A number of reports have described new-onset diabetes in temporal association with atypical antipsychotic treatment (Koller et al, Reference Koller, Schneider and Bennett2001, Reference Koller, Cross and Doraiswamy2003; Reference Koller and DoraiswamyKoller & Doraiswamy, 2002). In many of the reported cases, diabetes was noted in relatively young patients (mean age about 40 years) and was diagnosed within 3–6 months of first prescription of the atypical antipsychotic medication. Evaluation of the relatedness of diabetes to atypical antipsychotic use from case reports is complicated by a number of factors, including the increasing prevalence of diabetes in the general population and data indicating that a substantial number of individuals with diabetes are undiagnosed.
Data from the National Health and Nutrition Examination Survey (NHANES) III (1988–1994) indicate that diagnosed diabetes was present in 5.1% of the US adult population (Reference Harris, Flegal and CowieHarris et al, 1998). Subsequent reports from the Center for Disease Control have described a continued increase (about 30%) in the prevalence of diabetes during the 1990s, with the largest increase (70%) in individuals in the 30–39 year age range (Reference Mokdad, Ford and BowmanMokdad et al, 2000). Further data from NHANES III suggested that diabetes was undiagnosed in as many as a third of patients (Reference Harris, Flegal and CowieHarris et al, 1998). Results from another large survey support this finding that approximately half of patients in Australia with diabetes were undiagnosed (Reference Dunstan, Zimmet and WelbornDunstan et al, 2002).
The prevalence of diagnosed diabetes in patients with schizophrenia appears to exceed that of the general population by 2-fold (Reference Dixon, Weiden and DelahantyDixon et al, 2000). Patients with schizophrenia generally have poorer physical health (Reference Brown, Birtwistle and RoeBrown et al, 1999; Reference OsbornOsborn 2001), and less than adequate overall health care (Reference Phelan, Stradins and MorrisonPhelan et al, 2001; Reference Wang, Demier and KesslerWang et al, 2002a ) compared with the general population. The symptoms of the psychosis itself may hinder the ability or willingness of the patient to communicate potential physical probems (Reference Felker, Yazel and ShortFelker et al, 1996; Reference Jeste, Gladsjo and LindamerJeste et al, 1996). Thus, it is likely that the prevalence of unrecognised diabetes in patients with schizophrenia is at least as high as that in the general population.
Reasons for an increased prevalence of diabetes among patients with schizophrenia remain speculative. However, Dixon et al (Reference Dixon, Weiden and Delahanty2000) reported that in a survey of several large databases containing medical information on patients with schizophrenia, the patients with diabetes were more likely to be older, non-White, and to have hypertension – findings consistent with those in the general population. In a more recent review of 45 published case reports of new-onset diabetes in patients receiving atypical agents, Jin et al (Reference Jin, Meyer and Jeste2002) noted that 84% of the patients were overweight at baseline assessment, 42% had a positive family history of diabetes and 49% had high-risk ethnic backgrounds (African or Hispanic). The assessment of case reports is complicated by several factors, which include inconsistent reporting of important demographic and other variables that might affect glycaemic control, reporting bias and lack of an adequate control group. In addition, case reports cannot be used to determine causal relationships between individual therapies and treatment-emergent diabetes.
Weight gain – a body mass index (BMI) of more than 25 kg/m2 – is a risk factor for diabetes (Reference Chan, Rimm and ColditzChan et al, 1994; Reference Colditz, Willett and RotnitzkyColditz et al, 1995), and weight gain can occur during treatment with most of the atypical antipsychotic medications (Reference Allison, Mentore and HeoAllison et al, 1999). However, in some cases, new-onset diabetes has been reported in patients without weight gain (Reference Koller, Schneider and BennettKoller et al, 2001; Reference HendersonHenderson, 2002; Reference Koller and DoraiswamyKoller & Doraiswamy, 2002). Further, no association between weight gain and new-onset diabetes was noted in a naturalistic study of patients receiving clozapine (Reference Henderson, Cagliero and GrayHenderson et al, 2000). These observations have led to further speculation that some of the atypical antipsychotic medications may increase risk for diabetes by a weight-independent mechanism.
Given the growing interest in a possible association between diabetes and antipsychotic medications, a systematic re-evaluation of risk factor profiles of patients with treatment-emergent diabetes (TED) is warranted. In this retrospective analysis of a large clinical trials database, our objectives were: (a) to identify patients with schizophrenia who exhibited TED; (b) to compare the entry characteristics, including pre-randomisation risk factor profiles, of TED patients with those who maintained normal glucose tolerance during treatment; and (c) to examine the influence of treatment-emergent weight gain or therapy assignment on the development of TED.
METHOD
Patient population and study designs
Twenty-four studies were identified from the olanzapine clinical trial database in which patient weight and post-randomisation plasma glucose measurements were available at multiple time points. For many of the studies, the details of the study designs, patient characteristics (age, gender, race, illness characteristics), and efficacy and safety results have been previously published (Beasley et al, Reference Beasley, Sanger and Satterlee1996a ,Reference Beasley, Tollefson and Tran b ; Tollefson et al, Reference Tollefson, Beasley and Tran1997, Reference Tollefson, Dellva and Mattler1999, Reference Tollefson, Birkett and Kiesler2001; Reference Tran, Hamilton and KuntzTran et al, 1997). Briefly, study participants were in-patients or out-patients, aged 18–65 years, diagnosed with DSM–III–R or DSM–IV schizophrenia or related disorders (American Psychiatric Association, 1987, 1994), and had provided written informed consent after the study design and possible adverse events were described. Participation criteria were similar among the pooled trials, except that studies examining clozapine were limited to patients with treatment-refractory disease (Tollefson et al, Reference Tollefson, Dellva and Mattler1999, Reference Tollefson, Birkett and Kiesler2001) and, in several studies comparing olanzapine with risperidone, entry criteria excluded patients with cardiovascular disease from participation in the original trial (Reference Tran, Hamilton and KuntzTran et al, 1997). All studies included a medication wash-out period of 2–9 days and a double-masked treatment period of 6–52 weeks, followed by an olanzapine open-label extension phase in some cases. For studies with medication crossover, only the initial monotherapy treatment period was included in the analyses. During the double-masked treatment period, all patients received therapeutic doses of a single antipsychotic medication (olanzapine 5–25 mg/day, haloperidol 5–20 mg/day, risperidone 4–12 mg/day, clozapine 200–600 mg/day) or placebo.
Non-fasting glucose measurements
Non-fasting glucose levels were analysed by Covance Inc. using a photometric chemistry analyser (Hitachi 747–200; Roche Diagnostics, Indianapolis, Indiana, USA). The frequency of sample collection was specified by each study protocol. In general, two samples were obtained pre-randomisation and after that, samples were usually obtained weekly for the first 6 weeks and monthly or bi-weekly thereafter. In case of multiple glucose measurements for the same visit, only the maximum observation was considered. The analyses included all measurements up to and including the day after the last day of treatment.
Classification of patients
Patients with only baseline glucose values and those with pre-existing diabetes at entry (clinical diagnosis of diabetes, such as taking antidiabetic medications at baseline such as insulin, sulphonylurea, metformin, thiazolidinediones or α-glucosidase inhibitor) or two pre-randomisation glucose values of ≥11.1 mmol/l were excluded from the analyses. Patients (n=27) with a single glucose measurement ≥11.1 mmol/l at entry were not excluded because these individuals lacked a confirmatory second value prior to drug assignment. A single glucose value of ≥11.1 mmol/l at entry was, however, considered suggestive of underlying dysglycaemia in the assessment of pre-existing risk factors for diabetes (see Categorical risk factors, below).
Post-baseline non-fasting glucose values were used to classify or categorise patients as exhibiting:
-
(a) treatment emergent diabetes (TED), defined as two non-fasting glucose values of ≥11.1 mmol/l at any time after baseline, final glucose ≥11.1 mmol/l, initiation of antidiabetic medication, or a new clinical diagnosis of diabetes;
-
(b) uncertain glucose tolerance (UGT), defined as two or more glucose values ≥ 7.8 mmol/l but one or no glucose value ≥11.1 mmol/l at any time prior to end-point;
-
(c) normal glucose tolerance (NGT).
A non-fasting glucose value of ≥7.8 mmol/l was chosen as the threshold for UGT based on several lines of evidence:
-
(a) post-prandial glucose levels of individuals with normal glucose tolerance rarely exceed 7.8 mmol/l (American Diabetes Association, 2001);
-
(b) individuals with glucose values of 7.8 mmol/l or greater in a standard 2 h oral glucose tolerance test (OGTT) are considered to have impaired glucose tolerance (American Diabetes Association, 2002);
-
(c) non-fasting capillary glucose values 7.8 mmol/l or higher have reasonable sensitivity (62–65%) and specificity (95–96%) for identifying individuals with diabetes subsequently confirmed by OGTT or fasting blood sugar (Reference Rolka, Narayan and ThompsonRolka et al, 2001).
Categorical risk factors
Patients possessing one or more of the following risk factors (American Diabetes Association, 2002) for diabetes at baseline were identified: age ≥45 years, baseline BMI ≥27 kg/m2, non-White ethnicity, hypertension based on clinical diagnosis or use of antihypertensive medication, or non-fasting glucose levels suggestive of underlying dysglycaemia, e.g. a single pre-randomisation glucose value ≥11.1 mmol/l. Height was available for BMI calculation for approximately 80% of the patients. Where BMI could not be calculated, data from these patients were not included in these analyses. This analysis began before the BMI threshold as a risk factor for diabetes was lowered from 27 kg/m2 to 25 kg/m2 in the American Diabetes Association (ADA) Clinical Practice Recommendations (American Diabetes Association, 2002). Baseline glycaemic status of individual patients was based on the mean of two pre-randomisation measures for 4425 of the 5013 patients evaluated (88.3%).
Statistical methods
Data from 24 studies from the olanzapine clinical trial database were pooled for these analyses. The prevalence of baseline risk factors within the TED v. the NGT group or within the UGT v. the NGT group was compared by Fisher's exact test. Imbalances in risk factors that are continuous variables (such as age, mean baseline glucose, maximum baseline glucose and baseline BMI) were tested by F-test. Weight gain was analysed by a last observation carried forward (LOCF) method.
To account for variation in observation times for individual therapy groups, a time-to-event analysis using the Cox proportional hazards model was employed to assess the risk of TED. Specifically, the Cox model assessed the impact of mean non-fasting glucose values or the presence of pre-existing risk factors for diabetes on the subsequent risk of being identified with TED. The Cox proportional hazards model was also used to assess the impact of weight gain and therapy assignment on the risk of being identified with TED v. ‘not TED’ (UGT plus NGT cohorts). Because of the small number of events in individual therapy groups, treatment group results were compared between olanzapine and non-olanzapine groups (including haloperidol, risperidone and placebo). Unless otherwise specified, the Cox proportional hazards model included a single test covariate (baseline mean non-fasting glucose concentration, baseline risk factors for diabetes, treatment-emergent weight gain, or therapy assignment) along with study protocol. The study protocol was also included as a stratification variable in the model to control for effects of pooling data from several clinical trials.
RESULTS
Categorisation of patients
Of the 5529 patients enrolled, 149 patients were identified with pre-existing diabetes and were excluded from the TED analysis. Post-randomisation glucose values were available for 5013 patients not known to be diabetic by diagnosis, use of antidiabetic medication, or pre-randomisation glucose values. The majority (60%) of these patients received olanzapine, followed by haloperidol (24%), risperidone (8%), placebo (4%) and clozapine (4%) (Table 1). After randomisation, most patients (n=4637, 92.5%) appeared to maintain normoglycaemia and were considered to have NGT. Of the remaining patients, 94 (1.9%) were identified with TED and 282 (5.7%) exhibited an intermediate level of hyperglycaemia and, in the absence of more definitive testing, were considered to have UGT (Table 1). The mean post-randomisation observation time varied among the individual therapy assignments with a mean of 205 (s.d. 283) days (median 86 days), and a maximum observation time of 1775 days (Table 1). The mean weight gain for each therapy at end-point (LOCF) is also presented in Table 1.
Table 1 Post-randomisation glycaemic categories and median observation time by therapy assignment
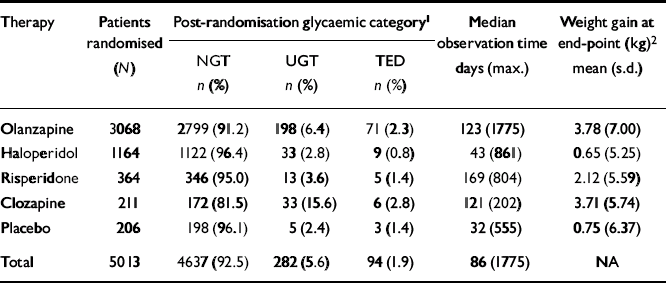
| Therapy | Patients randomised (N) | Post-randomisation glycaemic category1 | Median observation time days (max.) | Weight gain at end-point (kg)2 mean (s.d.) | ||
|---|---|---|---|---|---|---|
| NGT n (%) | UGT n (%) | TED n (%) | ||||
| Olanzapine | 3068 | 2799 (91.2) | 198 (6.4) | 71 (2.3) | 123 (1775) | 3.78 (7.00) |
| Haloperidol | 1164 | 1122 (96.4) | 33 (2.8) | 9 (0.8) | 43 (861) | 0.65 (5.25) |
| Risperidone | 364 | 346 (95.0) | 13 (3.6) | 5 (1.4) | 169 (804) | 2.12 (5.59) |
| Clozapine | 211 | 172 (81.5) | 33 (15.6) | 6 (2.8) | 121 (202) | 3.71 (5.74) |
| Placebo | 206 | 198 (96.1) | 5 (2.4) | 3 (1.4) | 32 (555) | 0.75 (6.37) |
| Total | 5013 | 4637 (92.5) | 282 (5.6) | 94 (1.9) | 86 (1775) | NA |
Risk factors for treatment-emergent diabetes
Risk factors at study entry
At study entry, mean non-fasting glucose levels for TED patients were significantly higher than for NGT patients (Table 2). Over half (61%) of the patients subsequently identified with TED had mean glucose values ≥6.1 mmol/l and over 30% had values ≥7.8 mmol/l at entry (Fig. 1). In comparison, 13% of NGT patients had mean glucose values ≥6.1 mmol/l and only 1.5% had values ≥ 7.8 mmol/l at entry. After randomisation of the patients with a single glucose measurement ≥11.1 mmol/l at entry (n=27) who were not excluded from the analysis, 9 were categorised in the TED group, 5 were categorised in the UGT group, and 13 were categorised in the NGT group.
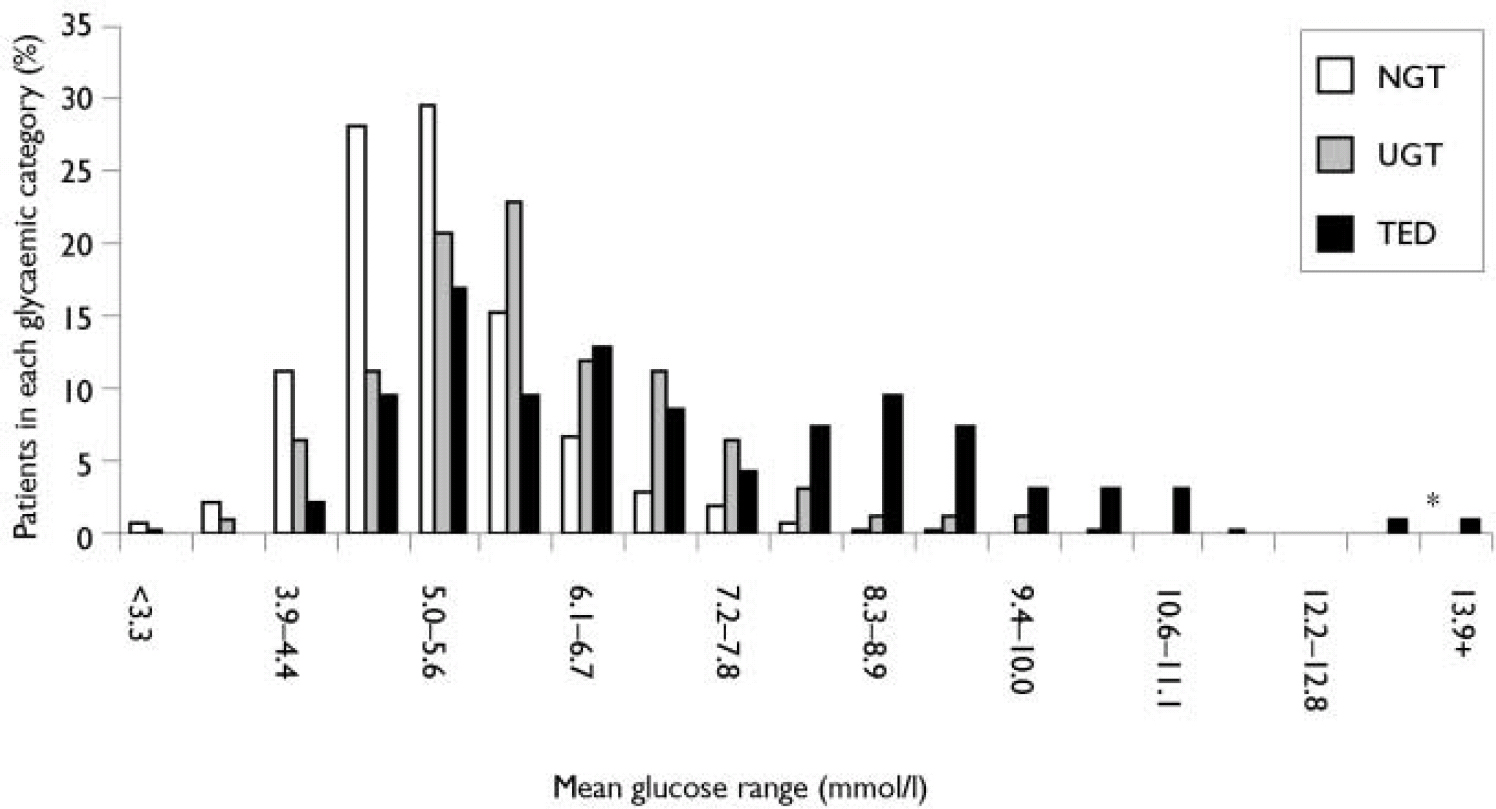
Fig. 1 Distribution of baseline mean non-fasting glucose levels by post-randomisation glycaemic category: normal glucose tolerance (NGT), treatment-emergent diabetes (TED) and uncertain glucose, tolerance (UGT). Asterisk denotes 2 patients with a single glucose measurement at entry.
Table 2 Comparison of entry characteristics of patients with treatment-emergent diabetes, uncertain glucose tolerance and normal glucose tolerance
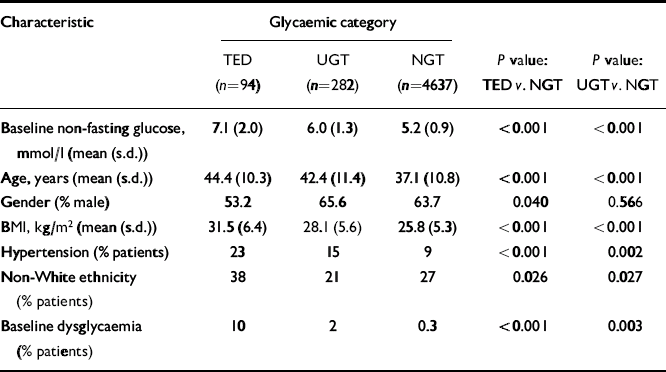
| Characteristic | Glycaemic category | ||||
|---|---|---|---|---|---|
| TED (n=94) | UGT (n=282) | NGT (n=4637) | P value: TED v. NGT | P value: UGT v. NGT | |
| Baseline non-fasting glucose, mmol/l (mean (s.d.)) | 7.1 (2.0) | 6.0 (1.3) | 5.2 (0.9) | <0.001 | <0.001 |
| Age, years (mean (s.d.)) | 44.4 (10.3) | 42.4 (11.4) | 37.1 (10.8) | <0.001 | <0.001 |
| Gender (% male) | 53.2 | 65.6 | 63.7 | 0.040 | 0.566 |
| BMI, kg/m2 (mean (s.d.)) | 31.5 (6.4) | 28.1 (5.6) | 25.8 (5.3) | <0.001 | <0.001 |
| Hypertension (% patients) | 23 | 15 | 9 | <0.001 | 0.002 |
| Non-White ethnicity (% patients) | 38 | 21 | 27 | 0.026 | 0.027 |
| Baseline dysglycaemia (% patients) | 10 | 2 | 0.3 | <0.001 | 0.003 |
Patients subsequently identified as having TED were significantly older, more obese and more likely to be hypertensive, non-White, female, or have baseline dysglycaemia than NGT patients (Table 2). Sixty-four per cent of TED patients possessed multiple risk factors for diabetes compared with 21% of NGT patients (Fig. 2). Baseline characteristics of patients subsequently identified with TED demonstrated that substantial numbers had baseline non-fasting glucose levels ≥ 7.8 mmol/l or multiple pre-existing risk factors for diabetes in each of the individual treatment groups (Table 2). Approximately half of the cases of TED were identified within 3 months of trial entry. For these ‘early’ TED patients, the entry glucose was 7.9 (s.d. 2.2) mmol/l and 71% possessed at least two risk factors for diabetes at entry.
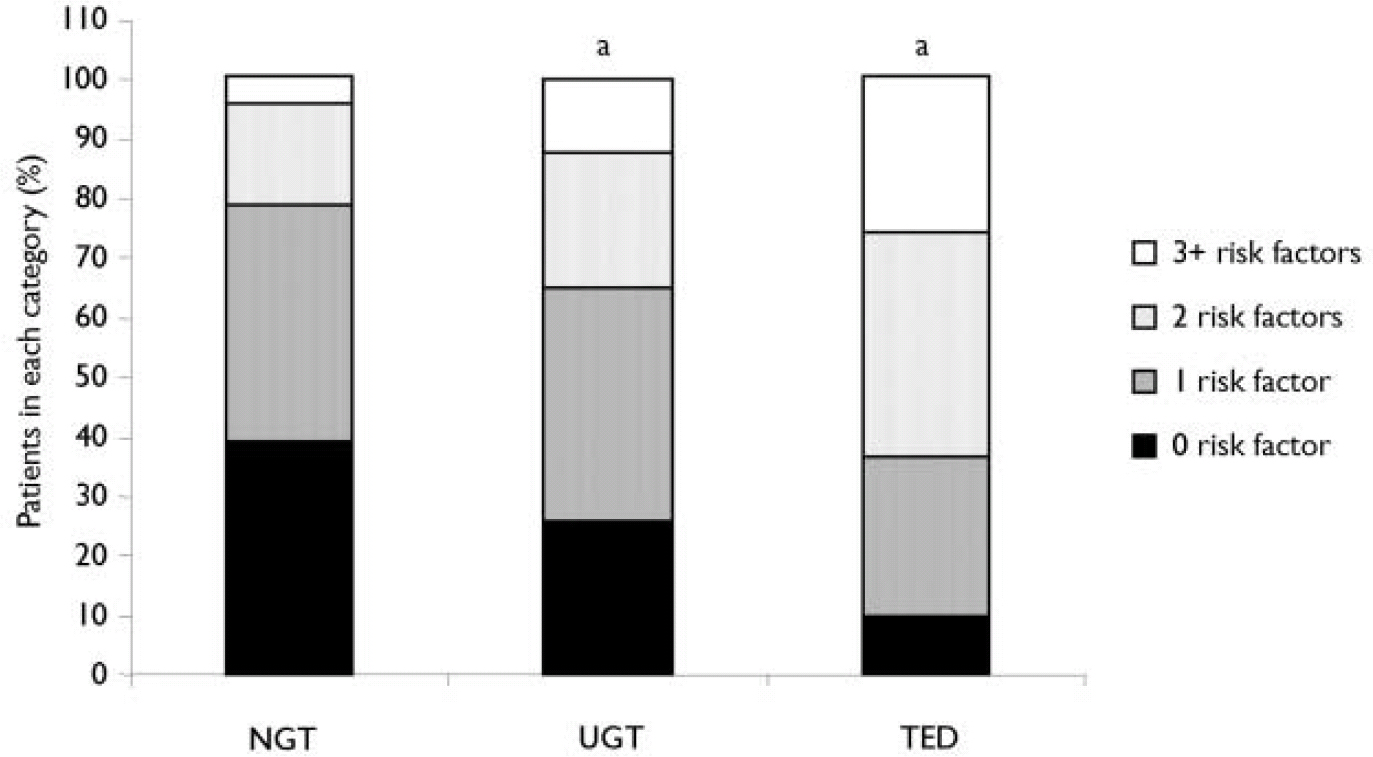
Fig. 2 Baseline risk factors for diabetes in the three post-randomisation glycaemic categories: normal glucose tolerance (NGT), treatment-emergent diabetes (TED) and uncertain glucose tolerance (UGT).a P<0.001, TED or UGT v. NGT.
As expected, entry non-fasting glucose had a highly significant impact on the risk of TED. The risk of being identified with TED was substantially greater for patients with entry non-fasting glucose ≥7.8 mmol/l: hazard ratio (HR) 31.9; 95% CI 19.6–52.0; P<0.001. Even at lower entry glucose levels, the risk of TED was still markedly elevated. For example, in patients with non-fasting glucose of ≥6.7 mmol/l, the risk of TED was elevated (HR 11.85, 95% CI 7.7–18.3; P<0.001) (Fig. 3). Further, the risk for TED was 9 times greater for patients with baseline random plasma glucose ≥ 6.7 mmol/l (HR 9.6, 95% CI 6.2–14.8; P<0.001).
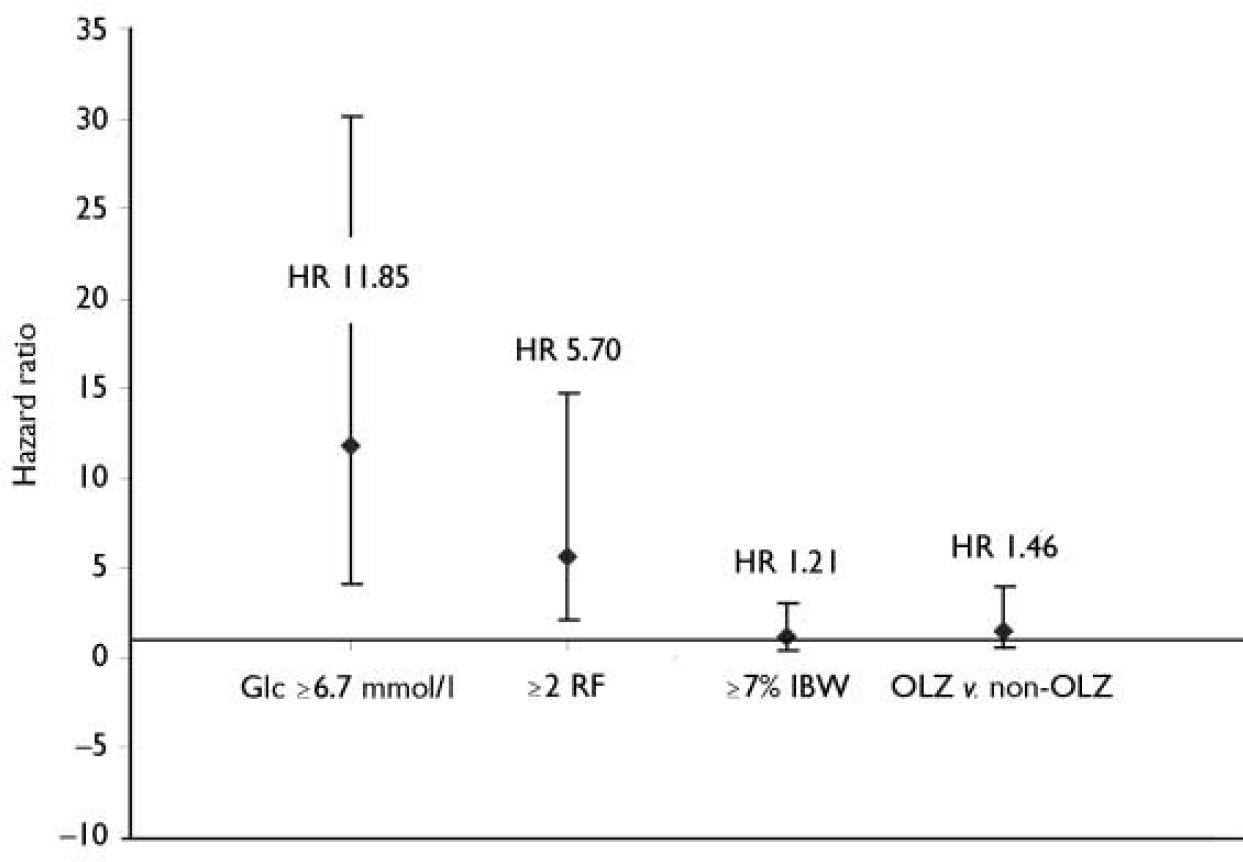
Fig. 3 Hazard ratio (HR) from Cox proportional hazards model evaluating the risk for treatment-emergent diabetes. The model included one of the following covariates: mean non-fasting glucose concentration (GIc) ≥6.7 mmol/l; two or more baseline risk factors (RF) for diabetes; weight gain ≥7% initial body weight (IBW); or therapy - olanzapine (OLZ) v. non-olanzapine (haloperidol, risperidone and placebo).
The presence of multiple baseline risk factors for diabetes (age, BMI, non-White ethnicity, hypertension and dysglycaemia) also had a highly significant impact on the risk of being identified with TED. Without adjusting for entry non-fasting glucose in the Cox proportional hazards model, patients with two or more risk factors at entry were nearly 6 times more likely to be identified with TED (HR 5.70, 95% CI 3.6–9.0; P<0.001) than patients with one or no risk factor.
An interaction between entry non-fasting glucose value and number of pre-existing diabetes risk factors would be expected. Among patients with entry glucose values ≥7.8 mmol/l and two or more baseline risk factors for diabetes, 40% (26 of 64 patients) were identified with TED. In contrast, less than 1% (26 of 3795) of patients with ≤1 risk factor and an entry glucose <7.8 mmol/l were identified with TED. Furthermore, for patients with entry glucose <7.8 mmol/l, the likelihood of being identified with TED was greater if multiple (two or more) baseline risk factors were present: 3.2% (34 out of 1068) of patients with normal glucose and multiple risk factors were identified with TED.
Of the 94 TED patients, nine appeared to lack risk factors for diabetes at study entry. However, within this subgroup, detailed review revealed that seven patients were overweight (BMI 26.5 to 26.9 kg/m2 or weight > 118 kg), over 35 years of age, or had questionable entry non-fasting glucose levels (range 7.8–10 mmol/l). The two remaining patients experienced substantial weight gain (>13 kg) prior to identification of TED.
A subset of patients (n=282) with repeated post-randomisation glucose levels ≥7.8 mmol/l, but an insufficient hyperglycaemia to meet criteria for TED were identified. This appeared to be a heterogeneous group in terms of glycaemic control and because confirmatory testing data (e.g. fasting plasma glucose or OGTT) were not available to define glycaemic status more precisely, these patients were considered to have UGT and were analysed separately. Overall, this group possessed entry characteristics (Table 2) and risk factor profiles (Fig. 2) intermediate to those of the TED and NGT groups. At study entry, the mean non-fasting glucose for patients identified as possessing UGT was significantly higher than NGT patients and 37% of the UGT patients had entry glucose values ≥6.1 mmol/l, with 7%≥7.8 mmol/l (Table 2 and Fig. 1). The number and percentage of patients identified with UGT in individual therapy groups with baseline mean non-fasting glucose ≥7.8 mmol/l or with 2 or more baseline risk factors for diabetes are presented in Table 3.
Table 3 Number and percentage of patients in each glycaemic category with baseline mean non-fasting glucose values of ≥7.8 mmol/l or over, or two or more pre-existing risk factors, for each treatment group
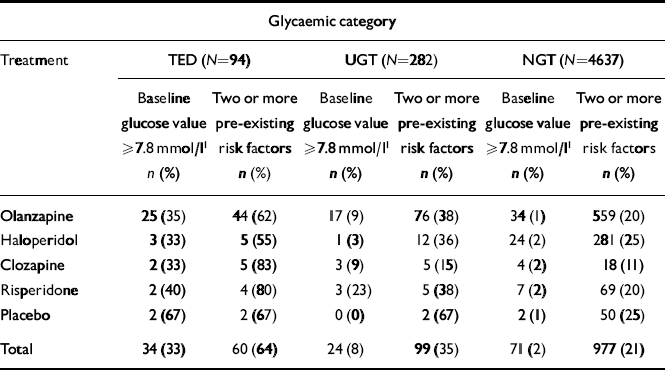
| Glycaemic category | ||||||
|---|---|---|---|---|---|---|
| Treatment | TED (N=94) | UGT (N=282) | NGT (N=4637) | |||
| Baseline glucose value ≥7.8 mmol/l1 n (%) | Two or more pre-existing risk factors n (%) | Baseline glucose value ≥7.8 mmol/l1 n (%) | Two or more pre-existing risk factors n (%) | Baseline glucose value ≥7.8 mmol/l1 n (%) | Two or more pre-existing risk factors n (%) | |
| Olanzapine | 25 (35) | 44 (62) | 17 (9) | 76 (38) | 34 (1) | 559 (20) |
| Haloperidol | 3 (33) | 5 (55) | 1 (3) | 12 (36) | 24 (2) | 281 (25) |
| Clozapine | 2 (33) | 5 (83) | 3 (9) | 5 (15) | 4 (2) | 18 (11) |
| Risperidone | 2 (40) | 4 (80) | 3 (23) | 5 (38) | 7 (2) | 69 (20) |
| Placebo | 2 (67) | 2 (67) | 0 (0) | 2 (67) | 2 (1) | 50 (25) |
| Total | 34 (33) | 60 (64) | 24 (8) | 99 (35) | 71 (2) | 977 (21) |
Post-randomisation risk factors
Patients identified as having TED gained slightly more weight than NGT patients (3.9 kg v. 2.7 kg, baseline to end-point, LOCF). However, observation times were longer for TED patients compared with the overall NGT group (data not shown). To adjust for differences in observation time, a time-to-event analysis was performed using a Cox proportional hazards model. In this analysis, the impact of weight gain (7% or more of the patient's initial body weight) as a categorical covariate on the risk of being identified with TED did not achieve statistical significance (HR 1.21, 95% CI 0.77–1.90, P=0.414; Fig. 3), without adjusting for baseline glucose concentration or number of pre-existing risk factors.
The risk of TED for patients receiving olanzapine v. non-olanzapine interventions (risperidone, haloperidol and placebo) was also assessed using the Cox proportional hazards model. As there were relatively few TED events in individual non-olanzapine treatment groups, the risk of TED was evaluated between patients receiving olanzapine and a pooled cohort of patients receiving the other non-olanzapine interventions (Table 1). Because clozapine, like olanzapine, has been suggested to be more closely associated with treatment-emergent diabetes than other antipsychotic medications, clozapine was omitted from the non-olanzapine group to avoid the potential for increasing the risk of diabetes in the non-olanzapine group. Using the Cox proportional hazards model, without adjusting for baseline random plasma glucose level, baseline number of risk factors or weight gain, the short-term risk for TED patients treated with olanzapine was not significantly greater than in a pooled cohort of patients receiving risperidone, haloperidol and placebo (HR 1.46, 95% CI 0.83–2.57, P=0.186; Fig. 3). In a separate analysis that included baseline glucose concentration, number of baseline risk factors and weight gain as continuous covariates, the risk for TED was also not significantly different between the olanzapine and non-olanzapine treatment groups (P=0.220). In this multivariate analysis, both baseline glucose values and number of pre-existing risk factors remained highly significant (P<0.001) covariates, whereas treatment-emergent weight gain was not significant (P=0.311).
DISCUSSION
In this retrospective analysis of a large clinical trial database of patients with schizophrenia, 94 cases of treatment-emergent diabetes (about 2% of the patient population) were identified. The annualised rates of TED were about 3% for patients treated with olanzapine, haloperidol and risperidone. The patients in the placebo group had an annualised TED rate of about 5%, which was not statistically different from the rate in the olanzapine treatment group. Only the patients treated with clozapine had a significantly greater rate (about 11% per year; P=0.022 v. olanzapine). Assuming that the definition of TED used in this study truly reflects more conventional definitions of diabetes, the rates of new diabetes seen were significantly greater than the rate that would be expected in the general population (about 0.3% per year in US adults, with a peak incidence of about 1% per year in the elderly; Reference Harris, Flegal and CowieHarris et al, 1998). Although it is possible that the definition of TED used for these analyses might have led us to underestimate the actual incidence of diabetes (see the paragraph discussing study limitations, below), the rates seen in our study are consistent with those reported in other studies of patients with schizophrenia. Annualised rates of diabetes of 1–7% have been reported in several epidemiology studies (Reference Caro, Ward and LevintonCaro et al, 2002; Reference Lee, Fowler and KadlubekLee et al, 2002; Reference Buse, Cavazzoni and HornbuckleBuse et al, 2003). The increased incidence of diabetes relative to the general population seen in these studies is present regardless of the type of antipsychotic drug prescribed. In addition, the elevated rate of diabetes seen in the placebo group in our study is consistent with the significantly increased risk of diabetes in patients with mental illness (Reference Tabata, Kikuoka and KikuokaTabata et al, 1987; Reference Mukherjee, Decina and BocolaMukherjee et al, 1996; Reference Dixon, Weiden and DelahantyDixon et al, 2000).
At entry into the clinical trials, patients in this study subsequently identified with TED possessed significantly higher non-fasting glucose levels and were much more likely to have multiple risk factors for diabetes than patients who maintained NGT. In general, TED patients were significantly older, more obese and more likely to be non-White, hypertensive or have non-fasting glucose levels suggestive of pre-existing dysglycaemia (e.g. single pre-randomisation glucose value greater than 11.1 mmol/l) at study entry than patients who appeared to maintain normal glucose levels (NGT patients). Overall, results of this analysis suggest that the majority of patients who were identified with TED were likely to have pre-existing, unrecognised glycaemic abnormalities or to have had a greater burden of pre-existing risk factors for diabetes than patients who appeared to maintain normoglycaemia.
Weight gain has been established as a risk factor for diabetes (Reference Chan, Rimm and ColditzChan et al, 1994; Reference Colditz, Willett and RotnitzkyColditz et al, 1995), and weight gain has been observed during treatment with many antipsychotics (Reference Allison, Mentore and HeoAllison et al, 1999). However, some reports have failed to demonstrate a relationship between weight gain and new-onset diabetes temporally associated with atypical antipsychotic treatment (Koller et al, Reference Koller, Schneider and Bennett2001, Reference Koller, Cross and Doraiswamy2003; Reference HendersonHenderson, 2002; Reference Koller and DoraiswamyKoller & Doraiswamy, 2002). A direct effect of atypical antipsychotic medications to promote dysglycaemia has been postulated (Koller et al, Reference Koller, Schneider and Bennett2001, Reference Koller, Cross and Doraiswamy2003; Reference HendersonHenderson, 2002); however, in a prospective randomised study of healthy volunteers (n=48) treated for approximately 2.5 weeks with olanzapine or risperidone, there was no significant change in insulin secretion or insulin sensitivity in the active therapy groups after adjusting for the impact of weight gain (Reference Sowell, Mukhopadhyay and CavazzoniSowell et al, 2002). In the current analysis, weight gain during the trials did not have a statistically significant effect on the risk of TED, although patients with TED gained slightly more weight than those who maintained NGT. Evaluation of the relationship between weight gain and risk of diabetes might be confounded if significant numbers of individuals with unrecognised pre-existing diabetes were present or if the population was already at high risk of diabetes (Reference Wannamethee and ShaperWannamethee & Shaper, 1999). Even among individuals without pre-existing diabetes but who are at high risk for the disorder, it may be difficult to measure a significant impact of further weight gain (Reference Wannamethee and ShaperWannamethee & Shaper, 1999). In our analysis, a substantial number of TED patients appeared to have a high likelihood of underlying glycaemic abnormalities or possess multiple risk factors for diabetes at baseline (for example, about a third of patients in the TED group had entry non-fasting glucose values ≥ 7.8 mmol/l and about two-thirds had two or more baseline risk factors). This, coupled with the relatively short duration of observation, might have contributed to the non-significant impact of weight gain in the Cox proportional hazards analysis.
There has been increasing interest in a possible differential risk for diabetes among patients taking different antipsychotic medications. When considering case reports involving patients treated with atypical antipsychotics, the largest number are for patients using olanzapine and clozapine (Reference HendersonHenderson, 2002; Reference Jin, Meyer and JesteJin et al, 2002). However, there are now reports of hyperglycaemia or diabetes during treatment with risperidone (Reference Melamed, Mazek and ElizurMelamed et al, 1998; Reference Croarkin, Jacobs and BainCroarkin et al, 2000; Reference Wirshing, Pierre and EyelerWirshing et al, 2001), quetiapine (Reference Sobel, Jaggers and FranzSobel et al, 1999; Reference Procyshyn, Pande and TseProcyshyn et al, 2000) and ziprasidone (Yang et al, 2002). However, because case reports often lack information on family history and additional factors that might affect glucose regulation, are subject to reporting bias and do not have a reference or control group, causal relationships between individual antipsychotics and treatment-emergent diabetes cannot be determined from case reports. Although numbers of case reports regarding specific agents differ, results from several large retrospective cohort analyses have been inconsistent regarding differences in risk of diabetes among users of various antipsychotic medications (Reference Gianfrancesco, Grogg and MahmoudGianfrancesco et al, 2002; Reference Kornegay, Vasilakis-Scaramozza and JickKornegay et al, 2002; Reference Koro, Fedder and L'ItalienKoro et al, 2002; Reference Lage and KemnerLage & Kemner, 2002; Reference Sernyak, Leslie and AlarconSernyak et al, 2002; Reference Wang, Glynn and GanzWang et al, 2002b ; Reference Buse, Cavazzoni and HornbuckleBuse et al, 2003). This retrospective analysis of data from olanzapine clinical trials found that patients treated with olanzapine did not have a significantly greater risk of TED compared with a non-olanzapine cohort whose treatment did not include clozapine. This result is consistent with some reports comparing the relative risk of developing diabetes during treatment with olanzapine v. other antipsychotics (Reference Lage and KemnerLage & Kemner 2002; Reference Smith, Lindenmayer and KhanSmith et al, 2002; Reference Buse, Cavazzoni and HornbuckleBuse et al, 2003), but not with other reports (Reference Caro, Ward and LevintonCaro et al, 2002; Reference Gianfrancesco, Grogg and MahmoudGianfrancesco et al, 2002; Reference Koro, Fedder and L'ItalienKoro et al, 2002; Reference MeyerMeyer, 2002; Reference Newcomer, Haupt and FucetolaNewcomer et al, 2002; Reference Sernyak, Leslie and AlarconSernyak et al, 2002).
All retrospective analyses have inherent limitations, and several limitations specific to the current study warrant discussion. Non-fasting glucose measurements have limited sensitivity for detecting diabetes (American Diabetes Association, 2002; Reference Rolka, Narayan and ThompsonRolka et al, 2001). Consequently, the current analysis probably represents a minimal estimate of the number of cases of TED. Inclusion of the UGT post-randomisation category may ameliorate this limitation to some extent in terms of the descriptive findings; however, without definitive diagnostic testing, limited conclusions regarding the true frequency of abnormal glycaemic events can be drawn from this heterogeneous group. It must also be acknowledged that reasonable alternative classification paradigms for identifying patients with TED or UGT could be employed: for example, use of 6.7 mmol/l glucose as the lower limit for UGT (Reference Rolka, Narayan and ThompsonRolka et al, 2001), or exclusion of 27 patients with a single glucose value > 11.1 mmol/l at study entry. In addition, alternative terminology could be applied to the post-randomisation glycaemic categories, as our TED criteria do not strictly meet ADA criteria for diabetes in absence of reported symptoms (American Diabetes Association, 2002). The clinical trials database also lacked information on prior antipsychotic treatment history and a number of important risk factors for diabetes (family history, previous history of impaired glucose tolerance or lipid profile) as these data were not collected in a systematic fashion. Therefore, the risk factor assessment may well represent an underestimate of the true pre-existing risk burden. Furthermore, some of the between-group comparisons for patients receiving different treatments were limited by differences in sample sizes and duration of observation. Finally, a major limitation is that the clinical trials used in this analysis were not intended to assess risk factors for diabetes or to look for treatment-emergent diabetes, and caution is warranted when extrapolating results of this analysis to a more general practice setting. Nevertheless, the clinical trials were randomised and masked, and unlike a number of the retrospective cohort studies noted above, more detailed baseline risk factor information was available for study participants. Retrospective analyses cannot definitively answer all questions regarding a potential link between schizophrenia and diabetes, nor can this type of analysis resolve whether there are subtle differences in risk for diabetes among users of different antipsychotic medications. We hope, however, that the results of this analysis may provide important preliminary information regarding antipsychotic therapy and the relative impact of pre-existing risk factors for diabetes, short-term weight gain and use of olanzapine on the short-term risk of marked glycaemic abnormalities or diabetes.
In summary, results of this retrospective analysis suggest that over the short term (generally less than 1 year's exposure, with a median exposure time of less than 6 months), elevated baseline non-fasting glucose level and presence of multiple risk factors for diabetes appear to have a major impact on the risk of being identified with TED, whereas the impact of treatment-emergent weight gain on short-term TED risk was relatively small and was not statistically significant. Patients treated with olanzapine did not have a significantly greater risk of short-term TED compared with a pooled cohort of patients receiving risperidone, haloperidol and placebo. Overall, the risk factors for diabetes in patients with schizophrenia overlap those in the general population.
Acknowledgements
Appreciation is expressed to Drs Margaret Sowell and Cindy Coe Taylor for. assistance with preparation of the manuscript. Dr Buse has received honoraria, consulting fees and research grants from Eli Lilly, Pfizer and Novartis. Since. October 2001, by institutional policy aimed at minimising potential dualities. of interest in the conduct of clinical trials, these funds are received under. contract with the University of North Carolina School of Medicine and are not. of direct financial benefit to Dr Buse. In 2002, abstracts of this study were. presented at the American Diabetes Association, the Collegium International. Neuro-Psychopharmacologicum, and the Institute on Psychiatric Services annual. meetings.









eLetters
No eLetters have been published for this article.