Despite the effects of location on life chances (Reference DorlingDorling, 2001), there is little evidence of geographical patterning in rates of the most common mental disorders of anxiety and depression (Reference McCullochMcCulloch, 2001; Reference Wainwright and SurteesWainwright & Surtees, 2003; Weich et al, Reference Weich, Holt and Twigg2003a ,Reference Weich, Twigg and Holt b ). One contradictory finding is the higher prevalence of common mental disorders in urban compared with rural or suburban areas in UK studies (Reference Meltzer, Gill and PetticrewMeltzer et al, 1995; Reference Paykel, Abbott and JenkinsPaykel et al, 2000; Reference Lehtinen, Michalak and WilkinsonLehtinen et al, 2003). Suicide rates are also higher in urban areas in England and Wales (Reference Saunderson, Haynes and LangfordSaunderson et al, 1998; Reference Middleton, Gunnell and FrankelMiddleton et al, 2003). A recent study in Sweden found a linear association between increasing population density and first-admission rate for depression (Reference Sundquist, Frank and SundquistSundquist et al, 2004). In this study we investigated whether (a) there are statistically significant associations between living in rural UK electoral wards and onset and maintenance of episodes of common mental disorders, (b) rural/non-rural gradients are greatest among those not employed or on low income and (c) these associations are confounded by age, gender, ethnicity, socio-economic status, or household composition.
METHOD
Data were gathered in the first two waves of the British Household Panel Survey (BHPS), which began in 1991 (Reference Taylor, Brice and BuckTaylor et al, 1999). The BHPS is an annual survey of individuals aged 16 and over in a representative sample of private households in England, Wales and Scotland. First-wave members were selected via a two-stage, stratified clustered probability sample. Efforts are made to reinterview all original sample members each year. Individual original sample members aged 16-74 at wave 1 who completed the General Health Questionnaire (GHQ; Reference Goldberg and WilliamsGoldberg & Williams, 1988) at both waves 1 and 2 were included in this study. Coordinators of the BHPS provided permission for and facilitated the linkage of BHPS data to other geographically referenced data-sets via each individual's electoral ward of residence at wave 1. This process did not threaten the anonymity of individual sample members.
Assessment of the onset and maintenance of episodes of common mental disorders
Common mental disorders were assessed using the self-administered 12-item GHQ (Reference Goldberg and WilliamsGoldberg & Williams, 1988). Designed as a case-finding measure in community settings, where sensitivity and specificity are about 80%, the GHQ has been validated against standardised clinical interviews. We followed evidence that the common mental disorders are validly represented as a single dimension encompassing comorbid symptoms of anxiety and depression (Reference KruegerKrueger, 1999; Reference Vollebergh, Iedema and BijlVollebergh et al, 2001; Reference Kendell and JablenskyKendell & Jablensky, 2003). The GHQ has been widely used for epidemiological research in general population samples and is robust to retest effects (Reference PevalinPevalin, 2000).
Each GHQ item has four response categories. For example, responses to the question, ‘Have you recently been feeling unhappy and depressed?’ are ‘not at all’, ‘no more than usual’, ‘rather more than usual’ and ‘much more than usual’. Items are scored in two ways, by the ‘GHQ method’ as present or absent (1 point for either of the latter two of the four potential responses and 0 otherwise) or by the Likert method (responses code in order as 0, 1, 2 or 3). This score represents the probability of being identified as having non-psychotic psychiatric morbidity if interviewed with a standardised clinical interview (Reference Goldberg and WilliamsGoldberg & Williams, 1988). We took a score of 3 or more (out of 12) by the GHQ method to determine caseness (Reference Goldberg and WilliamsGoldberg & Williams, 1988; Reference Weich and LewisWeich & Lewis, 1998), i.e. the presence of a common mental disorder. Likert scores (range 0-36) more closely approximate a normal distribution and were used when the GHQ score was treated as a continuous outcome.
When analysing GHQ score as a dichotomous outcome, data were stratified according to case status at wave 1. ‘Episode onset’ refers to those who did not meet case criteria at wave 1 but who did meet them at wave 2. ‘Episode maintenance’ describes individuals who met case criteria at both waves 1 and 2. In each instance, individuals meeting these outcome criteria were compared with those of similar case status at wave 1.
Individual- and household-level risk factors
In keeping with previous studies (Reference Weich and LewisWeich & Lewis, 1998; Reference Lorant, Deliege and EatonLorant et al, 2003), age, gender, marital status, ethnicity, education, employment status, financial strain and the number of current physical health problems were all included as potential individual-level confounders of associations between area-level exposures and rates of common mental disorders.
Recent studies have reported significant variation in rates of common mental disorders between households even after taking into account individual-level confounders (Reference Weich, Holt and TwiggWeich et al, 2003a ). Some exposures can only be assigned to the household level, such as overcrowding, household type, housing tenure and structural housing problems. This is not so for others, particularly income, for which data are most commonly aggregated at the household level (Reference Weich, Lewis and JenkinsWeich et al, 2001). Another example is occupational social class, where stronger associations with rates of common mental disorders have been found for the social class of the head of the household than for individual social class, particularly among women (Reference Weich and LewisWeich & Lewis, 1998). Household characteristics were assessed at wave 1 and included structural housing problems, household income, car access, housing tenure, social class (by head of household), overcrowding (more than two household members per bedroom) and household type (based on household composition). Structural housing problems were defined as any major problem or two or more minor problems from a list comprising damp, condensation, leaking roof and/or rotting wood. The BHPS data-set includes net income data, which have been validated against official UK income distribution figures (Reference Jarvis and JenkinsJarvis & Jenkins, 1995). Low income was defined as household income below half the median income for the sample.
Spatial scale
There were three potential ‘area’ levels above households within this data-set: electoral ward, postcode sector (the primary sampling unit for the BHPS) and region. Electoral wards (2400 addresses on average with a mean population of 5222 (s.d.=3899)) are currently the smallest geographical area at which BHPS data are available. Sensitivity analyses were undertaken by substituting each of the other two geographical levels for wards. The BHPS investigators and authors therefore agreed a method for matching respondents and characteristics of electoral wards, without disclosure of information that might permit identification of respondents.
Area-level characteristics
Electoral wards were characterised in two ways: using the UK Office for National Statistics (ONS) classification of wards (Reference Wallace and DenhamWallace & Denham, 1996) and population density, defined as the number of 25- to 64-year-olds per km2. Both measures were derived from the 1991 census; the density measure was based on reworked 1991 census data which attempted to adjust for the census undercount (Reference Simpson and DorlingSimpson & Dorling, 1994).
The ONS classification of wards (Reference Wallace and DenhamWallace & Denham, 1996) comprises 14 principal groups and 43 clusters, based primarily on demographic and socio-economic composition (Table 1). More than 30 census variables were used to generate this classification, including age, ethnicity, household composition, education, housing tenure, employment status and the proportion of residents working in different occupations (including agriculture, forestry and fishing). Although no direct measures of the physical environment were used, proportions of respondents living in terraced and purpose-built housing were included. Groups and clusters were derived using two-stage cluster analysis, followed by a k-means procedure with iteration to ensure that wards were assigned to the cluster with the smallest dissimilarity between it and the cluster centroid (Reference Wallace and DenhamWallace & Denham, 1996; Reference Bailey, Charlton and DollamoreBailey et al, 1999). The final classification was designed to ensure that clusters were homogeneous and sufficiently populous to permit the study of geographical patterns. Groups and clusters were given names by the originators of the classification ‘for ease of reference, based on the general characteristics of cluster members… combined with [their] geographic attributes’ (Reference Bailey, Charlton and DollamoreBailey et al, 1999). These names are shorthand rather than precise descriptions. A full list of groups and clusters, and portraits of each, are available elsewhere (Reference Wallace and DenhamWallace & Denham, 1996; Reference Bailey, Charlton and DollamoreBailey et al, 1999).
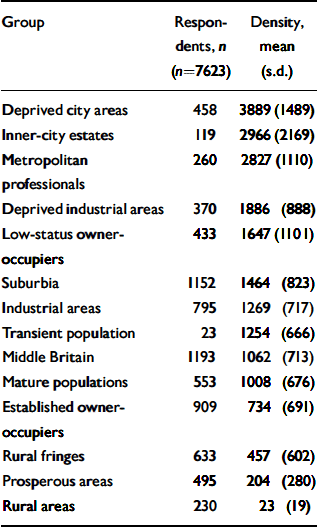
Table 1 Numbers of respondents at baseline (wave 1) and population densities (persons aged 25-64 per km2) for each of the 14 principal groups identified in the Office for National Statistics classification of wards
| Group | Respondents, n (n=7623) | Density, mean (s.d.) |
|---|---|---|
| Deprived city areas | 458 | 3889 (1489) |
| Inner-city estates | 119 | 2966 (2169) |
| Metropolitan professionals | 260 | 2827 (1110) |
| Deprived industrial areas | 370 | 1886 (888) |
| Low-status owner-occupiers | 433 | 1647 (1101) |
| Suburbia | 1152 | 1464 (823) |
| Industrial areas | 795 | 1269 (717) |
| Transient population | 23 | 1254 (666) |
| Middle Britain | 1193 | 1062 (713) |
| Mature populations | 553 | 1008 (676) |
| Established owner-occupiers | 909 | 734 (691) |
| Rural fringes | 633 | 457 (602) |
| Prosperous areas | 495 | 204 (280) |
| Rural areas | 230 | 23 (19) |
Using the ONS classification of wards, three rural groups were identified a priori on the basis of their geographical distribution and the identities of their clusters (Reference Wallace and DenhamWallace & Denham, 1996). These three groups (‘rural fringe’, ‘rural area’ and ‘prosperous area’) were aggregated to produce a single dummy variable representing ‘ONS rural grouping’. It was not possible to identify specific ‘urban’ areas in this way. As Table 1 shows, the three rural groups were those with the lowest population densities. The mean population density in this ‘ONS rural grouping’ was significantly lower than that in the remaining 11 ONS groups (difference between means 1242.5, 95% CI 1179.1-1305.9, P<0.001).
Statistical analysis
Multilevel models were developed using MLwiN software (Centre for Multilevel Modelling, University of Bristol, Bristol, UK; see http://www.mlwin.com/index.html). We analysed onset of episodes of common mental disorders separately from episode maintenance. In each instance a null, random-effects model was derived for persons nested in households, with households nested within wards (Reference Snijders and BoskerSnijders & Bosker, 1999). Individual-, household- and ward-level exposures were added to the models in subsequent analyses.
General Health Questionnaire scores were analysed first as a dichotomous outcome (cases v. non-cases) using multilevel logistic regression. These analyses were undertaken using a logit link function and assumed non-constant, between-individual variance based on a Bernoulli distribution (Reference GoldsteinGoldstein, 1995). However, the properties of binomial distributions (including Bernoulli) differ from those of continuous normally distributed outcomes. In particular, the variance associated with the intercept term is neither constant across groups nor independent of the mean value within the groups. Therefore it is not possible to ascertain the true variance of the intercept term at higher levels or (hence) to directly quantify total variance associated with models of this nature. We addressed these difficulties by means of a logit model based on the notion of a continuous latent variable, in which a threshold defines the binary outcome (see Reference Snijders and BoskerSnijders & Bosker, 1999: p. 223). We therefore assumed an underlying standard logistic distribution for the binary outcome (onset or not, maintenance or not across the two waves) at the individual level (level 1). Level 1 variance on this latent variable was always standardised to the standardised logistic variance of π2/3=3.29. When unexplained random variance at level 2 was indicated as r 0 2, the proportion of the total unexplained variance occurring at this level was estimated (from a two-level null random intercept model) as r 0 2/(r 0 2+3.29). In each of the logistic models, the constant term is the logit (loge of the odds) of a person in the base (reference) category being an individual experiencing either the ‘onset’ or ‘maintenance’ of a common mental disorder. The proportion of each onset or maintenance group was therefore estimated from the constant term in the null model, which is equal to ln(P/1+P).
In the logistic models, parameters were estimated using second-order Taylor expansion with predictive quasi-likelihood. This estimation procedure is considered superior to first- or second-order marginal quasi-likelihood when clusters, such as households, are small (Reference GoldsteinGoldstein, 1995). Markov chain Monte-Carlo methods may further improve the accuracy of such estimates but the method involves intensive computation and was only used here in the discussion of higher-level variation. Statistical significance of individual fixed estimates was tested using a Wald test against a χ2 distribution. Since difficulties may be encountered due to the distribution of parameter estimates when the variances are close to zero (negative variances cannot exist), 95% interval estimates (the ‘credible interval’) derived from Markov chain Monte-Carlo procedures are also reported for random model parameters.
General Health Questionnaire scores at wave 2 were also analysed as a continuous outcome, using hierarchical linear regression, controlling for GHQ score at wave 1. Intraclass correlation was used to assess stability of GHQ scores across waves and to indicate the scale of unobserved symptom fluctuation. We also considered the possibility that any rural/non-rural difference in common mental disorders might result from inherently greater between-ward variability in GHQ scores in rural areas. We ran separate null, random-effects linear regression models using Markov chain Monte-Carlo methods for ONS-defined rural and non-rural wards on cross-sectional data from wave 1 with GHQ score as a continuous outcome.
RESULTS
A total of 9518 individuals aged 16-74 participated in the BHPS at wave 1. Of these, 8980 (94%) completed the GHQ at wave 1 and 7659 also did so at wave 2 (85% of those who completed the GHQ at wave 1 and 80% of the total baseline sample). For analysis of episode onset, 5809 individuals were nested within 3679 households, within 615 wards. For analysis of episode maintenance, 1850 individuals were nested within 1566 households, within 511 wards. The baseline prevalence of common mental disorders in the study sample was 24.6%. Among those with non-case status at wave 1, 14.3% (95% CI 13.3-15.3) were found to have case status at wave 2. Of those who had case status at wave 1, 54.3% (95% CI 51.8-56.8) had the same status at wave 2.
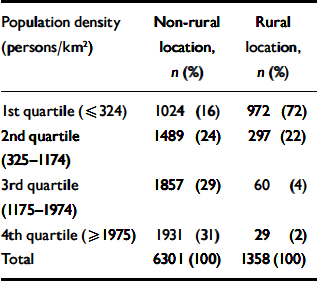
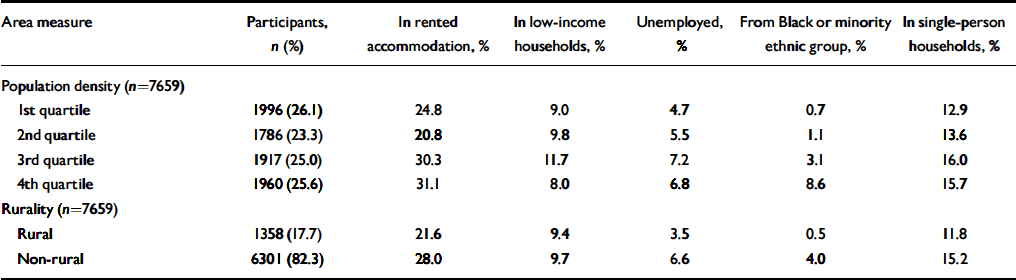
Among individuals living in ‘rural’ wards (using the ONS group classification), 72% were living in wards with population densities in the bottom quartile for the study sample, 22% in the third population density quartile, 4% in the second and 2% in the most densely populated quartile (Table 2). Most indices of ward-level deprivation are higher in the 3rd and top quartile, with the exception of the percentage of low-income households (Table 3). The proportion of residents from Black and minority ethnic groups increased sharply with population density and was eight times greater (4.0%) in non-rural compared with rural wards (0.5%).
Table 2 Distribution of study participants' area of residence according to population density and urban/rural location based on the Office for National Statistics classification of wards
| Population density (persons/km2) | Non-rural location, n (%) | Rural location, n (%) |
|---|---|---|
| 1st quartile (≤324) | 1024 (16) | 972 (72) |
| 2nd quartile (325-1174) | 1489 (24) | 297 (22) |
| 3rd quartile (1175-1974) | 1857 (29) | 60 (4) |
| 4th quartile (≥1975) | 1931 (31) | 29 (2) |
| Total | 6301 (100) | 1358 (100) |
Table 3 Socio-economic and demographic characteristics of electoral wards at baseline (wave 1) according to population density quartile and Office for National Statistics rural/non-rural classification
| Area measure | Participants, n (%) | In rented accommodation, % | In low-income households, % | Unemployed, % | From Black or minority ethnic group, % | In single-person households, % |
|---|---|---|---|---|---|---|
| Population density (n=7659) | ||||||
| 1st quartile | 1996 (26.1) | 24.8 | 9.0 | 4.7 | 0.7 | 12.9 |
| 2nd quartile | 1786 (23.3) | 20.8 | 9.8 | 5.5 | 1.1 | 13.6 |
| 3rd quartile | 1917 (25.0) | 30.3 | 11.7 | 7.2 | 3.1 | 16.0 |
| 4th quartile | 1960 (25.6) | 31.1 | 8.0 | 6.8 | 8.6 | 15.7 |
| Rurality (n=7659) | ||||||
| Rural | 1358 (17.7) | 21.6 | 9.4 | 3.5 | 0.5 | 11.8 |
| Non-rural | 6301 (82.3) | 28.0 | 9.7 | 6.6 | 4.0 | 15.2 |
Onset and maintenance of episodes of common mental disorders
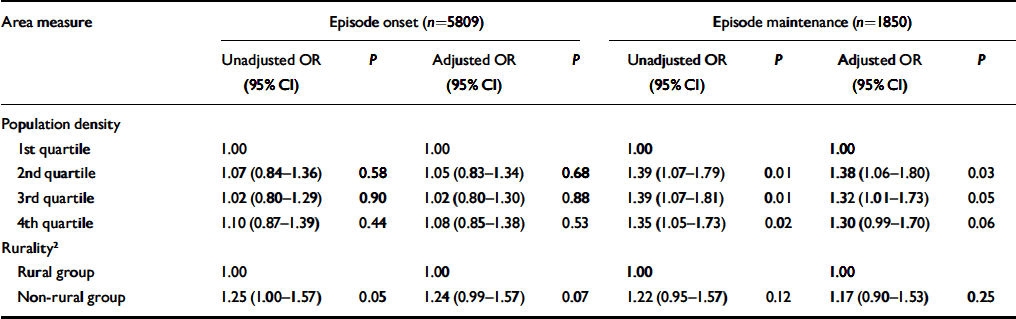
Population density was significantly associated with the maintenance of episodes of common mental disorders but not their onset (Table 4). In neither case, however, was there any evidence that the association was linear. Adjusting for individual and household characteristics had little effect on these associations. Table 4 shows that rates of both episode onset and maintenance were lower in rural than non-rural wards. Although the size of the non-rural/rural gradient was similar for both episode onset and maintenance, only the former reached statistical significance, before adjusting for individual and household characteristics.
Table 4 Associations between the onset and maintenance of episodes of common mental disorders and ward-level rurality and population density unadjusted and adjusted for individual- and household-level risk factors 1
| Area measure | Episode onset (n=5809) | Episode maintenance (n=1850) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted OR (95% CI) | P | Adjusted OR (95% CI) | P | Unadjusted OR (95% CI) | P | Adjusted OR (95% CI) | P | |||||||
| Population density | ||||||||||||||
| 1st quartile | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||||
| 2nd quartile | 1.07 (0.84-1.36) | 0.58 | 1.05 (0.83-1.34) | 0.68 | 1.39 (1.07-1.79) | 0.01 | 1.38 (1.06-1.80) | 0.03 | ||||||
| 3rd quartile | 1.02 (0.80-1.29) | 0.90 | 1.02 (0.80-1.30) | 0.88 | 1.39 (1.07-1.81) | 0.01 | 1.32 (1.01-1.73) | 0.05 | ||||||
| 4th quartile | 1.10 (0.87-1.39) | 0.44 | 1.08 (0.85-1.38) | 0.53 | 1.35 (1.05-1.73) | 0.02 | 1.30 (0.99-1.70) | 0.06 | ||||||
| Rurality 2 | ||||||||||||||
| Rural group | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||||
| Non-rural group | 1.25 (1.00-1.57) | 0.05 | 1.24 (0.99-1.57) | 0.07 | 1.22 (0.95-1.57) | 0.12 | 1.17 (0.90-1.53) | 0.25 | ||||||
1. Risk factors include age, gender, marital status, ethnicity, education, employment status, financial strain, number of current physical health problems, structural housing problems, household income, car access, housing tenure, social class (by head of household), overcrowding and household type.
2. Rurality was defined according to the Office for National Statistics classification of wards.
Score on GHQ as a continuous outcome
The intraclass correlation coefficient for GHQ score at waves 1 and 2 was +0.44. Although there were no statistically significant differences in the change in mean GHQ score between waves across population-density groups, the increase in GHQ scores in non-rural wards was significantly greater than in rural wards (Table 5). This difference remained after adjusting for individual and household characteristics.
Table 5 Associations between ward characteristics and total General Health Questionnaire (GHQ) score at wave 1 and wave 2 according to rurality and population density, and regression coefficients for GHQ score at wave 2 adjusted for GHQ score at wave 1 (Adjusted-1) and adjusted for GHQ score at wave 1, age, gender and individual and household-level risk factors 1 (Adjusted-2)
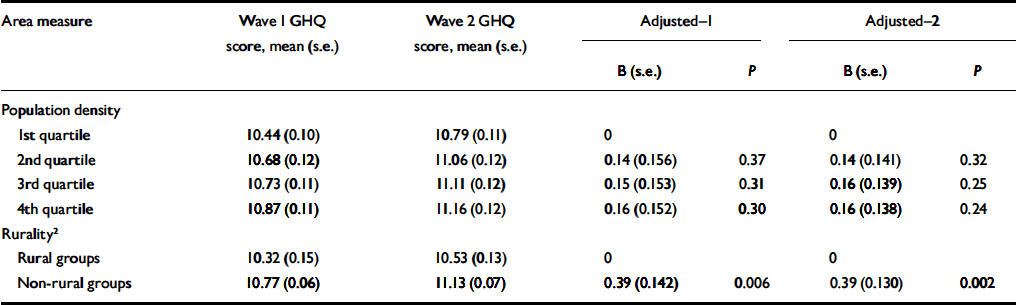
| Area measure | Wave 1 GHQ score, mean (s.e.) | Wave 2 GHQ score, mean (s.e.) | Adjusted-1 | Adjusted-2 | ||||
|---|---|---|---|---|---|---|---|---|
| B (s.e.) | P | B (s.e.) | P | |||||
| Population density | ||||||||
| 1st quartile | 10.44 (0.10) | 10.79 (0.11) | 0 | 0 | ||||
| 2nd quartile | 10.68 (0.12) | 11.06 (0.12) | 0.14 (0.156) | 0.37 | 0.14 (0.141) | 0.32 | ||
| 3rd quartile | 10.73 (0.11) | 11.11 (0.12) | 0.15 (0.153) | 0.31 | 0.16 (0.139) | 0.25 | ||
| 4th quartile | 10.87 (0.11) | 11.16 (0.12) | 0.16 (0.152) | 0.30 | 0.16 (0.138) | 0.24 | ||
| Rurality 2 | ||||||||
| Rural groups | 10.32 (0.15) | 10.53 (0.13) | 0 | 0 | ||||
| Non-rural groups | 10.77 (0.06) | 11.13 (0.07) | 0.39 (0.142) | 0.006 | 0.39 (0.130) | 0.002 | ||
1. Risk factors include age, gender, marital status, ethnicity, education, employment status, financial strain, and number of current physical health problems, structural housing problems, household income, car access, tenures, social class (by head of household), overcrowding and household type.
2. Rurality was defined according to the Office for National Statistics classification of wards.
The effects of ward population density or ONS rural/non-rural location (Table 5) did not vary with either baseline employment status or household income in their associations with change in GHQ score between assessments. Using cross-sectional data from wave 1, ward-level variances in GHQ score were 0.17 (credible interval 0.001-0.74, P=0.43) in ONS-defined rural areas and 0.18 (CI 0.002-0.48, P=0.18) in non-rural areas.
DISCUSSION
Main findings
Those living in rural areas experience better mental health than their non-rural counterparts to an extent that was numerically modest but statistically significant. This difference was most evident when studying mean GHQ score at each wave. Although this remained statistically significant after adjusting for numerous potential confounders, the actual difference amounted to approximately one-half of one point on the GHQ.
With the exception of episode maintenance, the clearest gradients in rates of common mental disorders and in change in GHQ score between waves were found when rurality was defined using the ONS classification of wards rather than population density. However, our findings also indicate that there was a high rate of episode remission among participants with common mental disorders at baseline living in wards in the bottom quartile of population density. In contrast to our cross-sectional findings (Reference Weich, Twigg and HoltWeich et al, 2003b ), we found no evidence that the effects of geographical location on change in GHQ score between assessments varied with employment status or household income. These results highlight the complexity of comparing outcomes in ‘urban’ and ‘rural’ environments, in part because there is little agreement about how these should be defined (Reference Macintyre, Ellaway and CumminsMacIntyre et al, 2002; Reference Weich, Blanchard and PrinceWeich et al, 2002; Reference Middleton, Gunnell and FrankelMiddleton et al, 2003; Reference van Osvan Os, 2004).
These findings are consistent with cross-sectional research showing little geographical patterning in the prevalence of common mental disorders. Although it might be argued that our results lack clinical significance, even very small differences in risk are cumulatively important in public health terms when multiplied by the numbers exposed (Reference RoseRose, 1992).
Classifying ‘rural’ and ‘urban’ areas
Urban and rural areas differ in ways that encompass both the physical and social environments, ranging from factors such as access to education, employment, transport, healthcare and leisure facilities to noise, crowding, rates of crime and fear of crime (Reference Wandersman and NationWandersman & Nation, 1998). Although rurality is often defined on the basis of population density (e.g. Reference Sundquist, Frank and SundquistSundquist et al, 2004; Reference WangWang, 2004), our findings indicate that this may result in misclassification. More than one-fifth of participants classified as living in a ‘rural’ area on the basis of ward socio-demographic composition (including percentage employed in agriculture) and geographical location fell outside of the bottom quartile for population density. Participants living in ‘non-rural’ areas were distributed fairly uniformly across all population density quartiles, with only one-third living in the most densely populated wards. Levels of population density are not evenly distributed across the country and there are small pockets of high density in otherwise remote areas (Reference Middleton, Gunnell and FrankelMiddleton et al, 2003).
As in a study that contrasted population density with a measure of remoteness from population concentrations (Reference Middleton, Gunnell and FrankelMiddleton et al, 2003), our findings would have differed substantially had we defined rurality according to population density alone. Although some researchers have developed alternative quantitative measures of rurality (such as geographical remoteness), others have resorted to using interviewers' impressions of rurality to overcome the perceived limitations associated with ward-level population density (Reference Meltzer, Gill and PetticrewMeltzer et al, 1995; Reference Paykel, Abbott and JenkinsPaykel et al, 2000).
The complexities of comparing ‘rural’ and ‘urban’ areas
Area-level studies based on aggregate measures of socio-economic deprivation consistently portray rural areas as less deprived and healthier than urban areas. Recent evidence indicates that this may be a statistical artefact resulting from the smaller size of rural wards and their greater internal (i.e. between-individual) variability with respect to deprivation. Although rural wards are more internally heterogeneous, even over areas smaller than wards, there is less variation in deprivation between rural areas than their urban counterparts (Reference Haynes and GaleHaynes & Gale, 2000). In other words, affluent and deprived individuals are more likely to live in close proximity in rural than urban areas. Previous research found that associations between area-level socio-economic deprivation and worse health emerged for rural areas when wards were aggregated to approximate the greater size of urban wards (Reference Haynes and GaleHaynes & Gale, 2000). This was not the case when areas smaller than wards were studied or when different indices of deprivation were employed. These findings support our decisions to study ‘rural areas’ as a single group and to control for socio-economic status at both individual and household levels. In this study, ward-level variance in GHQ score at wave 1 was almost identical in rural and non-rural areas. This argues against the possibility that the main study finding of a small rural/non-rural difference in common mental disorders was a result of a small number of affluent, healthy, rural wards.
In the present study, the only evidence of an adverse effect of population density on mental health was a statistically significant but non-linear association with episode maintenance. This contrasts with a substantial excess in hospital admissions for depression among those living in the most densely populated parts of Sweden (Reference Sundquist, Frank and SundquistSundquist et al, 2004). Notwithstanding the different outcomes in these studies, the discrepant findings might partly result from the far steeper gradient in population density in Sweden. The ratio of mean population densities for the top and bottom quintiles in the study by Sundquist et al (Reference Sundquist, Frank and Sundquist2004) was 120, compared with less than 10 in the present study. Likewise, the ‘urban’ density criterion of ≥ people per km2 used in a Canadian study (Reference WangWang, 2004) suggests lower population densities compared with the UK, although the author admitted that this cut-off may have been too low. The relative lack of variability in population density in Britain may preclude the emergence of associations with mental health outcomes and/or the detection of statistically significant effects. More importantly, definitions of rurality in Britain that rely exclusively on population density might fail to detect important differences in physical and social contexts.
Cross-national comparisons are particularly problematic, given historic, socio-economic and ethnic differences in rural and urban populations in different countries (Reference Costello, Keeler and AngoldCostello et al, 2001). Studies in New Zealand (Reference Romans-Clarkson, Walton and HerbisonRomans-Clarkson et al, 1990), the USA (Reference Blazer, George and LandermanBlazer et al, 1985), Scandinavia (Reference Lehtinen, Michalak and WilkinsonLehtinen et al, 2003) and Canada (Reference WangWang, 2004) found no evidence of statistically significant rural/non-rural differences in the prevalence of common mental disorders, although a modest difference emerged after adjusting for residents' characteristics in one study (Reference WangWang, 2004). Interpreting findings based on treated incidence is also inherently difficult given differences in service provision and pathways to care in urban and rural areas (Reference Sundquist, Frank and SundquistSundquist et al, 2004).
Strengths and limitations of the study
Cross-sectional studies may conceal associations between risk factors and either the onset or outcome of episodes of disorder. Previous findings suggest that social and economic risk factors may have a greater impact on the duration of episodes of common mental disorders than on their onset (Reference Weich and LewisWeich & Lewis, 1998; Reference Lorant, Deliege and EatonLorant et al, 2003). This is one of the first prospective studies of rural/non-rural differences in rates of common mental disorders in Britain. The multilevel structure of the data-set allowed us to include household as a distinct level between place (ward) and the individual, which many studies overlook (Reference McCullochMcCulloch, 2001; Reference Wainwright and SurteesWainwright & Surtees, 2003). Our estimates of standard errors for associations between area-level exposures and individual-level outcomes were less prone to bias than those arising from studies in which individual- and household-level exposures were conflated (Reference McCullochMcCulloch, 2001; Reference Wainwright and SurteesWainwright & Surtees, 2003). The BHPS is arguably the largest most comprehensive and representative survey ever of individuals and households in the UK.
Choice of spatial scale
A particular challenge facing studies of this nature is defining the appropriate spatial scale over which contextual characteristics are supposed to affect mental health. ‘Neighbourhoods’ are difficult to define (Reference Burrows and BradshawBurrows & Bradshaw, 2001; Reference O'CampoO'Campo, 2003) and it may be argued that wards are far too large to detect contextual influences. This view is consistent with evidence of statistically significant associations between rates of common mental disorders and specific features of the built environment assessed across small areas, after adjustment for characteristics of individual residents (Reference HalpernHalpern, 1995; Reference Weich, Blanchard and PrinceWeich et al, 2002). We had no alternative to the use of wards in this study and although residents may not equate wards with ‘neighbourhoods’, they are more than arbitrary administrative boundaries. In Britain wards are used for electoral purposes, with voters in each ward electing local government representatives.
Measuring the common mental disorders
The study was limited by use of the GHQ rather than a standardised clinical interview. However, traditional objections to findings not based on clinical diagnostic categories are reduced by evidence that the common mental disorders are validly represented as a single dimension encompassing the comorbid conditions of anxiety and depression (Reference KruegerKrueger, 1999; Reference Vollebergh, Iedema and BijlVollebergh et al, 2001; Reference Kendell and JablenskyKendell & Jablensky, 2003). Furthermore, it may be argued that even if our findings are not readily translated into absolute incidence and maintenance rates for specific categorical disorders, they are indicative of rates of ‘at-risk mental states’ which are intimately related to, and highly correlated with, these disorders (Reference van Osvan Os, 2004). Nevertheless, associations between poverty and the common mental disorders are generally larger in studies using standardised clinical interviews (Reference Meltzer, Gill and PetticrewMeltzer et al, 1995).
Since the GHQ is sensitive to recent change in psychological functioning, false positives might have included individuals with mild or transient psychological disturbance. By contrast, individuals with chronic symptoms of anxiety and depression may be given non-case status (false negatives). This misclassification should have biased associations towards the null. Although physical ill health also leads to false positives, study findings were adjusted for the number of current physical health problems. Those in lower occupational grades (Reference Stansfeld, North and WhiteStansfeld et al, 1995) may underreport psychiatric symptoms on the GHQ compared with responses to a standardised clinical interview. Although this may have led to an underestimate of the extent of confounding by individual socio-economic status, it was unlikely to have altered our main findings. We are unaware of response bias to the GHQ between urban and rural residents.
Defining episodes of disorder
The study was limited by the absence of data on the duration of episodes of anxiety and depression. ‘Episode onset’ was defined as the presence of common mental disorder at wave 2 (T2) among participants who did not meet criteria for caseness at wave 1 (T1) on the GHQ. Many (if not most) of these were likely to have been relapses rather than first inceptions. ‘Episode maintenance’ was defined as the proportion who met criteria for caseness at T1 that also met criteria for caseness at T2. We recognise that this may be viewed as implying continuous morbidity throughout the year and the term ‘maintenance’ was only used in the absence of any widely recognised alternative. Without interval data, it is possible that some individuals in the case group at T1 remitted and then relapsed between assessments, and that a proportion of people in the ‘episode onset’ group experienced multiple episodes between assessments. Episodes that began and then remitted between waves may have been missed among those identified as not meeting case criteria at both waves. However, the high intraclass (within individual) correlation in GHQ scores at T1 and T2 (r=+0.44) suggests only limited intraparticipant fluctuation in case status between waves.
Likewise, participants' exposure status was classified using information collected at wave 1. Some participants may have moved between urban and rural locations, between areas of differing population density, or in or out of employment between assessments. The present analyses therefore take no account of the duration of exposure to these risk factors. Were this type of mobility random, our results would have been biased towards the null. Although the modest numbers who moved into employment were likely to have been healthier than those who remained out of work (and vice versa), little is known about the effects of geographical mobility on patterns of psychiatric morbidity. This type of misclassification was unlikely to have a profound effect on our findings and is common to all cohort studies of this nature.
Understanding place and mental health
In general, the effects of place on rates of the common mental disorders appear modest (Weich et al, Reference Weich, Holt and Twigg2003a ,Reference Weich, Twigg and Holt b , Reference Weich, Twigg and Lewis2005; Reference Wainwright and SurteesWainwright & Surtees, 2004). The present findings confirm this counter-intuitive phenomenon and fail to support the view that the effects of place vary with individual and household characteristics (Reference Amato and ZuoAmato & Zuo, 1992; Reference Macintyre, Ellaway and CumminsMacIntyre et al, 2002; Reference Weich, Twigg and HoltWeich et al, 2003b ; Reference van Osvan Os, 2004). Nevertheless we found statistically significant longitudinal differences in rates of the common mental disorders in rural and non-rural areas. Although we adjusted for household composition (and therefore living alone), we were not able to control for other factors that might differentially affect mental health in urban and rural areas, including social support and social networks, access to transport and healthcare, and stigma associated with mental health problems. Further research is needed to better understand these differences, and how these might affect individuals' mental health.
Acknowledgements
We thank Professor Nick Buck of the Institute for Social and Economic Research for his help in obtaining and managing the BHPS data-set with ward-level indices. The Wellcome Trust funded this study. The data used in this publication were made available through the UK Data Archive. The data were originally collected by the ESRC Research Centre on Microsocial Change at the University of Essex, now incorporated within the Institute for Social and Economic Research. Neither the original collectors of the data nor the Archive bear any responsibility for the analyses or interpretations presented here.








eLetters
No eLetters have been published for this article.