Generalised anxiety disorder (GAD) is characterised by excessive and uncontrollable worry and central nervous system hyperarousal. 1 Prevalence is 5.7% in epidemiologic surveys. Reference Kessler, Berglund, Demler, Jin and Walters2 GAD is typically chronic, is associated with increased risk for functional and medical disability, Reference Revicki, Travers, Wyrwich, Svedsater, Locklear and Mattera3 and is the most costly of all the anxiety disorders. Reference Revicki, Travers, Wyrwich, Svedsater, Locklear and Mattera3 Although there are empirically supported treatments (pharmacotherapy; cognitive–behavioural therapy), up to half of patients do not benefit Reference Lydiard, Monnier, Heimberg, Turk and Mennin4,Reference Huppert, Sanderson, Stein, Hollander and Rothbaum5 – a finding that highlights the importance of pursuing novel treatments for GAD.
Repetitive transcranial magnetic stimulation (rTMS) is a neuromodulation therapy that has been studied increasingly in recent years as a treatment for a variety of psychiatric disorders. During rTMS a magnetic coil is placed near the scalp to alter the electrical activity of brain regions and associated circuits. High-frequency (>5 Hz) pulses excite and low-frequency (<1 Hz) ones inhibit the adjacent cortex, with more complex activation and network connectivity alterations occurring in more remote brain regions. Reference Tracy, de Sousa de Abreu, Nalesnik, Mao, Lage and Shergill6,Reference Siebner, Hartwigsen, Kassuba and Rothwell7 The original US Food and Drug Administration (FDA) indication was for treatment-resistant major depressive disorder using high-frequency pulses over the left dorsolateral prefrontal cortex (DLPFC). Meta-analytic research supports the efficacy of these stimulation parameters for depression (see for example Berlim et al Reference Berlim, van den Eynde, Tovar-Perdomo and Daskalakis8 ) as well as alternative parameters at the DLPFC, such as low-frequency right-sided stimulations. Reference Berlim, Van den Eynde and Jeff Daskalakis9 Research suggests that anxiety symptoms also improve in patients with major depressive disorder following rTMS. Reference Diefenbach, Bragdon and Goethe10 However, little is known about the use of rTMS to treat anxiety disorders, and no randomised controlled trials (RCTs) have investigated the efficacy of rTMS for GAD.
GAD is characterised by abnormal fronto-limbic circuitry, Reference Mochcovitch, da Rocha Freire, Garcia and Nardi11,Reference Hilbert, Lueken and Beesdo-Baum12 supporting the potential use of rTMS to target this circuit. A variety of symptom provocation tasks have been used to study functional brain activity in GAD. For example, worry, the hallmark symptom of GAD, is associated with increased activation in the prefrontal cortex (PFC), and decreased activation in amygdala, in both patients with GAD and healthy controls; however, this worry-related neural activity continues only in patients with GAD even after the worry-induction period has ended. Reference Paulesu, Sambugaro, Torti, Danelli, Ferri and Scialfa13
The precise biological mechanism by which rTMS improves psychiatric symptoms remains poorly understood. It is possible that there is a direct effect on neural networks thereby improving emotion regulation processes. For example, in healthy volunteers, DLPFC stimulation alters activation of, and functional connectivity between, the DLPFC and ventromedial PFC (VMPFC) during emotional decision-making. Reference Baumgartner, Knoch, Hotz, Eisenegger and Fehr14 Additional proposed biological mechanisms include epiphenomena such as normalisation of neuroendocrine and/or neurotrophic factors, Reference Baeken and De Raedt15 which have also been shown to change via DLPFC neuromodulation in healthy volunteers (see for example Baeken et al Reference Baeken, Vanderhasselt and De Raedt16 ). To date there has been only one published open trial of rTMS for treating GAD. Reference Bystritsky, Kaplan, Feusner, Kerwin, Wadekar and Burock17 Ten patients completed six sessions (twice weekly for 3 weeks) of low-frequency rTMS at the right DLPFC. Functional neuroimaging during a gambling decision-making task was used to select the DLPFC stimulation site, and right DLPFC activation was evident for all ten participants. At post-treatment, 60% of the patients met criteria for remission (Hamilton Rating Scale for Depression <8 and Clinical Global Impression-Improvement ⩽2). Reference Bystritsky, Kaplan, Feusner, Kerwin, Wadekar and Burock17 Results were largely maintained at 6-month follow-up. Reference Bystritsky, Kerwin and Feusner18 Although these outcomes are encouraging, only limited conclusions can be drawn given the absence of a control group. The current study is the first RCT for rTMS treatment for GAD (clinicaltrials.gov: NCT01659736). Low-frequency stimulation was targeted at the right DLPFC, located using structural neuronavigation. The number and timing of treatment sessions was chosen to align with the FDA-approved protocol for depression to protect against null effect because of inadequate dose. It was predicted that for patients receiving active rTMS there would be evidence of higher rates of treatment response and remission and larger improvements in anxiety, worry and depressive symptoms compared with patients undergoing placebo rTMS (using a ‘sham’ coil). In addition this study sought to explore the impact of neuromodulation on DLPFC activation and its association with symptom change. A leading cognitive theory suggests that GAD is characterised by intolerance of uncertainty – wherein uncertain or ambiguous situations create intense emotional responses that precipitate worry. Reference Dugas, Gagnon, Ladouceur and Freeston19 Based upon this theory we chose to study neuroactivation in GAD under conditions of stressful uncertainty using a gambling decision-making task. This task was adapted from the task employed in previous rTMS GAD research, where it was shown to reliably activate DLPFC. Reference Bystritsky, Kaplan, Feusner, Kerwin, Wadekar and Burock17 It was hypothesised that there would be a larger change in DLPFC activations in the active v. sham group and that this change in DLPFC activation would correlate significantly with symptom improvements.
Method
Participants
Participants were adults (age ⩾18) diagnosed with GAD as the principal or coprincipal disorder of at least moderate severity (Clinical Global Impression – severity scale (CGI-S) ⩾4). Reference Guy20 Additional symptom inclusion criteria were similar to those used in the previous open trial of rTMS for GAD: Reference Bystritsky, Kaplan, Feusner, Kerwin, Wadekar and Burock17 the Hamilton Rating Scale for Anxiety (HRSA) Reference Shear, Vander Bilt, Rucci, Endicott, Lydiard and Otto21 ⩾18 and 17-item Hamilton Rating Scale for Depression (HRSD) Reference Hamilton22 ⩽17. Exclusion criteria were brain trauma or disorder; serious and/or unstable medical illness (for example cardiac disease, thyroid disease); post-traumatic stress disorder (current); substance use disorder (past 6 months); lifetime bipolar, psychotic, developmental or obsessive–compulsive disorder; judged too psychiatrically unstable to participate (for example acute suicidality); any contraindication for magnetic resonance imaging (MRI, such as metal in the body) and/or rTMS (such as history of epilepsy); and concurrent psychotherapy. Concurrent pharmacotherapy was stabilised (type and dose) for 3 months prior to study entry with the exception of benzodiazepines as needed, which were stabilised on a daily dose for at least 2 weeks (based on the medication half-life). Patients were required to keep medication use stable throughout the treatment phase of the study and weekly assessments via interview confirmed that all patients adhered to this requirement.
Participants were recruited through an out-patient clinic specialising in the treatment of anxiety and related disorders, as well as newspaper advertisements, internet (for example Google ads, Craigslist, clinicaltrials.gov), community flyers, physician referral and media coverage. The study CONSORT diagram is presented in online Fig. DS1. Of the 34 patients who met study entry criteria, 8 withdrew prior to randomisation. Participants who withdrew prior to randomisation did not differ from those randomised on any pre-treatment clinical or demographic variable (all Ps >0.05). Of the 26 patients randomised to treatment, 14 were allocated to active rTMS and 12 to sham. However, data were excluded when patients adhered to the treatment schedule for fewer than 25 consecutive treatment sessions (i.e. there was a gap ⩾7 days in treatments sessions between sessions 6 and 26) resulting in exclusion of data from one patient (active). Thus, the final sample used in data analyses included 13 in the active and 12 in the sham group.
Measures
Diagnostic status was determined using the Mini-International Neuropsychiatric Interview (MINI). Reference Sheehan, Lecrubier, Sheehan, Amorim, Janavs and Weiller23 Principal/coprincipal diagnoses were determined using the Clinician's Severity Rating (CSR) with a 0 to 8 scale rated from ‘absent’ to ‘very severe’. Reference Brown, Di Nardo, Lehman and Campbell24 The CGI-S was used to assess global illness (1, normal, not at all ill to 7, extremely ill). The CGI – improvement scale (CGI-I) Reference Guy20 was rated from 1 very much improved to 7, very much worse.
Primary outcome measure
The HRSA was the primary outcome measure and the structured interview guide was used. Reference Shear, Vander Bilt, Rucci, Endicott, Lydiard and Otto21 Responder status was defined as ⩾50% HRSA improvement, and remission total HRSA <8 and a CGI-I score of 1 (very much improved) or 2 (much improved). These response and remission criteria were used in a previous study of rTMS for GAD. Reference Bystritsky, Kaplan, Feusner, Kerwin, Wadekar and Burock17
Secondary outcome measures
Self-reported worry was assessed using the Penn State Worry Questionnaire (PSWQ) Reference Meyer, Miller, Metzger and Borkovec25 and depression using the Depression Anxiety Stress Scales-Depression Subscale (DASS-DEP). Reference Lovibond and Lovibond26 Clinician-rated depression was assessed using the structured interview guide version of the 17-item HRSD. Reference Williams27
Treatment
rTMS
Treatment was administered using the FDA-Cleared Neurostar TMS Therapy System, but applied non-FDA-approved location and intensity parameters for a non-FDA approved indication. rTMS was delivered at a frequency of 1 Hz for 15 min (900 pulses/session) with the intensity at 90% of the resting motor threshold, to the right DLPFC, for 30 sessions (5 days/week for 6 weeks; 27 000 total pulses).
Sham
Participants receiving sham rTMS completed the same procedures as those in the active rTMS group, but treatments were administered using the Neuronetics XPLOR coil. This sham coil looks and sounds like the active coil to preserve the double-masking, but the intensity of the magnetic stimulus is far below the level needed to produce clinical benefit.
Structural MRI and neuronavigation
We obtained anatomical MRI brain images using a Siemens 3T Allegra MRI machine. T 1-weighted brain structure images were collected using a 3D MPRAGE pulse sequence (repetition time (TR) = 2300 ms, echo time (TE) = 2.74 ms, inversion time (TI) = 900 ms, flip angle 8°, field of view (FOV) = 176 × 256 mm, matrix 176 × 256 × 176, voxel size 1 × 1 × 1 mm, pixel bandwidth 190 Hz; total scan time 7 min 37 s).
The right DLPFC target for all patients was identified based on Montreal Neurological Institute (MNI) coordinates for the right DLPFC target (x = 42, y = 36, z = 32) provided by Bystritsky and colleagues Reference Bystritsky, Kaplan, Feusner, Kerwin, Wadekar and Burock17 as the mean pre-treatment peak voxel from their group functional-MRI (fMRI) data in patients with GAD. A high-resolution single-subject structural scan in MNI space (Colin-27 template, ch2.nii, voxel size 1 × 1 × 1 mm), along with a 3 mm radius target sphere image cantered at the right DLPFC MNI coordinate were non-rigidly coregistered to the patient's T 1 image in native space using the SPM8 normalise function. The normalise function thereby transformed the right DLPFC target sphere from its location in MNI space, to its corresponding location in patient native space.
A frameless stereotactic neuronavigation system (visor2, ANT Neuro, Enschede, Netherlands; http://www.ant-neuro.com) was used to guide the coil to the patient's right DLPFC brain target. The neuronavigation system was comprised of visor2 software running on a laptop computer (EliteBook 8560w, Hewlett Packard, Palo Alto, California, USA) connected to an infrared positioning camera (NDI, Waterloo, Ontario, Canada).
fMRI task
The fMRI task was adapted from a gambling task designed to induce anxiety related to uncertainty during decision-making. Reference Bystritsky, Kaplan, Feusner, Kerwin, Wadekar and Burock17 In the current study patients were presented with two cards – red v. blue – and instructed to ‘look for a pattern’ and predict which card would be drawn next. Patients were given 50 points and told that they could win or lose points (2 points per trial) based upon correct or incorrect predictions. No monetary value was associated with point wins or losses. Unknown to the participants, trials were presented in two blocks: win blocks (75% of the time the patient's choice is correct) and lose blocks (75% of the time the patient's choice is incorrect). There were eight events in each block and six blocks of each condition. Given the set win/lose parameters, all patients ended with a total loss of 16 points. fMRI analyses were conducted on the completers sample (n = 9 active, n = 10 sham), and excluded three additional patients in sham (n = 2 ended the MRI prematurely; n = 1 had incidental findings on structural MRI with no clinical manifestation). Thus, fMRI data were analysed for n = 9 in the active group and n = 7 in the sham group.
Blood oxygenation level-dependent (BOLD) contrast was obtained with T 2*-weighted echo planar imaging (EPI) sequence (TR = 1860 ms, TE = 27 msec, flip angle 70, FOV 22 cm, 64 × 64 acquisition matrix) with 36 contiguous axial functional slices of 3 mm thickness with 1 mm gap, yielding 3.4 × 3.4 × 4 mm voxels.
Procedure
Study procedures were approved by the Hartford Hospital Institutional Review Board (DIEF003523HI) and all patients gave written informed consent prior to participation. The CONSORT checklist is provided in online Fig. DS2. A licensed clinical psychologist completed masked assessments. Clinician and self-reports were collected at pre-, post- and 3-month follow-up, with a subset of measures collected weekly (sessions 1, 6, 11, 16, 21 and 30). The study design initially included a 6-month follow-up assessment, however, a new study was initiated part-way through the current trial offering active treatment for sham non-responders after the 3-month follow-up (clinicaltrials.gov: NCT01815099). Given that 6-month follow-up data were collected for only a subset of participants, 3-month follow-up was used as the end-point in the current analyses. Patients completed MRI at pre- and post-treatment. Adverse events were assessed using a checklist at each visit during the first 2 weeks of treatment, and weekly thereafter. Adverse events spontaneously reported were also recorded. In this parallel-group design, patients were randomised (1:1 ratio) using a computerised random number generator in groups of 10 for the first 20 participants. Once 20 participants were enrolled, a randomisation schedule was created to replace for attrition. Sample size was set a priori as 10 participants per group based upon feasibility for pilot study data collection. Initially patients were not going to be replaced for attrition; however, given the high drop-out rate the protocol was revised to replace for patient attrition. A licensed clinical psychologist who had no direct patient contact developed and held the randomisation schedule. The schedule was shared only with the rTMS technician responsible for coil selection. Thus, the treating psychiatrist, evaluator and patients were not informed of treatment condition assignment.
Data analytic plan
Clinical outcomes
Baseline demographic and clinical characteristics were compared by treatment group using between-group t-tests. Frequency counts of response and remission status were compared using chi-square. These analyses were conducted for treatment completers (n = 9/13 and n = 10/12 for active and sham, respectively) as well as an intent-to-treat (ITT) sample (n = 13 active and n = 12 sham) including participants who had attended at least one rTMS session. Repeated measures analysis of variance (ANOVA) was used to determine changes in primary and secondary outcome measures. For these analyses a series of two group (active v. sham) × three time (pre-, post-, follow-up) ANOVAS were conducted for the ITT sample after using multiple imputation procedures Reference Rubin28 to replace missing data. We conducted ANOVAs for the completers sample as well. Results from the completers analyses differed from the ITT analyses primarily on the number of analyses reaching statistical significance, presumably because of lower power. For parsimony, only the ITT analyses are reported here. The completers analyses are available in the online supplement (Supplement DS1 and Tables DS1 and DS2). The primary result of interest is the group × time interaction. Statistically significant interactions were followed by within-group paired t-tests (pre-to-post, pre-to-follow-up) and effect sizes (Cohen's d, interpreted as 0.30 small; 0.50 medium; and 0.80 large). Reference Cohen29 Given that this is a pilot study with small samples, statistical trends (P<0.10) are also reported for hypothesis generation purposes. The frequencies of adverse events were compared using chi-square. For all chi-square analyses, results with at least one cell with n<5 participants should be interpreted cautiously.
Imaging data analysis
The fMRI data were processed using SPM8, including motion correction using the INRIAlign toolbox, normalisation to MNI template and smoothing (5 mm3 full-width at half maximum (FWHM) Gaussian kernel). Data were then analysed using a general linear model (GLM) approach. For each individual, the win and lose blocks were modelled as separate regressors. However, based on the previous results, Reference Bystritsky, Kaplan, Feusner, Kerwin, Wadekar and Burock17 a contrast defining both regressors as main effect (compared with baseline) was defined. Individual contrast images were entered into a group (active v. sham) × time (pre- v. post-treatment) repeated measure ANOVA to assess a group × time interaction. Since this report is focused on the activation change in the stimulation site, group results were masked with a customised right DLPFC BrainMap volume-of-interest (thresholded at 25%). Reference Nielsen and Hansen30
Results
Pre-treatment characteristics and attrition
Pre-treatment demographic and clinical characteristics for the ITT sample are presented in Table 1. The two groups were matched well on demographic variables and most clinical variables; however, patients randomly assigned to the active group presented with more severe anxiety and worry. One-third of patients randomised to the active group and one-fifth of those assigned to the sham group discontinued the study prior to completing 30 sessions. This difference was not statistically significant (χ2(1,n = 25) = 0.68, P = 0.409). Those patients who dropped out did not differ from those who completed treatment on any pre-treatment demographic or clinical variables. In addition, within the completers sample, the active and sham groups did not differ on pre-treatment demographic or clinical variables (online Table DS1).
Table 1 Demographic and clinical characteristics by group for intent-to-treat sample
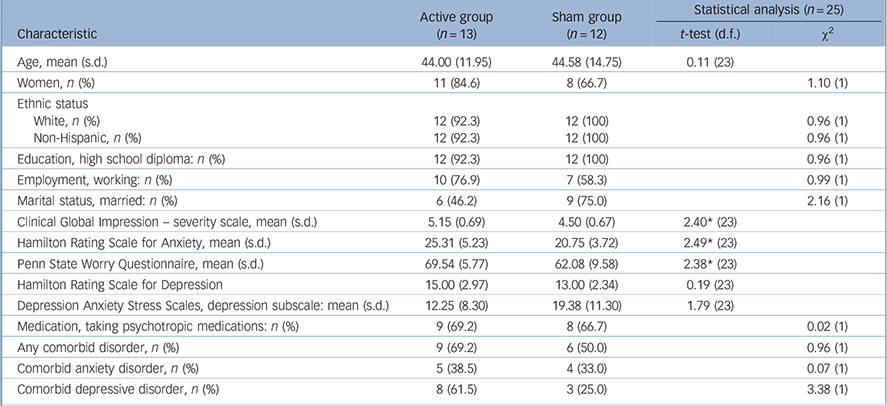
| Active group (n = 13) |
Sham group (n = 12) |
Statistical analysis (n = 25) | ||
|---|---|---|---|---|
| Characteristic | t-test (d.f.) | χ2 | ||
| Age, mean (s.d.) | 44.00 (11.95) | 44.58 (14.75) | 0.11 (23) | |
| Women, n (%) | 11 (84.6) | 8 (66.7) | 1.10 (1) | |
| Ethnic status | ||||
| White, n (%) | 12 (92.3) | 12 (100) | 0.96 (1) | |
| Non-Hispanic, n (%) | 12 (92.3) | 12 (100) | 0.96 (1) | |
| Education, high school diploma: n (%) | 12 (92.3) | 12 (100) | 0.96 (1) | |
| Employment, working: n (%) | 10 (76.9) | 7 (58.3) | 0.99 (1) | |
| Marital status, married: n (%) | 6 (46.2) | 9 (75.0) | 2.16 (1) | |
| Clinical Global Impression – severity scale, mean (s.d.) | 5.15 (0.69) | 4.50 (0.67) | 2.40* (23) | |
| Hamilton Rating Scale for Anxiety, mean (s.d.) | 25.31 (5.23) | 20.75 (3.72) | 2.49* (23) | |
| Penn State Worry Questionnaire, mean (s.d.) | 69.54 (5.77) | 62.08 (9.58) | 2.38* (23) | |
| Hamilton Rating Scale for Depression | 15.00 (2.97) | 13.00 (2.34) | 0.19 (23) | |
| Depression Anxiety Stress Scales, depression subscale: mean (s.d.) | 12.25 (8.30) | 19.38 (11.30) | 1.79 (23) | |
| Medication, taking psychotropic medications: n (%) | 9 (69.2) | 8 (66.7) | 0.02 (1) | |
| Any comorbid disorder, n (%) | 9 (69.2) | 6 (50.0) | 0.96 (1) | |
| Comorbid anxiety disorder, n (%) | 5 (38.5) | 4 (33.0) | 0.07 (1) | |
| Comorbid depressive disorder, n (%) | 8 (61.5) | 3 (25.0) | 3.38 (1) | |
* P<0.05.
Responder and remitter status
Participants who completed treatment
At post-treatment significantly more patients met responder status in the active (7/9, 77.8%) v. the sham group (2/10, 20.0%) (χ2(1,n = 19) = 6.34, P = 0.012). A similar pattern emerged for remitter status at post-treatment (active group = 3/9, 33.3%, sham group 1/10, 10.0%); however, this difference was not statistically significant (χ2(1,n = 19) = 1.55, P = 0.213). At 3-month follow-up there were significantly more responders (χ2(1,n = 18) = 11.46, P = 0.001) and remitters (χ2(1,n = 18) = 9.00, P = 0.003) in the active (7/9, 77.8% responders; 6/9, 67.7% remitters) v. the sham group (0/10, 0% responders; 0/9, 0% remitters).
Intent-to-treat analysis
In the ITT analysis (using the last available assessment as end-point) response rates were significantly higher in the active v. sham group (active 8/13, 61.5%, sham group 2/12, 16.7%; χ2(1,n = 25) = 5.24, P = 0.022) at post-treatment. However, remitter rates did not differ significantly in the active (4/13, 30.8%) v. sham group (1/12, 8.3%) (χ2(1,n = 25) = 1.96, P = 0.161) at post-treatment. At 3-month follow-up there were significantly more responders in the active (8/13, 61.5%) v. the sham group (0/12, 0%) (χ2(1,n = 25) = 10.86, P = 0.001) as well as significantly more remitters in the active (7/13, 53.8%) v. the sham group (0/12, 0%) (χ2(1,n = 25) = 8.97, P = 0.003).
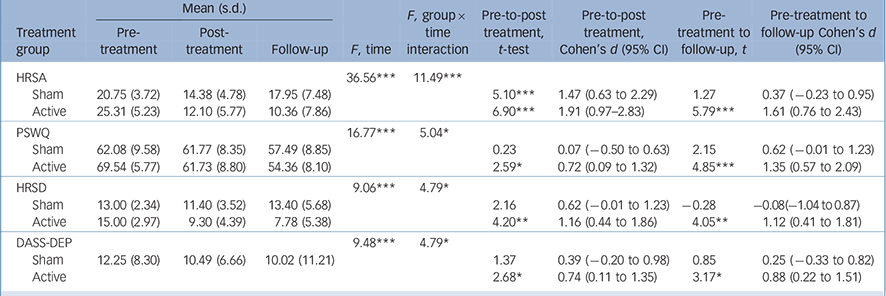
Primary and secondary outcomes
Results for the primary and secondary outcomes in the ITT sample are presented in Table 2. Planned contrasts showed that for the HRSA, patients in both the active and sham groups experienced large and statistically significant improvements at post-treatment, but these gains were maintained only in the active group at follow-up. For all secondary symptom variables, only the active group demonstrated statistically significant improvements at post-treatment and follow-up assessments. Effect sizes for secondary symptoms ranged from moderate to large in the active and negligible to moderate in the sham group.
Table 2 Intent-to-treat omnibus tests and planned contrasts for primary and secondary outcomes a
| Mean (s.d.) |
F, group × time interaction |
Pre-to-post treatment, t-test |
Pre-to-post treatment, Cohen's d (95% CI) |
Pre- treatment to follow-up, t |
Pre-treatment to follow-up Cohen's d (95% CI) |
||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment group |
Pre- treatment |
Post- treatment |
Follow-up | F, time | |||||
| HRSA | 36.56*** | 11.49*** | |||||||
| Sham | 20.75 (3.72) | 14.38 (4.78) | 17.95 (7.48) | 5.10*** | 1.47 (0.63 to 2.29) | 1.27 | 0.37 (−0.23 to 0.95) | ||
| Active | 25.31 (5.23) | 12.10 (5.77) | 10.36 (7.86) | 6.90*** | 1.91 (0.97–2.83) | 5.79*** | 1.61 (0.76 to 2.43) | ||
| PSWQ | 16.77*** | 5.04* | |||||||
| Sham | 62.08 (9.58) | 61.77 (8.35) | 57.49 (8.85) | 0.23 | 0.07 (−0.50 to 0.63) | 2.15 | 0.62 (−0.01 to 1.23) | ||
| Active | 69.54 (5.77) | 61.73 (8.80) | 54.36 (8.10) | 2.59* | 0.72 (0.09 to 1.32) | 4.85*** | 1.35 (0.57 to 2.09) | ||
| HRSD | 9.06*** | 4.79* | |||||||
| Sham | 13.00 (2.34) | 11.40 (3.52) | 13.40 (5.68) | 2.16 | 0.62 (−0.01 to 1.23) | −0.28 | −0.08(−1.04 to 0.87) | ||
| Active | 15.00 (2.97) | 9.30 (4.39) | 7.78 (5.38) | 4.20** | 1.16 (0.44 to 1.86) | 4.05** | 1.12 (0.41 to 1.81) | ||
| DASS-DEP | 9.48*** | 4.79* | |||||||
| Sham | 12.25 (8.30) | 10.49 (6.66) | 10.02 (11.21) | 1.37 | 0.39 (−0.20 to 0.98) | 0.85 | 0.25 (−0.33 to 0.82) | ||
| Active | 2.68* | 0.74 (0.11 to 1.35) | 3.17* | 0.88 (0.22 to 1.51) | |||||
HRSA, Hamilton Anxiety Rating Scale; PSWQ, Penn State Worry Questionnaire; HRSD, Hamilton Rating Scale for Depression; DASS-DEP, Depression Anxiety Stress Scales Depression Subscale.
a. Active group n = 13; Sham group n = 12. All analyses were computed using multiple imputation.
* P<0.05,
** P<0.01,
*** P<0.001.
Adverse events
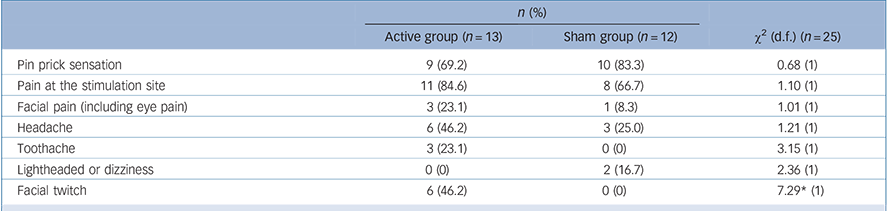
The frequency of adverse events was similar in the active and sham groups, with pin prick or pain at the stimulation site being the most commonly reported events (Table 3). The only statistically significant difference between groups was the presence of facial twitch. One serious adverse event occurred when a patient in the active group was admitted to hospital for evaluation of chest pain; however, the event was determined to be unrelated to the study intervention.
Table 3 Frequency of patients reporting adverse events at any time point
| n (%) | |||
|---|---|---|---|
| Active group (n = 13) | Sham group (n = 12) | χ2 (d.f.) (n = 25) | |
| Pin prick sensation | 9 (69.2) | 10 (83.3) | 0.68 (1) |
| Pain at the stimulation site | 11 (84.6) | 8 (66.7) | 1.10 (1) |
| Facial pain (including eye pain) | 3 (23.1) | 1 (8.3) | 1.01 (1) |
| Headache | 6 (46.2) | 3 (25.0) | 1.21 (1) |
| Toothache | 3 (23.1) | 0 (0) | 3.15 (1) |
| Lightheaded or dizziness | 0 (0) | 2 (16.7) | 2.36 (1) |
| Facial twitch | 6 (46.2) | 0 (0) | 7.29* (1) |
* P<0.01.
fMRI
A repeated measures ANOVA demonstrated a significant group × time interaction in the right DLPFC (x = 42, y = 41, z = 25; F (1,56) = 8.07, P = 0.006; online Fig. DS3) such that activation in this region significantly increased after active treatment (t(1,8) = −3.65, P = 0.006) and tended to decrease after sham treatment (t(1,6) = 2.104, P = 0.08). Moreover, the changes in right DLPFC activation correlated significantly with changes in worry symptoms (PSWQ, r = −0.55, P = 0.027) and tended to correlate with anxiety symptoms (HRSA, r = 70.47, P = 0.067) such that greater symptom improvement was associated with greater increases in right DLPFC activation from pre- to post-treatment. Changes in right DLPFC activations were not associated with changes in depressive symptoms (HRSD, r = −0.42, P = 0.103; DASS-DEP r = −0.23, P = 0.391).
Discussion
Main finding and interpretation
Results from this first RCT of neuromodulation in GAD provide preliminary evidence for the efficacy of rTMS. Pre-to-post-treatment effect sizes for symptom changes were uniformly larger in the active v. sham group. This interaction reached statistical significance for anxiety, worry and depressive symptoms. Response and remission rates were also higher in the active v. sham group. Only one prior open trial investigated rTMS in GAD; Reference Bystritsky, Kaplan, Feusner, Kerwin, Wadekar and Burock17 the outcomes of that study (i.e. high response rates and large pre-to-post anxiolytic effects) were similar to those of the current study. The same stimulation parameters were used (low-frequency right DLPFC), however, the current protocol entailed a higher number and frequency of rTMS sessions culminating in a net gain of 21 600 total pulses. As of yet the optimal treatment parameters for GAD are not known. In the current study, the intense treatment schedule was a common reason for refusal and withdrawal, and many enrolled participants experienced difficulty complying with the schedule. It will be important to identify efficacious dosing schedules that are more acceptable and feasible, such as accelerated rTMS administered over a course of a few days (see for example McGirr et al Reference McGirr, Van den Eynde, Tovar-Perdomo, Fleck and Berlim31 ).
The optimal stimulation target for treating GAD is also unknown. Support for the DLPFC comes from anxiolytic effects of rTMS in patients with major depressive disorder Reference Diefenbach, Bragdon and Goethe10 and changes in anxiety-related biological processes in healthy controls (see for example Baeken et al Reference Baeken, Vanderhasselt and De Raedt16 ). The emotion dysregulation model of GAD provides a theoretical rationale for DLPFC stimulation. Emotion regulation is the process of identifying and altering emotional experiences, and GAD patients demonstrate problems with these skills. Reference Mennin, Heimberg, Turk and Fresco32 The DLPFC plays a central role in emotion regulation processes via its connections with cortical and subcortical regions (for example dorsal anterior cingulate cortex, inferior frontal gyrus, ventral anterior cingulate cortex, VMPFC). In particular the connection with the VMPFC may mediate DLPFC stimulation and limbic activations. Reference Diekhof, Geier, Falkai and Gruber33 Neuromodulation of the DLPFC may therefore improve emotion regulation of anxiety by having an impact on the functioning of and/or communication within these frontolimbic networks.
In the current study a treatment course of low-frequency (inhibitory) stimulation of the right DLPFC was associated with increased activation in the target site during decision-making and neural activation changes were associated with changes in worry. While engaging in emotion regulation tasks, patients with GAD demonstrate hypoactivation in the PFC as well as the anterior cingulate cortex and decreased structural and functional connectivity between frontal and limbic regions. Reference Mochcovitch, da Rocha Freire, Garcia and Nardi11 These abnormalities may reflect deficient neurobiological ‘top–down’ emotional control. Given that these abnormalities are characterised in part by DLPFC hypoactivation in patients with GAD, Reference Ball, Ramsawh, Campbell-Sills, Paulus and Stein34 results from the current study are suggestive of DLPFC normalisation over treatment, which enhances top–down regulation over prefrontal and limbic areas. Improvements in GAD symptoms following pharmacotherapy or counselling also demonstrate normalisation in DLPFC activation, Reference Hoehn-Saric, Schlund and Wong35 as well as improved connectivity between the DLPFC and other prefrontal regions Reference Andreescu, Sheu, Tudorascu, Gross, Walker and Banihashemi36 and between the PFC and amygdala. Reference Maslowsky, Mogg, Bradley, McClure-Tone, Ernst and Pine37 Connectivity analyses of data from the current study are in process and may further elucidate the effect of rTMS treatment on GAD neurocircuitry.
Strengths and limitations
The current RCT is a substantial advancement and critical step toward empirically supporting rTMS for GAD. However, results should be considered preliminary because of the small sample sizes. Attrition rates were also higher than those in rTMS trials for major depressive disorder Reference Berlim, van den Eynde, Tovar-Perdomo and Daskalakis8 but in-line with pharmacotherapy trials in GAD. Reference Mitte38 Adverse events were largely similar between treatment conditions; however, facial twitch was more common in the active group. It will be important for future research to minimise this potential threat to unmasking (for example, by using a protocol to prevent disclosure of facial twitch to evaluators). In addition, the randomisation schedule did not equally distribute anxiety symptoms, with those patients with more severe anxiety being allocated to active treatment. However, the treatment effect in active rTMS was not consistent with a regression to the mean interpretation, as patients who benefitted typically reported symptoms within or close to remission. Although active treatment was superior to sham, there was a large acute anxiolytic effect in sham as well. Individuals with GAD are prone to placebo response, Reference Khan, Kolts, Rapaport, Krishnan, Brodhead and Browns39 and the effect size in the current study is consistent with the large placebo effect for neuromodulation found in patients with major depressive disorder. Reference Brunoni, Lopes, Kaptchuk and Fregni40 Importantly, improvements in the sham group were not maintained whereas patients receiving active rTMS tended to maintain or improve over follow-up. In a previous open trial rTMS outcomes were maintained over 6 months. Reference Bystritsky, Kerwin and Feusner18 However, long-term durability is not known and future research will need to investigate relapse risk and the potential use of maintenance rTMS as is often done clinically for patients with major depressive disorder.
It will also be important for future research to establish rTMS mechanisms of action. Data from the current study are informative as the first to report on neurobiological changes following rTMS treatment for GAD. However, the biological process underlying the mechanism by which inhibitory stimulation led to increased DLPFC activation during decision-making is not clear. It is hypothesised that excessive inhibition within the frontolimbic network is subsequently normalised after treatment, but this is purely speculative. It will also be important to explore the biological mechanisms of anxiety improvements following rTMS such as normalisation of neuroendocrine, neurotransmitter and/or neurotrophic factors. Reference Baeken and De Raedt15 This research will be facilitated by investigating the impact and predictors of neuromodulation on transdiagnostic biological and behavioural constructs consistent with the National Institute of Mental Health Research Domain Criteria initiative. Reference Cuthbert41 Such efforts will provide the foundation for more personalised and targeted neuromodulation treatments in the future.
Funding
This study was funded by a grant (number: 129522) from the Hartford HealthCare Research Funding Initiative to G.J.D. The funding source had no role in the study design; collection, analysis, interpretation of data; writing the report, or in making the decision to submit the article for publication. Material support was provided by Neuronetics. Neuronetics reviewed a draft of this report prior to submission and otherwise had no role in the study design; collection, analysis, interpretation of data; writing the report, or in making the decision to submit the article for publication.
Acknowledgements
We thank Joost van Ginkel for providing an SPSS macro and providing consultation on multiple imputation procedures.






eLetters
No eLetters have been published for this article.