31,Reference Klibanski, Neer, Beitins, Ridgway, Zervas and McArthur32,Reference Greenspan, Neer, Ridgway and Klibanski33 hyperprolactinaemic hypogonadism was generally accompanied by loss of bone mineral density. This iatrogenic complication will summate with other risk factors for bone pathology in intellectual disability, such as primary hypogonadism, anti-epileptic treatment and vitamin D deficiency, and could partly explain the high risk for osteoporosis and fractures seen in these indivduals. Reference Hawli, Nasrallah and El-Hajj Fuleihan34,Reference Schrager35,Reference Vanlint and Nugent36
Strengths and limitations of the study
This is the only study comparing metabolic and endocrine parameters between antipsychotic-treated and antipsychotic-naive individuals with intellectual disability and between individuals with intellectual disability and the general population. Moreover, its naturalistic setting in one NHS trust and wide inclusion criteria make its results generalisable with a strong degree of confidence. However, despite being a large study compared with others in this population, the sample size was not large enough to allow a robust comparison of diabetes rates between the antipsychotic-treated and the antipsychotic-naive group, something that could possibly explain the borderline statistical difference between the two groups. This problem could only be overcome by a multicentre study.
Implications
In summary, the Oxford Learning Disabilities Study provides an initial evidence base underpinning the safe use of antipsychotic drugs in the intellectual disability population. Whatever the efficacy of antipsychotic use in relation to challenging behaviour, they are still widely used. This would be difficult to defend in such a vulnerable population if it led to major metabolic side-effects. In fact, our findings offer significant reassurance in relation to cardiovascular and metabolic risk in the intellectual disability population. Nevertheless, there may be potential problems in a susceptible subgroup, hence regular monitoring of blood glucose, lipids and weight 37 should be instituted when prescribing antipsychotics to people who may already have risk factors for diabetes, and when using antipsychotics with a high metabolic impact.
The study identified hyperprolactinaemia as the commonest side-effect in antipsychotic-treated individuals with intellectual disability and hyperprolactinaemic hypogonadism as a complication of risperidone and amisulpride treatment, leading to bone loss in a population already at risk for osteoporosis and fractures. These findings should lead to screening for and management of hyperprolactinaemic hypogonadism, together with studies to investigate optimal prevention and treatment strategies in the intellectual disability population.
Funding
The study was supported by the Baily Thomas Charitable Fund.
Acknowledgements
We thank all the patients who participated in the study, the carers for their support, and Professor Fredrik Karpe and the NIHR Oxford Biomedical Research Centre for helpful advice and for allowing us to use the Oxford Biobank database. We also thank Dr Susan Manley and Professor Francesco Pezzella for useful comments.








eLetters
No eLetters have been published for this article.