Deviant neuropsychological performance of subjects symptomatically at risk of first-episode psychosis has been shown in several domains including verbal memory and executive functions, sustained attention, processing speed and possibly spatial working memory (Reference Carr, Halpin and LauCarr et al, 2000; Reference Wood, Pantelis and ProffittWood et al, 2003; Reference Hawkins, Addington and KeefeHawkins et al, 2004; Reference Brewer, Francey and WoodBrewer et al, 2005; Reference Francey, Jackson and PhillipsFrancey et al, 2005; Reference Niendam, Bearden and JohnsonNiendam et al, 2006; Reference Pukrop, Schultze-Lutter and RuhrmannPukrop et al, 2006; Reference Simon, Dvorsky and BoeschSimon et al, 2006). Furthermore, subtle, self-experienced cognitive–perceptive disturbances have been shown to be predictive of later schizophrenia, and to be common within the psychotic spectrum (Klosterkötter et al, Reference Klosterkötter, Ebel and Schultze-Lutter1996, Reference Klosterkötter, Hellmich and Steinmeyer2001). These subjective disturbances were suggested to characterise an even earlier state of the initial psychosis prodrome (Reference Ruhrmann, Schultze-Lutter and KlosterkötterRuhrmann et al, 2003) when compared to the symptomatic ‘ultra-high risk’ criteria (Reference Phillips, Yung and McGorryPhillips et al, 2000) especially developed to depict an imminent risk of psychosis. Yet, little is known about the possible association between subjective and objective cognitive disturbances and their relation to different prodromal states.
METHOD
Inclusion and exclusion criteria
A two-stage conceptualisation of the prodromal state was employed (Reference Ruhrmann, Schultze-Lutter and KlosterkötterRuhrmann et al, 2003) distinguishing an early initial prodromal state with a clearly increased, but not yet imminent, risk of psychosis from a late initial prodromal state with a somewhat imminent risk of psychosis.
An early initial prodromal state was defined by the presence of at least any one of the cognitive–perceptive basic symptoms found predictive for the development of schizophrenia in the Cologne Early Recognition study (Reference Klosterkötter, Hellmich and SteinmeyerKlosterkötter et al, 2001) as assessed with the Bonn Scale for the Assessment of Basic Symptoms (BSABS, Reference Gross, Huber and KlosterkötterGross et al, 1987) and, since June 2000, with the Schizophrenia Proneness Instrument, Adult version (SPI–A; Reference Schultze-Lutter, Addington and RuhrmannSchultze-Lutter et al, 2007), respectively: thought interferences, perseveration, pressure or blockages; disturbances of receptive language, decreased ability to discriminate between ideas and perception or fantasy and true memories, unstable ideas of reference, derealisation; visual or acoustic perception disturbances. For inclusion, these symptoms had to occur first at least 12 months earlier and at several times within one of the past 3 months. The presence of late initial prodromal #state-relevant symptoms served as an additional exclusion criterion.
In line with the ultra-high risk criteria (Reference Phillips, Yung and McGorryPhillips et al, 2000), a late initial prodromal state was defined by the presence of at least any one attenuated psychotic symptom (i.e. ideas of reference; odd beliefs or magical thinking; unusual perceptual experiences; odd thinking and speech; suspiciousness or paranoid ideation) with a score of 3–5 on the Structured Interview of Prodromal Syndromes (SIPS; Reference Miller, McGlashan and Lifshey RosenMiller et al, 2002) within the past three months, appearing several times per week for a period of at least 1 week, or the presence of at least one transient, spontaneously resolving psychotic symptom (brief limited intermittent psychotic symptoms, i.e. hallucinations; delusions; formal thought disorder; gross disorganised or catatonic behaviour) with a score of at least 4 for less than 1 week (interval between episodes at least 1 week) as assessed with the Positive and Negative Syndrome Scale (PANSS; Reference Kay, Fiszbein and OplerKay et al, 1987). The presence of early initial prodromal state-relevant basic symptoms did not serve as an exclusion criterion in this group.
Exclusion criteria for both groups were:
-
• current or past diagnosis of any psychotic disorder according to DSM–IV criteria (American Psychiatric Association, 1994)
-
• diagnosis of delirium, dementia, amnestic or other cognitive disorder, mental retardation, psychiatric disorders due to a somatic factor or related to psychotropic substances according to DSM–IV
-
• alcohol or drug abuse within the past 3 months according to DSM–IV
-
• diseases of the central nervous system (inflammatory, traumatic, epileptic).
Participants
One hundred and two subjects seeking help for mental problems at the Early Recognition and Intervention Centre for mental crises (FETZ) between September 1998 and August 2004 and who fulfilled criteria of either an early (n=33) or late initial prodromal state (n=69), gave written informed consent to participate in the study and completed the neuropsychological test battery (see below). The two samples did not differ in terms of their socio-demographic characteristics including premorbid IQ and presence of current non-psychotic DSM–IV axis I disorder (Table 1). At baseline, all participants had never been treated with a neuroleptic medication.
Table 1 Characteristics of sample
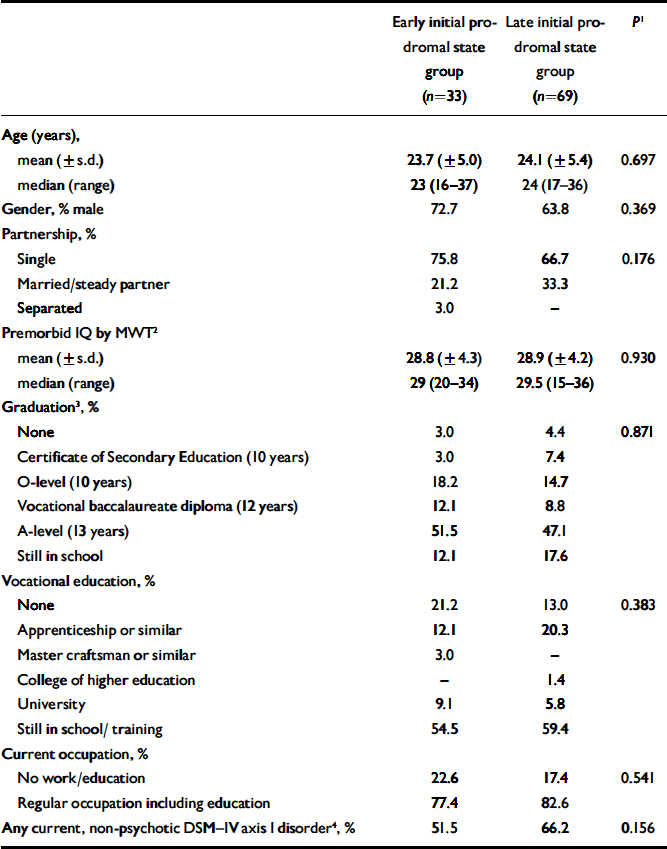
| Early initial prodromal state group (n=33) | Late initial prodromal state group (n=69) | P 1 | |
|---|---|---|---|
| Age (years), | |||
| mean (±s.d.) | 23.7 (±5.0) | 24.1 (±5.4) | 0.697 |
| median (range) | 23 (16-37) | 24 (17-36) | |
| Gender, % male | 72.7 | 63.8 | 0.369 |
| Partnership, % | |||
| Single | 75.8 | 66.7 | 0.176 |
| Married/steady partner | 21.2 | 33.3 | |
| Separated | 3.0 | — | |
| Premorbid IQ by MWT2 | |||
| mean (±s.d.) | 28.8 (±4.3) | 28.9 (±4.2) | 0.930 |
| median (range) | 29 (20-34) | 29.5 (15-36) | |
| Graduation3, % | |||
| None | 3.0 | 4.4 | 0.871 |
| Certificate of Secondary Education (10 years) | 3.0 | 7.4 | |
| O-level (10 years) | 18.2 | 14.7 | |
| Vocational baccalaureate diploma (12 years) | 12.1 | 8.8 | |
| A-level (13 years) | 51.5 | 47.1 | |
| Still in school | 12.1 | 17.6 | |
| Vocational education, % | |||
| None | 21.2 | 13.0 | 0.383 |
| Apprenticeship or similar | 12.1 | 20.3 | |
| Master craftsman or similar | 3.0 | — | |
| College of higher education | — | 1.4 | |
| University | 9.1 | 5.8 | |
| Still in school/training | 54.5 | 59.4 | |
| Current occupation, % | |||
| No work/education | 22.6 | 17.4 | 0.541 |
| Regular occupation including education | 77.4 | 82.6 | |
| Any current, non-psychotic DSM—IV axis I disorder4, % | 51.5 | 66.2 | 0.156 |
Instruments
Subjective psychopathology
Subtle, self-experienced, self-reported disturbances in attention, memory functions, thought processes, speech, auditory and visual perception as well as stress tolerance and basic mood that often remain solely in the self-perception of the patient and not evidenced in behaviour, i.e. basic symptoms, were assessed with the ‘Schizophrenia Proneness Instrument, Adult version’ (SPI–A; Reference Schultze-Lutter, Addington and RuhrmannSchultze-Lutter et al, 2007), which consists of six sub-scales. Five of them, i.e. affective–dynamic disturbances, cognitive–attentional impediments, cognitive disturbances, disturbances in experiencing self and surroundings, and perception disturbances, were used for the correlational analyses; the sixth, body perception disturbances, was not included in the analyses as no association between these coenesthetic disturbances and psychological performances was expected. The SPI–A is a semi-structured interview and was conducted by medical or psychological staff members of the FETZ who are well trained and experienced in the assessment of basic symptoms.
Objective neuropsychological measures
The neurocognitive test battery was conducted by fully qualified neuropsychologists and took approximately 2.5 h to complete. Patients were usually tested on 2 successive days in the morning to minimise fatigue.
Pattern recognition. A computerised version of a visual backward masking task with letters F, H, or T as target stimuli and one of four masking conditions, i.e. random dot pattern or letter pattern masking stimulus after short (42.75 ms) or long (104 ms) inter-stimulus intervals, provided a measure of visual information-processing in terms of the number of hits. The session consisted of 3 blocks of 30 trials each, including 6 trials of each masking condition and 6 no-mask control trials presented in random order.
Attention. The Continuous Performance Test (identical pairs version, CPT–IP; Reference CornblattCornblatt, 1996) provided a measure of sustained attention. The signal detection parameter d′ was calculated across 300 trials.
A dual tasking paradigm requiring the simultaneous solution of a visual and auditory task provided a measure of divided attention. In the first session, participants were instructed to pay 80% of their attention to the visual task, and during the second session to pay 80% of their attention to the auditory task. In both sessions the number of correct responses to the auditory task was recorded; correct responses in the second session were chosen for the analyses.
Working memory. The Letter Number Span (Reference Gold, Carpenter and RandolphGold et al, 1997) requires participants to sort letters from numbers within a sequence of alternating letters and numbers read to them, and to separately recall the letters and numbers in ascending orders. As a measure of working memory, during each trial of a computerised version of the Subject Ordered Pointing Task (SOPT, Reference PetridesPetrides, 1995) participants had to point to 1 of 12 objects, and the relative positions of the objects varied randomly across trials. Across 3 sessions of 12 trials the number of errors, i.e. pointing to an object already chosen on a previous trial, was calculated. Within each trial of the Delayed Response Task (Reference Pukrop, Matuschek and RuhrmannPukrop et al, 2003) for spatial working memory, a black dot was presented for 200 ms at 1 of 16 possible positions of a circle followed by two delay conditions (5 s, 15 s). During the delay period, participants had to solve arithmetic distractor tasks, and after the delay they were required to indicate on a touch-sensitive monitor the position of the dot previously presented in order to determine the Eucledian distance to the target.
Memory and learning. The Auditory Verbal Learning Test (AVLT; Reference LezakLezak, 1995) provided a verbal memory measure for immediate recall after one to five learning trials of word lists. The mean number of correct recalls across all five trials entered the analyses. A measure of visual memory was provided by the Rey–Osterrieth Complex Figure Test (ROFT; Reference ReyRey, 1941), calculating the delayed recall performance by a standardised scoring procedure.
Processing speed. The Digit Symbol Test (Reference Kaplan, Fein and MorrisKaplan et al, 1991) and Trail-Making Test A and B (Reference Reitan and WolfsonReitan & Wolfson, 1985) provided measures for the speed of visual information-processing and visuomotor coordination.
Executive functions. The mean percentage of perseverative and non-perseverative errors made in the Wisconsin Card Sorting Test (WCST; Reference Heaton, Chelune and TalleyHeaton et al, 1993) provided a measure of executive functions in terms of set shifting and problem-solving. Verbal executive functions were measured by a verbal fluency task, i.e. the mean sum of five lexical and semantic category tasks.
Data analysis
For reasons of statistical power, the number of comparisons and correlations was limited by using only the five SPI–A sub-syndromes and on one score for each neuropsychological test, i.e. 13 neurocognitive test parameters (see Table 2). To detect differences in subjective and objective cognitive deficits between different stages of the prodrome, group comparison between participants with early and late initial prodromal states were carried out. As SPI–A sub-syndromes are totals of ordinal data and a substantial proportion of neurocognitive data lacked normal distribution, this was generally done by Mann–Whitney tests. Adjustment for multiple testing according to Holm's sequential method (Reference HolmHolm, 1979) was carried out separately across the 13 neurocognitive and 5 psychopathologic comparisons.
Table 2 Correlation of subjective and objective measures in participants at-risk
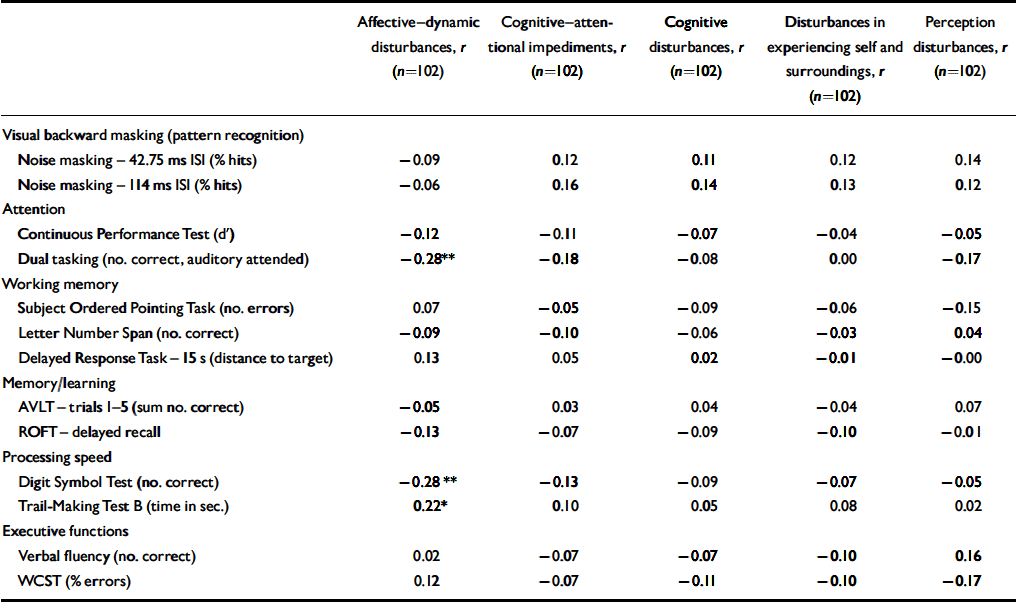
| Affective—dynamic disturbances, r (n=102) | Cognitive—attentional impediments, r (n=102) | Cognitive disturbances, r (n=102) | Disturbances in experiencing self and surroundings, r (n=102) | Perception disturbances, r (n=102) | |
|---|---|---|---|---|---|
| Visual backward masking (pattern recognition) | |||||
| Noise masking — 42.75 ms ISI (% hits) | -0.09 | 0.12 | 0.11 | 0.12 | 0.14 |
| Noise masking — 114 ms ISI (% hits) | -0.06 | 0.16 | 0.14 | 0.13 | 0.12 |
| Attention | |||||
| Continuous Performance Test (d′) | -0.12 | -0.11 | -0.07 | -0.04 | -0.05 |
| Dual tasking (no. correct, auditory attended) | -0.28** | -0.18 | -0.08 | 0.00 | -0.17 |
| Working memory | |||||
| Subject Ordered Pointing Task (no. errors) | 0.07 | -0.05 | -0.09 | -0.06 | -0.15 |
| Letter Number Span (no. correct) | -0.09 | -0.10 | -0.06 | -0.03 | 0.04 |
| Delayed Response Task — 15 s (distance to target) | 0.13 | 0.05 | 0.02 | -0.01 | -0.00 |
| Memory/learning | |||||
| AVLT — trials 1-5 (sum no. correct) | -0.05 | 0.03 | 0.04 | -0.04 | 0.07 |
| ROFT — delayed recall | -0.13 | -0.07 | -0.09 | -0.10 | -0.01 |
| Processing speed | |||||
| Digit Symbol Test (no. correct) | -0.28 ** | -0.13 | -0.09 | -0.07 | -0.05 |
| Trail-Making Test B (time in sec.) | 0.22* | 0.10 | 0.05 | 0.08 | 0.02 |
| Executive functions | |||||
| Verbal fluency (no. correct) | 0.02 | -0.07 | -0.07 | -0.10 | 0.16 |
| WCST (% errors) | 0.12 | -0.07 | -0.11 | -0.10 | -0.17 |
Spearman correlation analyses of subjective and objective data were employed to detect associations between self-reported cognitive disturbances and performance in neurocognitive tests across all participants at risk as well as separately for participants with an early and a late initial prodrome to determine if there were associations specific to one or other of the two groups. Furthermore, to detect potential common factors of subjective and objective cognitive deficits, a factor analysis (principal component analysis with varimax rotation) was performed.
RESULTS
Group comparisons
Participants with an early initial prodrome reported less severe disturbances, therefore participants with an early and those with a late initial prodromal state differed significantly on all SPI–A sub-scales with the exception of perception disturbances, which were the least endorsed of all sub-scales in both groups (Fig. 1). This finding remained even after adjustment for multiple testing with disturbances in experiencing self and surroundings differing most significantly (P(adjusted)=0.00035), followed by cognitive disturbances (P(adjusted)=0.004), affective–dynamic disturbances (P(adjusted)=0.006) and, finally, by cognitive–attentional impediments (P(adjusted)=0.010).
Despite participants with an early initial prodrome performing slightly better than those with a late initial prodrome in every task, the two groups did not differ significantly in performance on the neurocognitive tests except in executive function as assessed by the percentage of perseverative and non-perseverative errors in the WCST. The early initial prodrome group (Fig. 2) had significantly fewer errors. However this difference was no longer significant after adjusting for multiple testing (P(adjusted)=0.286).
Associations between subjective and objective measures
Within the whole at-risk sample, there were a few small but significant correlations of a less than moderate effect size (ρ<0.3; Reference BortzBortz, 1999) between affective–dynamic disturbances and neuropsychological parameters, i.e. divided attention as measured by the dual tasking test and processing speed as measured by both the Digit Symbol Test and Trail-Making Test B (Table 2). Additional analyses at an item level revealed that these correlations were due to self-reported reduced stress tolerance, especially with regard to novel demands (r=–0.314 to −0.256, P=0.001 to 0.011) as well as to working under pressure of time or rapidly changing different demands (r=–0.266 to −0.241, P=0.008 to 0.015). There were correlations between the Digit-Symbol Test and reduced tolerance to social everyday situations (r=–0.272, P=0.006) and between the dual tasking parameter and change in mood (r=–0.227, P=0.008) and decrease in positive emotional responsiveness (r=–0.240, P=0.017). All correlations reflected lower neuropsychological test performance being related to more severe self-reported disturbances. No correlations between neurocognitive measures and subjective cognitive or perceptive disturbances were observed (Table 2).
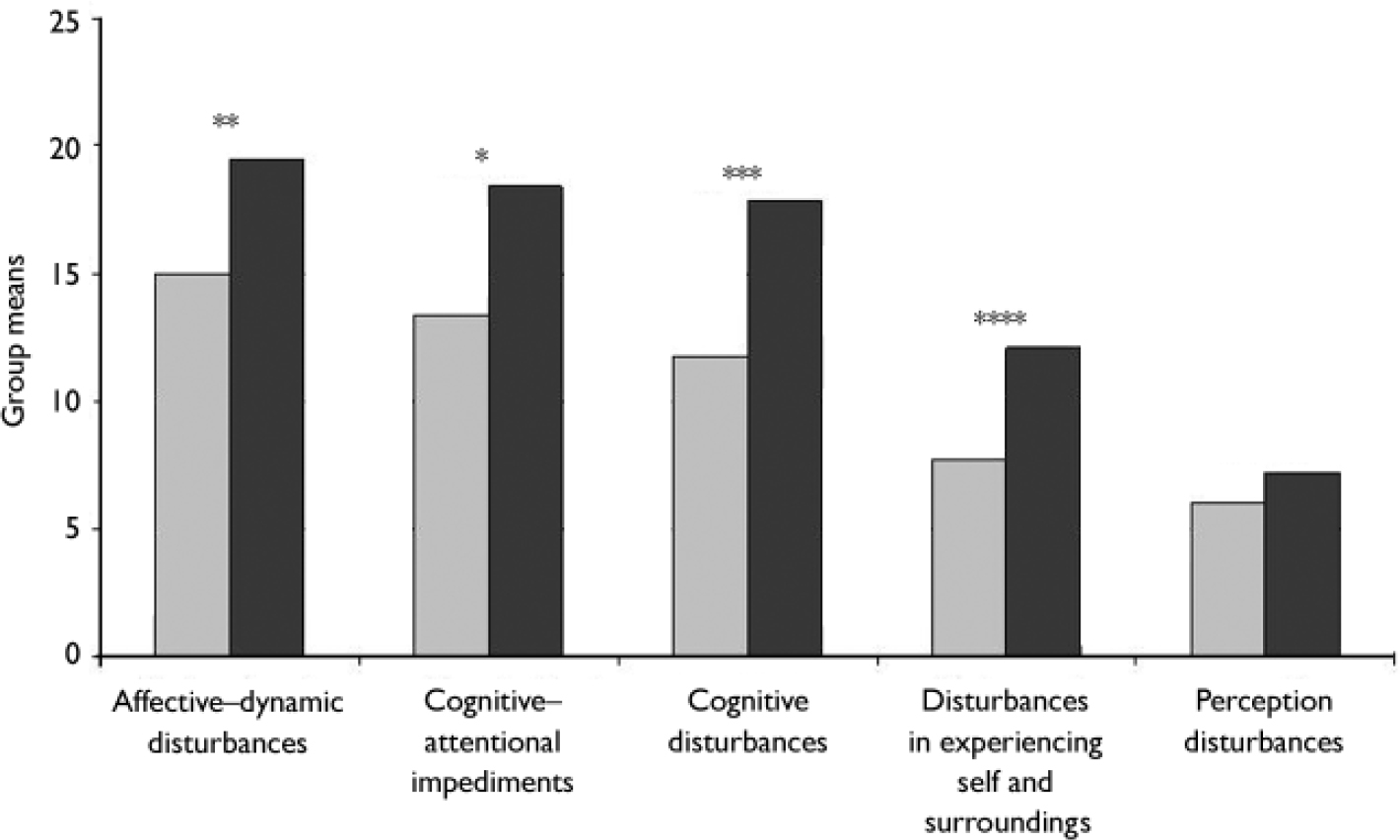
Fig. 1 Comparison of group means of the SPI–A subscale totals between participants with an early ░ and a late ▪ initial prodromal state. *P=0.005, **P=0.002, ***P=0.001, ****P=0.00007.
The general independence of subjective and objective deficits was also supported by the result of the factor analysis of the whole sample that converged after six iterations and generated a five-factor solution with 63.75% explained variance. Herein, the SPI–A sub-scales formed a factor of their own explaining 18.63% of the variance, and the 13 neurocognitive parameters framed altogether 4 factors of 5 to 2 included tests explaining between 15.26 and 5.84% of variance.
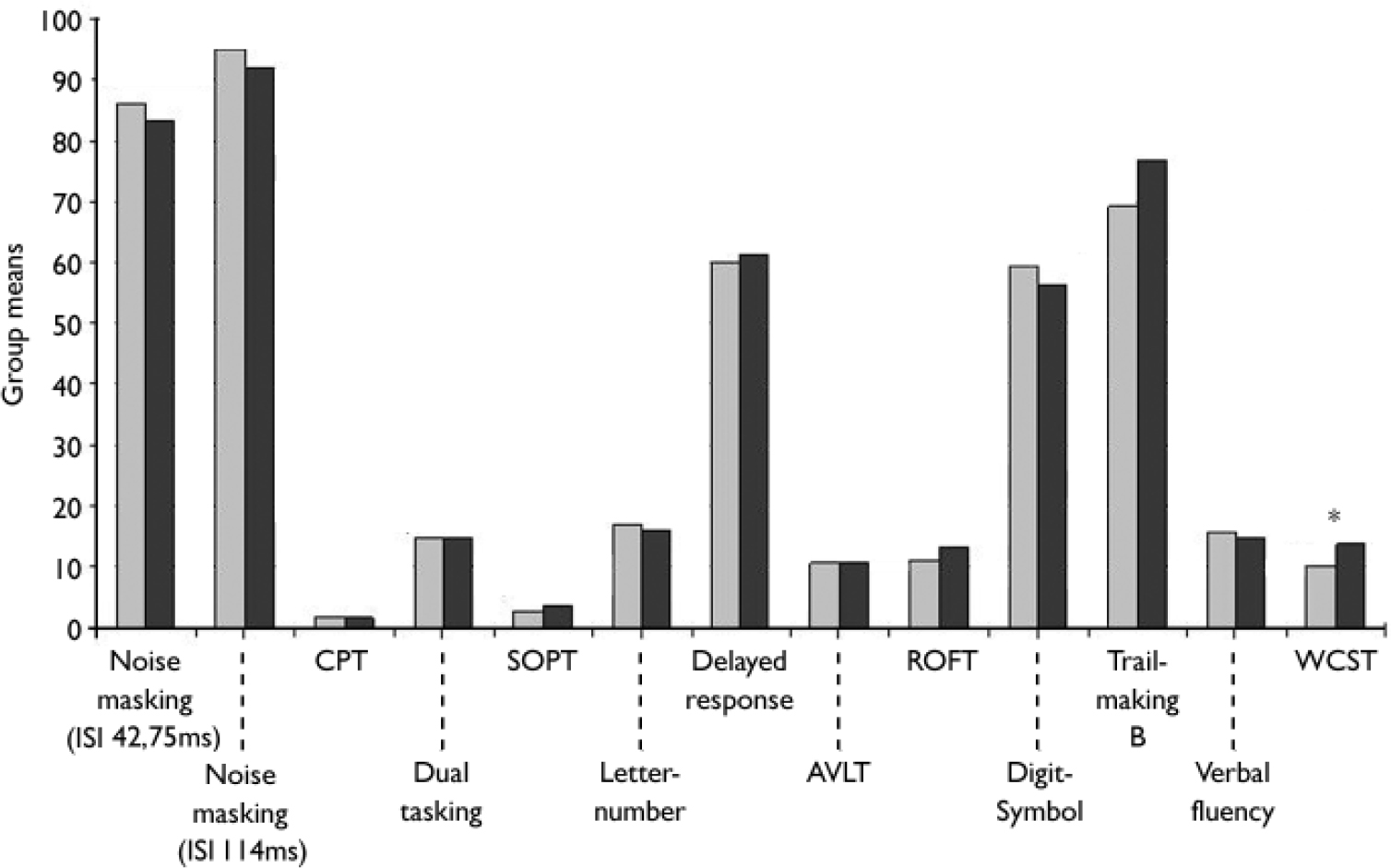
Fig. 2 Comparison of group means of neurocognitive measures between participants with an early ░ and a late ▪ initial prodromal state. AVLT, Auditory Verbal Learning Test; CPT, Continuous Performance Test; ROFT, Rey–Osterrieth Complex Figure Test; SOPT, Subject Ordered Pointing Task; WCST, Wisconsin Cardsorting Test. *P=0.022, **P<0.010.
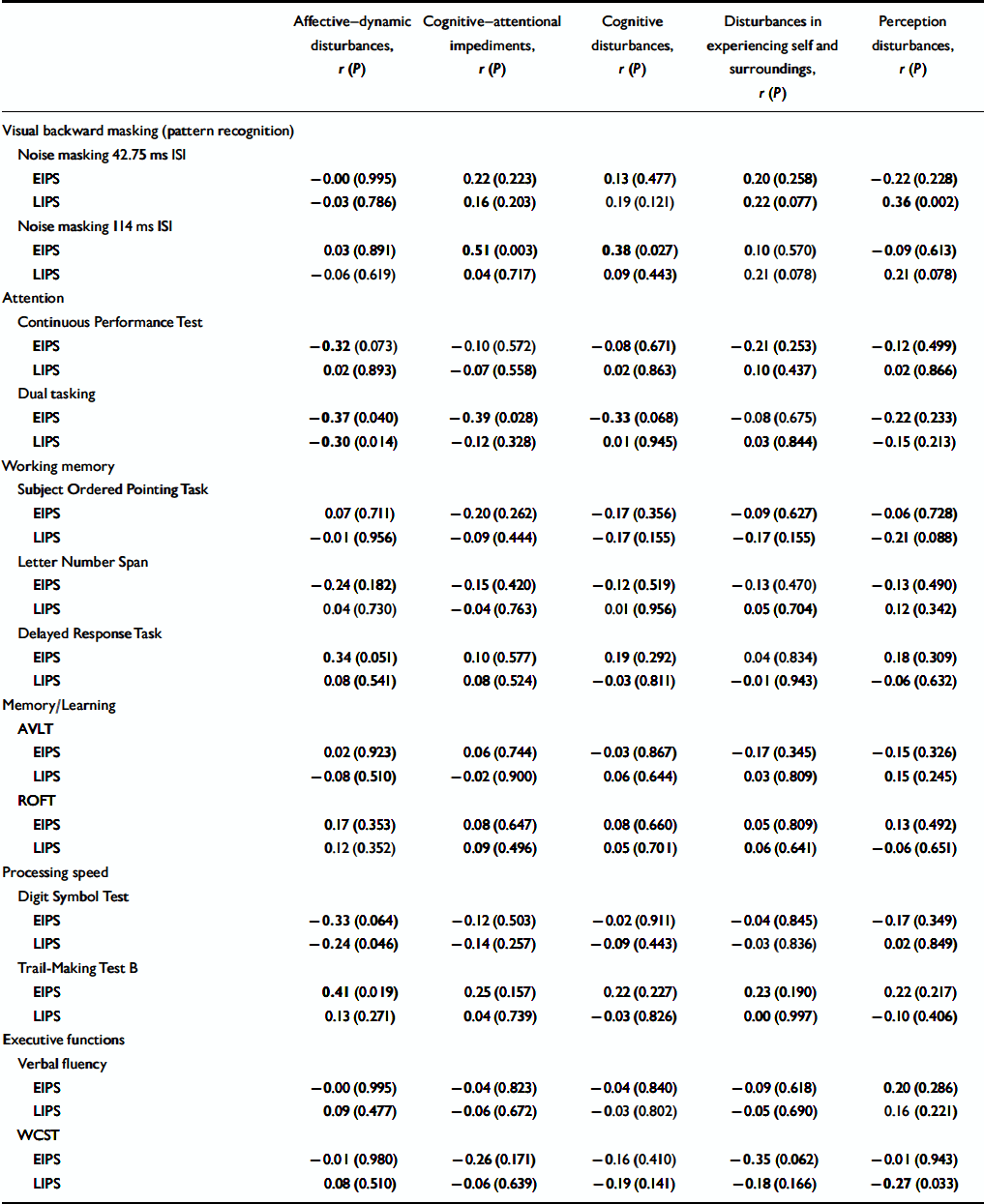
Examining correlations between subjective and objective measures separately for participants with an early and a late initial prodrome revealed that the association of the SPI–A affective–dynamic sub-syndrome and neurocognitive performance was stronger in participants with an early prodrome in which correlations of at least moderate effect were demonstrated not only for the three tasks but also for the CPT and the delayed response task, both of which involve a speed element (Table 3). Only two of the three correlations found for the whole sample reoccurred in the late initial prodromal state, with no significant correlation between affective–dynamic disturbances and Trail-Making Test B (Table 3).
Table 3 Correlation of subjective and objective measures in the early (n=33) and late (n=69) initial prodromal states
| Affective—dynamic disturbances, r (P) | Cognitive—attentional impediments, r (P) | Cognitive disturbances, r (P) | Disturbances in experiencing self and surroundings, r (P) | Perception disturbances, r (P) | |
|---|---|---|---|---|---|
| Visual backward masking (pattern recognition) | |||||
| Noise masking 42.75 ms ISI | |||||
| EIPS | -0.00 (0.995) | 0.22 (0.223) | 0.13 (0.477) | 0.20 (0.258) | -0.22 (0.228) |
| LIPS | -0.03 (0.786) | 0.16 (0.203) | 0.19 (0.121) | 0.22 (0.077) | 0.36 (0.002) |
| Noise masking 114 ms ISI | |||||
| EIPS | 0.03 (0.891) | 0.51 (0.003) | 0.38 (0.027) | 0.10 (0.570) | -0.09 (0.613) |
| LIPS | -0.06 (0.619) | 0.04 (0.717) | 0.09 (0.443) | 0.21 (0.078) | 0.21 (0.078) |
| Attention | |||||
| Continuous Performance Test | |||||
| EIPS | -0.32 (0.073) | -0.10 (0.572) | -0.08 (0.671) | -0.21 (0.253) | -0.12 (0.499) |
| LIPS | 0.02 (0.893) | -0.07 (0.558) | 0.02 (0.863) | 0.10 (0.437) | 0.02 (0.866) |
| Dual tasking | |||||
| EIPS | -0.37 (0.040) | -0.39 (0.028) | -0.33 (0.068) | -0.08 (0.675) | -0.22 (0.233) |
| LIPS | -0.30 (0.014) | -0.12 (0.328) | 0.01 (0.945) | 0.03 (0.844) | -0.15 (0.213) |
| Working memory | |||||
| Subject Ordered Pointing Task | |||||
| EIPS | 0.07 (0.711) | -0.20 (0.262) | -0.17 (0.356) | -0.09 (0.627) | -0.06 (0.728) |
| LIPS | -0.01 (0.956) | -0.09 (0.444) | -0.17 (0.155) | -0.17 (0.155) | -0.21 (0.088) |
| Letter Number Span | |||||
| EIPS | -0.24 (0.182) | -0.15 (0.420) | -0.12 (0.519) | -0.13 (0.470) | -0.13 (0.490) |
| LIPS | 0.04 (0.730) | -0.04 (0.763) | 0.01 (0.956) | 0.05 (0.704) | 0.12 (0.342) |
| Delayed Response Task | |||||
| EIPS | 0.34 (0.051) | 0.10 (0.577) | 0.19 (0.292) | 0.04 (0.834) | 0.18 (0.309) |
| LIPS | 0.08 (0.541) | 0.08 (0.524) | -0.03 (0.811) | -0.01 (0.943) | -0.06 (0.632) |
| Memory/Learning | |||||
| AVLT | |||||
| EIPS | 0.02 (0.923) | 0.06 (0.744) | -0.03 (0.867) | -0.17 (0.345) | -0.15 (0.326) |
| LIPS | -0.08 (0.510) | -0.02 (0.900) | 0.06 (0.644) | 0.03 (0.809) | 0.15 (0.245) |
| ROFT | |||||
| EIPS | 0.17 (0.353) | 0.08 (0.647) | 0.08 (0.660) | 0.05 (0.809) | 0.13 (0.492) |
| LIPS | 0.12 (0.352) | 0.09 (0.496) | 0.05 (0.701) | 0.06 (0.641) | -0.06 (0.651) |
| Processing speed | |||||
| Digit Symbol Test | |||||
| EIPS | -0.33 (0.064) | -0.12 (0.503) | -0.02 (0.911) | -0.04 (0.845) | -0.17 (0.349) |
| LIPS | -0.24 (0.046) | -0.14 (0.257) | -0.09 (0.443) | -0.03 (0.836) | 0.02 (0.849) |
| Trail-Making Test B | |||||
| EIPS | 0.41 (0.019) | 0.25 (0.157) | 0.22 (0.227) | 0.23 (0.190) | 0.22 (0.217) |
| LIPS | 0.13 (0.271) | 0.04 (0.739) | -0.03 (0.826) | 0.00 (0.997) | -0.10 (0.406) |
| Executive functions | |||||
| Verbal fluency | |||||
| EIPS | -0.00 (0.995) | -0.04 (0.823) | -0.04 (0.840) | -0.09 (0.618) | 0.20 (0.286) |
| LIPS | 0.09 (0.477) | -0.06 (0.672) | -0.03 (0.802) | -0.05 (0.690) | 0.16 (0.221) |
| WCST | |||||
| EIPS | -0.01 (0.980) | -0.26 (0.171) | -0.16 (0.410) | -0.35 (0.062) | -0.01 (0.943) |
| LIPS | 0.08 (0.510) | -0.06 (0.639) | -0.19 (0.141) | -0.18 (0.166) | -0.27 (0.033) |
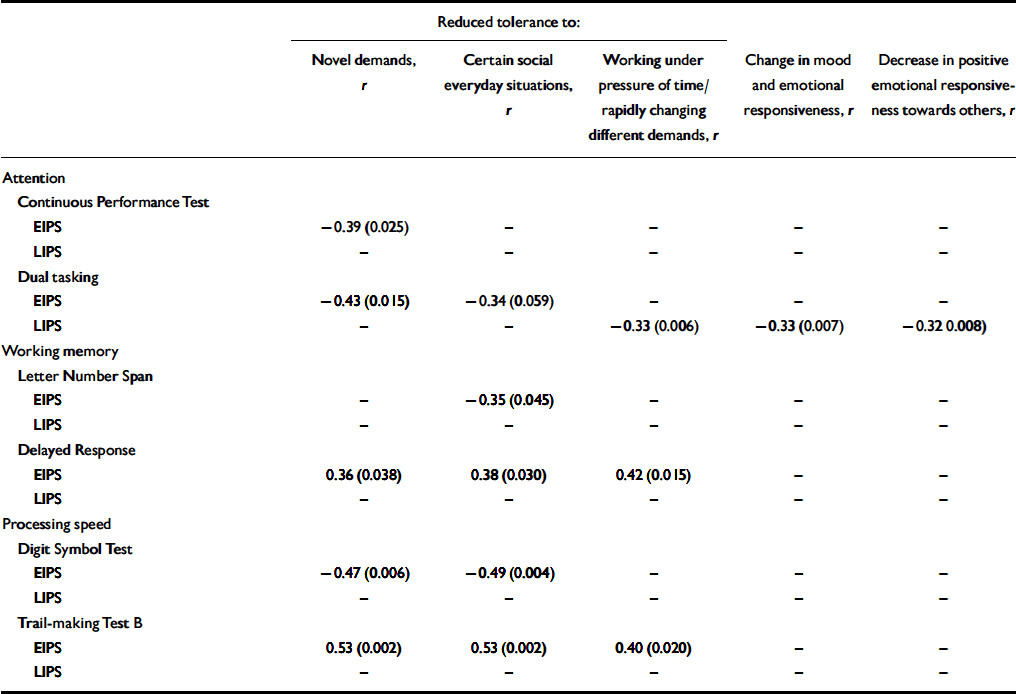
Similar to the whole sample, correlations of the affective–dynamic sub-scale and test performance at an item level were mainly due to self-reported reduced stress tolerance in the early initial prodromal state group, where they showed moderate to strong effects (Table 4). Within the late initial prodrome group, however, significant correlations at an item level became less frequent and more influenced by affect in that there are moderate correlations between dual tasking and mainly affective items of this sub-scale (Table 4). Again, as in the whole sample, correlations with this affective–dynamic sub-scale were in the expected direction.
Table 4 Affective—dynamic disturbances: correlations of at least moderate effect with objective measures in participants with an early or late initial prodromal state1
| Reduced tolerance to: | |||||
|---|---|---|---|---|---|
| Novel demands, r | Certain social everyday situations, r | Working under pressure of time/rapidly changing different demands, r | Change in mood and emotional responsiveness, r | Decrease in positive emotional responsiveness towards others, r | |
| Attention | |||||
| Continuous Performance Test | |||||
| EIPS | -0.39 (0.025) | — | — | — | — |
| LIPS | — | — | — | — | — |
| Dual tasking | |||||
| EIPS | -0.43 (0.015) | -0.34 (0.059) | — | — | — |
| LIPS | — | — | -0.33 (0.006) | -0.33 (0.007) | -0.32 0.008) |
| Working memory | |||||
| Letter Number Span | |||||
| EIPS | — | -0.35 (0.045) | — | — | — |
| LIPS | — | — | — | — | — |
| Delayed Response | |||||
| EIPS | 0.36 (0.038) | 0.38 (0.030) | 0.42 (0.015) | — | — |
| LIPS | — | — | — | — | — |
| Processing speed | |||||
| Digit Symbol Test | |||||
| EIPS | -0.47 (0.006) | -0.49 (0.004) | — | — | — |
| LIPS | — | — | — | — | — |
| Trail-making Test B | |||||
| EIPS | 0.53 (0.002) | 0.53 (0.002) | 0.40 (0.020) | — | — |
| LIPS | — | — | — | — | — |
In addition, there were few and inconsistent significant correlations between cognitive disturbances and pattern recognition (114 ms ISI, noise masking) and divided attention (dual tasking), respectively, in the early initial prodrome group and between perception disturbances and pattern recognition (42.75 ms ISI, noise masking) and executive function as measured by WCST-percentage of errors in the late initial prodrome group (Table 3). Except for dual tasking in an early initial prodromal state, where a lower test performance was associated with more severe subjective cognitive disturbances, contrary to expectations better test performance on neurocognitive measures was associated with more severe cognitive–perceptive basic symptoms. At the single item level of these four sub-scales, correlations with neurocognitive parameters were rare and so scattered that, with regard to the large number of 22 × 13 correlations, they have to be considered random.
DISCUSSION
Based on findings on time until conversion to psychosis in prodromal samples as defined by basic symptoms (Reference Klosterkötter, Hellmich and SteinmeyerKlosterkötter et al, 2001) and the ultra-high risk criteria (e.g. Reference Phillips, Yung and McGorryPhillips et al, 2000; Reference Miller, McGlashan and Lifshey RosenMiller et al, 2002), a two-stage definition of the psychosis prodrome was developed proposing an early and a late initial prodromal state; Reference Ruhrmann, Schultze-Lutter and KlosterkötterRuhrmann et al, 2003). As would be expected from this definition, the present data showed that the early initial prodromal state group was generally less impaired than the late initial prodromal group, with the exception of perception disturbances. These group differences were especially pronounced in the SPI–A sub-scales cognitive disturbances and disturbances in experiencing self and surroundings, although about half of ‘cognitive disturbances’ (3 of 6 items) and of ‘disturbances in experiencing self and surroundings’ (3 of 5 items) were – at a severity of at least ‘3’ – part of the inclusion criteria of the early but not the late initial prodromal group. Thus with inclusion criteria of the late initial prodromal state group being completely devoid of any precondition with regard to basic symptoms, these group differences cannot be related to the definition of prodromal groups that would have been in favour for higher values in the early prodrome group.
Studies on neurocognitive performance of participants with potentially prodromal symptoms differ in the definition of the prodrome and the way to evaluate their performances. Whereas most studies had employed ultra-high risk criteria that are broadly comparable to the late initial prodromal state criteria (Reference Carr, Halpin and LauCarr et al, 2000; Reference Wood, Pantelis and ProffittWood et al, 2003; Reference Hawkins, Addington and KeefeHawkins et al, 2004; Reference Brewer, Francey and WoodBrewer et al, 2005; Reference Francey, Jackson and PhillipsFrancey et al, 2005; Reference Niendam, Bearden and JohnsonNiendam et al, 2006; Reference Silverstein, Uhlhaas and EssexSilverstein et al, 2006), only two had considered both early and late initial prodromal state criteria (Reference Pukrop, Schultze-Lutter and RuhrmannPukrop et al, 2006; Reference Simon, Dvorsky and BoeschSimon et al, 2006), and one study had focused on participants with schizotypy in whom ‘incipient (prodromal) schizophrenia was strongly suspected, due to past or current micropsychotic episodes’ (Reference Parnas, Vianin and SaebyeParnas et al, 2001: p. 173). Furthermore, some studies used healthy control participants for direct statistical comparison (Reference Carr, Halpin and LauCarr et al, 2000; Reference Parnas, Vianin and SaebyeParnas et al, 2001; Reference Wood, Pantelis and ProffittWood et al, 2003; Reference Brewer, Francey and WoodBrewer et al, 2005; Reference Francey, Jackson and PhillipsFrancey et al, 2005; Reference Pukrop, Schultze-Lutter and RuhrmannPukrop et al, 2006; Reference Silverstein, Uhlhaas and EssexSilverstein et al, 2006), whereas others based their comparison on normative data (Reference Hawkins, Addington and KeefeHawkins et al, 2004; Reference Niendam, Bearden and JohnsonNiendam et al, 2006; Reference Simon, Dvorsky and BoeschSimon et al, 2006). Yet, despite these differences, results were generally consistent in that no deficits in performance of participants who are potentially prodromal was demonstrated for
-
• working memory (delayed response; Reference Pukrop, Schultze-Lutter and RuhrmannPukrop et al, 2006)
-
• executive function (WCST; Reference Carr, Halpin and LauCarr et al, 2000; Reference Pukrop, Schultze-Lutter and RuhrmannPukrop et al, 2006) and
-
• visual learning and memory (Reference Niendam, Bearden and JohnsonNiendam et al, 2006; Reference Pukrop, Schultze-Lutter and RuhrmannPukrop et al, 2006)
and that poorer performance was evidenced in
-
• processing speed (Trail-Making Test, Digit Symbol Test) in both early and late initial prodromal state (Reference Simon, Dvorsky and BoeschSimon et al, 2006) and participants with an ultra-high risk (Reference Hawkins, Addington and KeefeHawkins et al, 2004; Reference Niendam, Bearden and JohnsonNiendam et al, 2006),
-
• sustained attention (CPT) in partcipants with a late initial prodromal state and an ultra-high risk (Reference Hawkins, Addington and KeefeHawkins et al, 2004; Reference Francey, Jackson and PhillipsFrancey et al, 2005; Reference Pukrop, Schultze-Lutter and RuhrmannPukrop et al, 2006; Reference Simon, Dvorsky and BoeschSimon et al, 2006), but not those with an early initial prodromal (Reference Pukrop, Schultze-Lutter and RuhrmannPukrop et al, 2006; Reference Simon, Dvorsky and BoeschSimon et al, 2006),
-
• verbal memory (e.g. AVLT) and verbal fluency in participants both with early and late initial prodromal states and those with an ultra-high risk (Reference Carr, Halpin and LauCarr et al, 2000; Reference Hawkins, Addington and KeefeHawkins et al, 2004; Reference Brewer, Francey and WoodBrewer et al, 2005; Reference Niendam, Bearden and JohnsonNiendam et al, 2006; Reference Pukrop, Schultze-Lutter and RuhrmannPukrop et al, 2006; Reference Simon, Dvorsky and BoeschSimon et al, 2006) as well as partly in participants who are at genetically high risk (Reference Cosway, Byrne and ClaffertyCosway et al, 2000; Reference Whyte, Brett and HarrisonWhyte et al, 2006).
However, results on pattern recognition and especially on spatial working memory remain inconsistent. In one of our own studies (Reference Pukrop, Schultze-Lutter and RuhrmannPukrop et al, 2006), neither participants with an early initial prodromal state (n=38) nor those with a late initial prodromal state (n=90) had shown significant underperformance in pattern recognition. Broadly in line with this, participants with ultra-high risk were reported to not differ from controls in a pre-attentive perceptual organisation task similar to the pattern recognition task (Reference Silverstein, Uhlhaas and EssexSilverstein et al, 2006). Yet, 10 at-risk participants with schizotypy performed significantly better than controls in a visual binding test (Reference Parnas, Vianin and SaebyeParnas et al, 2001) that is also considered to test pre-attentive perceptual organisation (Reference Silverstein, Uhlhaas and EssexSilverstein et al, 2006). The most inconsistencies occurred in studies assessing spatial working memory performance. In some studies (Reference Parnas, Vianin and SaebyeParnas et al, 2001; Reference Pukrop, Schultze-Lutter and RuhrmannPukrop et al, 2006) there were no differences between subjects and controls; in one ultra-high risk sample (Reference Wood, Pantelis and ProffittWood et al, 2003) subjects performed more poorly, and in another, participants with ultra-high risk performed at a higher level than norms (Reference Hawkins, Addington and KeefeHawkins et al, 2004).
With the observed neurocognitive profile of at-risk subjects being only marginally affected by socio-demographic and clinical characteristics (Reference Niendam, Bearden and JohnsonNiendam et al, 2006; Reference Pukrop, Schultze-Lutter and RuhrmannPukrop et al, 2006; Reference Silverstein, Uhlhaas and EssexSilverstein et al, 2006), the findings so far were thought to be consistent with a primary involvement of left fronto-temporal networks in the prodromal phase (Reference Niendam, Bearden and JohnsonNiendam et al, 2006; Reference Pukrop, Schultze-Lutter and RuhrmannPukrop et al, 2006).
The focus of this current study is on the association between cognitive performance and self-perceived cognitive disturbances. The result in the late initial prodromal state group is in line with earlier findings showing no correlation between attenuated negative symptoms as measured with the SIPS and motor speed, verbal learning and memory, verbal working memory, visual learning and memory, reasoning and problem solving and processing speed in participants with ultra-high risk (Reference Niendam, Bearden and JohnsonNiendam et al, 2006). As attenuated and brief limited intermittent psychotic symptoms were defining 97% of these 45 participants with ultra-high risk and 4 of the 6 SIPS negative items involve aspects of reduced stress tolerance and constricted affect, results in this study of the participants with a late initial prodromal state and affective–dynamic disturbances are comparable. Previously, Wood et al (Reference Wood, Pantelis and Proffitt2003) also failed to show a significant correlation between negative symptoms as assessed by the Schedule for Assessment of Negative Symptoms total score and spatial working memory in 38 participants with ultra-high risk (73.7% with attenuated and brief limited intermittent psychotic symptoms). However, in this study examining only the 9 participants that had made a transition to psychosis, there was in fact a significant positive correlation between the two measures.
Although the association with subjectively reported affective–dynamic disturbances should be examined both longitudinally and in larger early initial prodromal state samples, our current study demonstrates that particularly in an assumed early stage of an evolving psychosis, subjectively reduced stress tolerance appears related to neurocognitive performance in tests including a speed element or time restriction. Furthermore, it may be that this clinically plausible finding might prove on an empirical level to occur only within an early state of the prodrome.
The second important observation is the fact that no correlation between subjective cognitive–perceptive disturbances and performance in neurocognitive tests was evidenced in the at-risk sample. Although correlations between both, cognitive–attentional impediments and cognitive disturbances, and divided attention (dual tasking) and pattern recognition (noise masking, 114 ms ISI), respectively, existed in participants with an early initial prodromal state, it was in opposite directions and thus non-conclusive, especially as both involved neurocognitive domains on which at-risk subjects performed in the normal range. No further associations, which could be considered not random, were detected in the late initial prodrome group.
Finally, within the transition sequence study on basic symptoms (Reference KlosterkötterKlosterkötter, 1992), cognitive basic symptoms were shown to convert to psychotic symptoms such as thought insertion, withdrawal and broadcast as well as verbal hallucinations, and perceptual basic symptoms rather to delusional perceptions. Thus, our finding that cognitive–perceptive basic symptoms, which seem more related to positive than to negative symptoms of psychosis, were unrelated to neuropsychological measures makes sense and is in line with the lack of relationship between attenuated psychotic symptoms on the SIPS and neurocognitive measures in participants with ultra-high risk (Reference Niendam, Bearden and JohnsonNiendam et al, 2006).
Thus, mean performance levels in objective tests did not appear to carry substantial information on subjectively experienced cognitive and perceptive performance. The impact of this on outcome and whether this will be supported in larger samples needs to be seen in future studies. Furthermore, the neurocognitive test battery was comprised of tests that detected deficits in patients with manifest psychosis. It may be that other neuropsychological tests may be more appropriate to examine cognitive deficits in this group. Finally, future studies should consider the early initial prodromal state group, who are generally less impaired and who have psychopathological disturbances that remain on a structural level of information processing without influencing thought content and observed speech (for example in terms of a paranoid ideation, ideas of reference or odd speech), to be a better starting point to study associations of objective and subjective cognitive deficits.
However, the apparent lack of association between symptoms currently used to define potentially prodromal states and neuropsychological measures offers an opportunity to refine the predictive power of current prodromal criteria. Only when information is non-redundant (i.e. when measures are not highly correlated) might adding a measure help to explain more variance in an outcome such as conversion to psychosis. For such a refinement of prediction of psychosis, processing speed as well as verbal memory and fluency seem to be the most promising candidates as yet since they have been consistently reported to be deficient in potentially prodromal states (Reference Carr, Halpin and LauCarr et al, 2000, Reference Hawkins, Addington and KeefeHawkins et al, 2004; Reference Brewer, Francey and WoodBrewer et al, 2005; Reference Niendam, Bearden and JohnsonNiendam et al, 2006; Reference Pukrop, Schultze-Lutter and RuhrmannPukrop et al, 2006; Reference Simon, Dvorsky and BoeschSimon et al, 2006).
In conclusion, our findings generally support earlier results showing lack of association between neurocognitive deficits and psychopathologic features in psychosis as well as a potential association between these two areas that might be more detectable in the very early state of the beginning illness. The results generally imply that there could be a benefit in adding neurocognitive measures to the currently mainly symptomatic definitions of the psychosis prodrome in order to improve prediction.
Acknowledgements
The psychopathological assessments since June 2000 were part of the baseline assessments of a study on the evaluation of the SPI–A, supported by a grant from the German Research Foundation (DFG; grant ID KL970/3 KL970/3-1 -1 and KL970/3 KL970/3-2) -2) to J.K., F.S.-L. and Professor E. M. Steinmeyer.









eLetters
No eLetters have been published for this article.