A main focus of studies examining the biological determinants of aggressive behaviour has been the association between low serotonergic (5-HT) function and impulsive aggression in studies of the cerebrospinal fluid (CSF) concentration of the 5-HT metabolite 5-hydroxyndoleacetic acid (5-HIAA; Reference Tuinier, Verhoevn and Van PraagTuinier et al, 1995) and from 5-HT drug challenge studies (Reference Markowitz, Coccaro, Hollander and SteinMarkowitz & Coccaro, 1995). In contrast, testosterone has been implicated primarily in aggressive rather than impulsive behaviour (Reference ArcherArcher, 1991). The notion that a low 5-HT level relates more to impulsivity and that testosterone relates more to aggression receives support from CSF studies (Reference Virkkunen, Rawlings and TakolaVirkkunen et al, 1994). We investigated relationships between impulsivity, aggression, 5-HT function and testosterone in controls and in offenders with personality disorders, minimising potential confounding factors due to organic brain conditions and drug and alcohol misuse, and tested the hypothesis that reduced 5-HT function would be seen only in the subgroup of aggressive offenders with high impulsivity and not in those with normal or low impulsivity.
METHOD
Subjects
Sixty male medication-free offenders with DSM-III-R (American Psychiatric Association, 1987) personality disorders detained in maximum-security hospitals in the UK, and 27 healthy male volunteers screened for DSM-III-R Axis I and II disorders, drawn from nursing/ancillary staff in the same institutions, were recruited. There were no differences between controls and patients in terms of mean (s.d.) age (controls=30.3 (6.0) years v. patients=29.7 (6.6) years; t=0.33, d.f.=85, P=0.71) or body mass index (weight (kg)/height (m2) ratios; t=0.55, d.f.=85, P=0.56).
All subjects were interviewed and screened for DSM-III-R Axis I and II disorders using the Structured Clinical Interview Diagnosis (SCID-I and II) schedules (Reference Spitzer, Williams and GibbonSpitzer et al, 1990). Exclusion criteria for all subjects included age >55 years, alcohol intake >50 units per week in the past year, illicit drug or steroid use in the last 5 years, psychotropic medication in the last 6 weeks, a history of head trauma and abnormal blood biochemistry. Additional exclusion criteria for controls were an Axis II diagnosis (SCID-II; Reference Spitzer, Williams and GibbonSpitzer et al, 1990).
Procedure
All subjects completed the Special Hospital Assessment of Personality and Socialisation (SHAPS; Reference BlackburnBlackburn, 1986) and psychometric assessments of trait impulsivity (Impulsiveness-Venturesomeness-Empathy, IVE, inventory; Reference Eysenck and EysenckEysenck & Eysenck, 1978; Barratt Impulsivity Scale, BIS; Reference Barratt, Spielberger and ButcherBarratt, 1985) and aggression (Buss-Durkee Hostility Inventory, BDHI; Reference Buss and DurkeeBuss & Durkee, 1957; Brown-Goodwin Lifetime History of Aggression, BGA, scale; Reference Brown, Goodwin and BallengerBrown et al, 1979). Depression and anxiety were assessed using the Beck Depression Inventory (BDI; Reference Beck, Ward and MendelsonBeck et al, 1961) and Spielberger's State-Trait Anxiety Inventory (STAI; Reference Spielberger, Gorusch and LushereSpielberger et al, 1970). Study participation was approved by the local hospital research and ethics committees.
Diagnostic/group assignment
The SHAPS was used as the primary instrument for group assignment among patients because DSM-III-R Axis II diagnoses were not mutually exclusive in this group. It has been validated in forensic samples (Reference BlackburnBlackburn, 1986) and provides distinct personality groupings, based on the orthogonal dimensions of belligerence (related to impulsivity and hostility) and withdrawal (relating to anxiety and introversion), within which several Axis II personality disorders will fall. Fifty-one patients were classified as SHAPS psychopaths (high belligerence scores) and nine as SHAPS non-psychopaths (low belligerence scores). The majority of the controls were classified as SHAPS non-psychopaths, but five had sub-scale scores that reflected some ‘psychopathic’ traits. The latter were not excluded because they did not meet DSM criteria for a diagnosis of Axis II personality disorders. Of the SHAPS psychopaths, 21 were primary (low withdrawal scores) and 30 were secondary psychopaths (high withdrawal scores).
The majority (42) of SHAPS psychopaths had Cluster B DSM-III-R diagnosis. Several SHAPS psychopaths, however, also met the criteria for one or more Cluster A/C diagnoses, including that for avoidant (3), dependent (6), paranoid (5) and passive aggressive (13) personality. Among the SHAPS non-psychopathic group, six met the criteria for obsessional, two for narcissistic and seven for schizoid personality disorders.
Serotonin drug challenge and biochemical measures
The responsivity of the 5-HT system was assessed using the prolactin (PRL) response to an oral 30-mg d-fenfluramine (dFEN) challenge, which is believed to act primarily at 5-HT2 receptor sites (Reference Di Renzo, Amoroso and TaglialatelaDiRenzo et al, 1989) and is more 5-HT-specific than d,l-fenfluramine (Reference Garattini, Caccia and MenniniGarattini et al, 1979). Sixty patients and 27 control subjects received dFEN, with 40 patients and 21 controls receiving a placebo (PBO) challenge in a single-blind fashion separated by at least 1 week. No significant order effects were observed in the baseline PRL levels or PRL responses to challenge with PBO or dFEN. Subjects attended the research room at about 10.30 a.m. after a light breakfast. An intravenous catheter was inserted in a forearm vein at 11.00 a.m. and kept open with a heparin lock. Blood samples were taken immediately after cannulation, then 60 minutes later immediately before oral administration of identical capsules that contained either dFEN or PBO (time zero), and at hourly intervals for a further 5 hours (+300 min).
Plasma PRL and cortisol (CORT) levels were obtained at all time points, testosterone on time zero samples and dFEN/norfenfluramine on samples from +60 or +300 min. Subjects remained awake and fasting and completed the Profile of Mood States (POMS; Reference McNair, Lorr and DropplemanMcNair et al, 1981) hourly.
Biochemical assay methods
Blood samples were placed on ice, with plasma separated at 2000 rpm and stored at ‒20°C until assay. The lower limit of detection for PRL (IRMA-NETRIA, London) was 24mIU/l, with intra- and inter-assay variability less than 2.4% and 4.8%, respectively. The respective values for CORT ([125I]radioimmunoassay, Bioclin, Cardiff) were 0.2μg/dl, 4.3% and 5.8%. For dFEN and norfenfluramine concentrations (gas-liquid chromatography) lower limits of detection were 0.5 and 2ng/ml, with intra- and inter-assay coefficients of variation of 6.9% and 2.7%, respectively. For testosterone (solidphase radioimmunoassay, DPC, Los Angeles, CA) the values were 0.04 ng/ml, 5.2% and 5.9%. The normal range was 2.8-6.0 ng/ml.
Data analysis
Impulsivity and aggression were difficult to separate, so in addition to analysing the rating scales separately, composite scores likely to weight towards either impulsivity or aggression were derived for each dimension. Impulsivity scores from the SHAPS, BIS and IVE were converted into z-scores and summed to form a composite impulsivity measure. Similarly, a composite aggression score was calculated from a summation of the z-scores on the BDHI motor aggression factor (assault, indirect aggression, verbal aggression and irritability), BGA total score and SHAPS aggression scale.
Testosterone values from the two occasions were averaged. As plasma dFEN and norfenfluramine were analysed separately and as a combined drug measure did not differ, only the combined drug measure is reported. The area under the curve (AUC) was calculated for hormonal and drug measures by the trapezoid method, corrected for baseline differences taken as +60 minutes after drug administration because this offered the most stable baseline with undetectable drug levels until after this point. Placebo-corrected hormonal AUC values were obtained by subtracting those after PBO from those after dFEN.
Statistical analysis
Data were analysed using SPSS for Windows Release 6 (SPSS Inc., Chicago, IL). Analysis of variance (ANOVA) was used with one between-group factor (controls, SHAPS psychopaths, SHAPS non-psychopaths) and one within-group factor (drug). As fewer subjects had both a placebo and dFEN challenge rather than a dFEN challenge alone, and there were few differences between the findings, we report the dFEN challenge data only unless a different result was obtained using placebo data, in which case both are presented. Least significant differences (LSD) were used for post hoc comparison. Where post hoc tests revealed significant group differences, t-tests were used to assess the magnitude of these significant differences. Drug levels were used as covariates. Correlations between variables were determined by Spearman's correlation coefficients. Unless stated otherwise, correlations relate to the total sample. Stepwise regression analyses were used to explain the relative contributions of variables that contributed to the variance in PRL response to fenfluramine, that is, the 5-HT function.
RESULTS
Psychometric characteristics of the sample
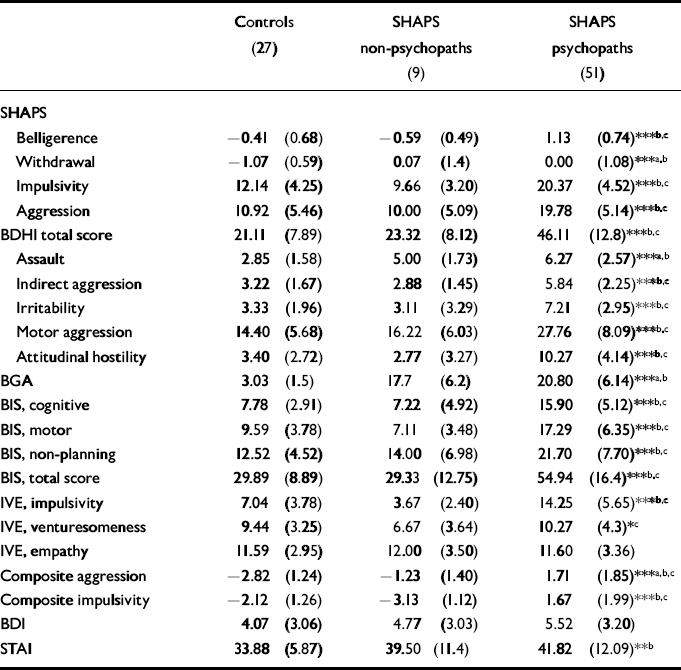
The SHAPS non-psychopaths and psychopaths had higher composite aggression scores than controls (F(2,84)=69.3, P<0.001) (see Table 1). The significantly higher aggression score among SHAPS psychopaths compared with controls and SHAPS non-psychopaths was due to to higher scores on the BDHI motor aggression subscale (F(2,84)=33.71, P<0.001) and BDHI attitudinal hostility scales (F(2,84)=38.59, P<0.001). On the BGA, SHAPS psychopaths and non-psychopaths had higher scores than controls (F(2,84)=105.37, P<0.001), but SHAPS psychopaths and non-psychopaths did not differ significantly on this measure. Significant differences between non-psychopaths and controls were attributable to higher scores in non-psychopaths on the BDHI assault item and the BGA. Primary and secondary psychopaths did not differ significantly in aggression ratings.
Table 1 Mean scores (s.d.) on psychometric measures
| Controls (27) | SHAPS non-psychopaths (9) | SHAPS psychopaths (51) | |
|---|---|---|---|
| SHAPS | |||
| Belligerence | -0.41 (0.68) | -0.59 (0.49) | 1.13 (0.74)*** b,c |
| Withdrawal | -1.07 (0.59) | 0.07 (1.4) | 0.00 (1.08)*** a,b |
| Impulsivity | 12.14 (4.25) | 9.66 (3.20) | 20.37 (4.52)*** b,c |
| Aggression | 10.92 (5.46) | 10.00 (5.09) | 19.78 (5.14)*** b,c |
| BDHI total score | 21.11 (7.89) | 23.32 (8.12) | 46.11 (12.8)*** b,c |
| Assault | 2.85 (1.58) | 5.00 (1.73) | 6.27 (2.57)*** a,b |
| Indirect aggression | 3.22 (1.67) | 2.88 (1.45) | 5.84 (2.25)*** b,c |
| Irritability | 3.33 (1.96) | 3.11 (3.29) | 7.21 (2.95)*** b,c |
| Motor aggression | 14.40 (5.68) | 16.22 (6.03) | 27.76 (8.09)*** b,c |
| Attitudinal hostility | 3.40 (2.72) | 2.77 (3.27) | 10.27 (4.14)*** b,c |
| BGA | 3.03 (1.5) | 17.7 (6.2) | 20.80 (6.14)*** a,b |
| BIS, cognitive | 7.78 (2.91) | 7.22 (4.92) | 15.90 (5.12)*** b,c |
| BIS, motor | 9.59 (3.78) | 7.11 (3.48) | 17.29 (6.35)*** b,c |
| BIS, non-planning | 12.52 (4.52) | 14.00 (6.98) | 21.70 (7.70)*** b,c |
| BIS, total score | 29.89 (8.89) | 29.33 (12.75) | 54.94 (16.4)*** b,c |
| IVE, impulsivity | 7.04 (3.78) | 3.67 (2.40) | 14.25 (5.65)*** b,c |
| IVE, venturesomeness | 9.44 (3.25) | 6.67 (3.64) | 10.27 (4.3)* c |
| IVE, empathy | 11.59 (2.95) | 12.00 (3.50) | 11.60 (3.36) |
| Composite aggression | -2.82 (1.24) | -1.23 (1.40) | 1.71 (1.85)*** a,b,c |
| Composite impulsivity | -2.12 (1.26) | -3.13 (1.12) | 1.67 (1.99)*** b,c |
| BDI | 4.07 (3.06) | 4.77 (3.03) | 5.52 (3.20) |
| STAI | 33.88 (5.87) | 39.50 (11.4) | 41.82 (12.09)** b |
Impulsivity scores
The SHAPS psychopaths had significantly higher composite impulsivity scores than controls and non-psychopaths (F(2,84)=59.50, P<0.001). This pattern of group differences applied to all sub-scales of the composite score. The SHAPS nonpsychopaths tended to have lower scores than controls on all impulsivity measures but the differences did not reach significance. Although secondary psychopathic patients had higher impulsivity scores than primary psychopaths, these differences did not reach significance.
Mood measures
There were no significant group differences in BDI score (F(2,84)=1.91, P=0.15). The SHAPS psychopaths, however, demonstrated significantly higher scores on the STAI (F(2,84)=5.05, P<0.01), which was attributable solely to the secondary psychopath group.
Biochemical/hormonal data
Despite differences in anxiety score, primary and secondary psychopaths did not differ on biochemical measures, so for ease of interpretation the psychopaths are presented as a single group. Mean hormonal concentrations and responses to challenge are shown in Table 2.
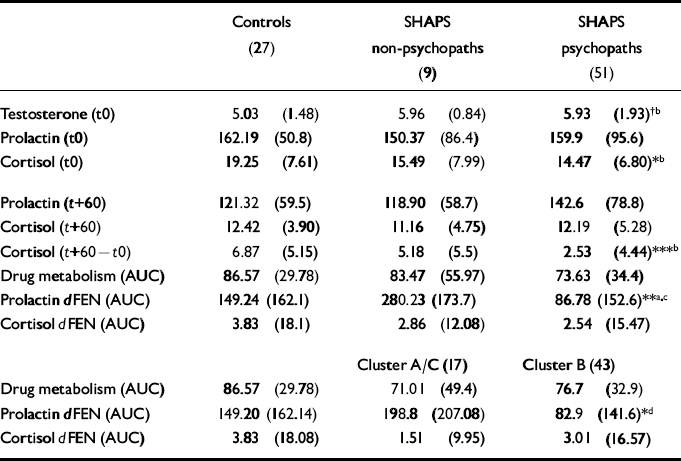
Table 2 Hormonal concentrations and response to challenge (mean (s.d.))
| Controls | SHAPS | SHAPS | |
|---|---|---|---|
| (27) | non-psychopaths | psychopaths | |
| (9) | (51) | ||
| Testosterone (t0) | 5.03 (1.48) | 5.96 (0.84) | 5.93 (1.93)† b |
| Prolactin (t0) | 162.19 (50.8) | 150.37 (86.4) | 159.9 (95.6) |
| Cortisol (t0) | 19.25 (7.61) | 15.49 (7.99) | 14.47 (6.80)* b |
| Prolactin (t+60) | 121.32 (59.5) | 118.90 (58.7) | 142.6 (78.8) |
| Cortisol (t+60) | 12.42 (3.90) | 11.16 (4.75) | 12.19 (5.28) |
| Cortisol (t+60 - t0) | 6.87 (5.15) | 5.18 (5.5) | 2.53 (4.44)*** b |
| Drug metabolism (AUC) | 86.57 (29.78) | 83.47 (55.97) | 73.63 (34.4) |
| Prolactin dFEN (AUC) | 149.24 (162.1) | 280.23 (173.7) | 86.78 (152.6)** a,c |
| Cortisol dFEN (AUC) | 3.83 (18.1) | 2.86 (12.08) | 2.54 (15.47) |
| Cluster A/C (17) | Cluster B (43) | ||
| Drug metabolism (AUC) | 86.57 (29.78) | 71.01 (49.4) | 76.7 (32.9) |
| Prolactin dFEN (AUC) | 149.20 (162.14) | 198.8 (207.08) | 82.9 (141.6)* d |
| Cortisol dFEN (AUC) | 3.83 (18.08) | 1.51 (9.95) | 3.01 (16.57) |
Prechallenge and baseline biochemical (hormonal) measures
There was a trend towards significant group differences (F(2,81)=2.49, P=0.08) in plasma testosterone, with SHAPS psychopaths showing higher mean levels than controls. These differences were due primarily to primary psychopaths (mean=6.24, s.d.=1.41) having higher testosterone levels than controls (mean=5.03, s.d.=1.48; t=-2.80, d.f.=44, P<0.01).
Plasma CORT concentrations at the time of cannulation were significantly higher in controls compared with psychopaths but not non-psychopaths (F(2,84)=4.41, P<0.01). However, this difference disappeared by baseline (+60 min) due to a significantly greater drop in CORT concentration between cannulation and baseline in controls compared with psychopaths (F(2,84)=7.84, P<0.001). Non-psychopaths showed an intermediate drop (Table 2).
There were no significant group differences in PRL concentration at cannulation (F(2,84)=0.06, P=0.98) or baseline (F(2,84)=0.99, P=0.37).
Drug challenge data
The challenge tests were well tolerated and the only observed group difference was on the POMS fatigue measure, where SHAPS psychopaths reported more fatigue than controls based on an analysis of the AUC data (mean=14.6, s.d.=19.0 v. mean=4.2, s.d.=10.1; F(2,80)=3.57, P<0.05).
Fenfluramine drug levels
There were no differences between controls and SHAPS-defined (F(2,84)=1.24, P=029) or DSM-III-R-defined groups (F(2,84)=1.10, P=0.33) on the drug metabolism AUC data.
Biochemical measures in controls and SHAPS category patients
Administration of dFEN resulted in significant PRL responses in an analysis of the PBO-corrected data (F(2,58)=6.08, P<0.01). By using the dFEN challenge occasion alone there was a significant effect of group (F(2,84)=6.17, P<0.01), with non-psychopathic patients having higher responses than either controls or psychopaths (Fig. 1 and Table 2). Differences between controls and psychopaths showed a trend towards significance (t=1.68, d.f.=76, P=0.09).
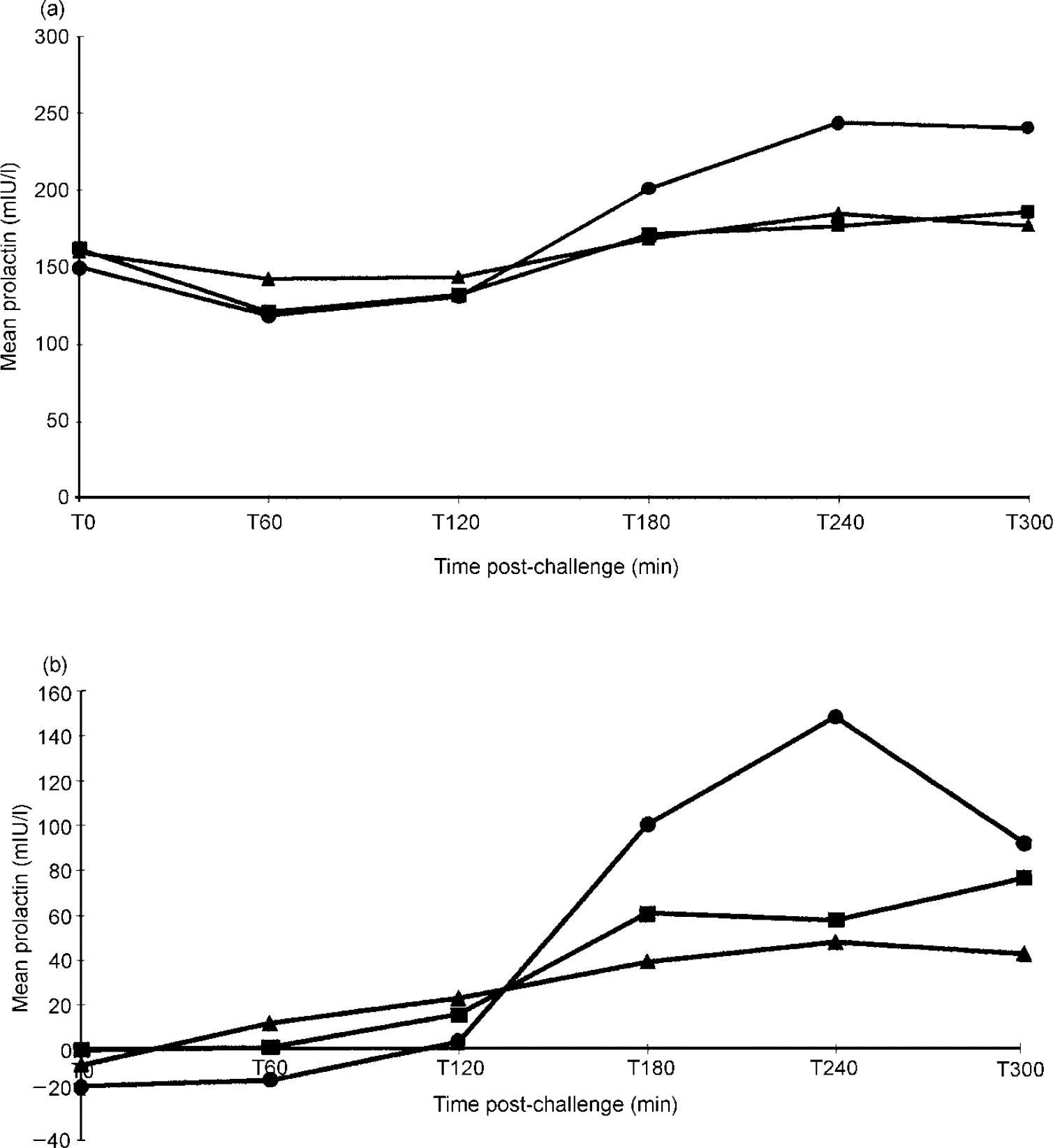
Fig. 1 Drug response curves in patients assessed using the Special Hospital Assessment of Personality and Socialisation (SHAPS): (a) prolactin response to d-fenfluramine; (b) placebo-corrected data for prolactin response to d-fenfluramine. ▴ Psychopaths: (a) n=51; (b) n=34; • non-psychopaths: (a) n=9; (b) n=6; ▪ controls: (a) n=27; (b) n=21.
Administration of dFEN did not result in a significant CORT AUC response (F(2,58)=0.15, P=0.85) and there was no difference between controls and SHAPS groups in CORT AUC values (F(2,84)=0.05, P=0.94).
Prolactin responses in controls and DSM-III-R personality disorders
There was a significant personality disorder cluster effect (F(2,84)=3.49, P<0.05), with Cluster B patients having lower mean PRL responses than Cluster A/C patients, whereas controls had intermediate responses (see Table 2). Covarying for drug level did not alter the findings (F(2,83)=0.05, P<0.05).
In a comparison of PRL AUCs in patients with a specific diagnosis of personality disorder, controls and the remaining patients with personality disorder who did not meet the criteria for that diagnosis, significant group differences were observed only for those with diagnoses of borderline (F(2,84)=3.58, P<0.05), schizoid (F(2,84)=8.62, P<0.001) and obsessional personality disorder (F(2,84)=5.51, P<0.01). A trend towards a significant group difference was observed for a diagnosis of antisocial personality disorder (F(2,84)=2.65, P=0.07). Stepwise multiple regression analysis confirmed that only those with borderline (low PRL response) and schizoid (high PRL response) personalities contributed significantly to the variance in PRL AUC response to dFEN (r 2=0.15; F(2,84)=7.74, P<0.01).
Prolactin response in controls and patients who self-harm
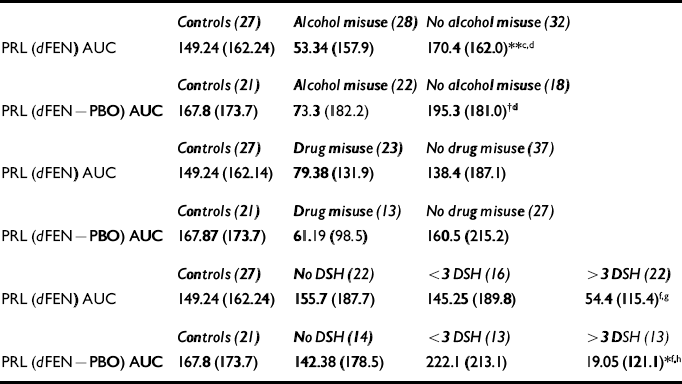
A comparison of controls (no self-harm), patients with no history of self-harm (n=22), patients with one or two attempts (n=16) and patients with multiple (more than three) attempts (n=22) revealed a trend towards lower PRL responses in high-frequency self-harmers compared with controls (F(3,83)=1.88, P=0.13), which was significant when the PBO-corrected AUC data were used (F(3,57)=3.21, P<0.05) (see Table 3).
Table 3 Mean prolactin responses (s.d.) to fenfluramine (dFEN)
| Controls (27) | Alcohol misuse (28) | No alcohol misuse (32) | ||
| PRL (dFEN) AUC | 149.24 (162.24) | 53.34 (157.9) | 170.4 (162.0)**c,d | |
| Controls (21) | Alcohol misuse (22) | No alcohol misuse (18) | ||
| PRL (dFEN — PBO) AUC | 167.8 (173.7) | 73.3 (182.2) | 195.3 (181.0)†d | |
| Controls (27) | Drug misuse (23) | No drug misuse (37) | ||
| PRL (dFEN) AUC | 149.24 (162.14) | 79.38 (131.9) | 138.4 (187.1) | |
| Controls (21) | Drug misuse (13) | No drug misuse (27) | ||
| PRL (dFEN — PBO) AUC | 167.87 (173.7) | 61.19 (98.5) | 160.5 (215.2) | |
| Controls (27) | No DSH (22) | <3 DSH (16) | > 3 DSH (22) | |
| PRL (dFEN) AUC | 149.24 (162.24) | 155.7 (187.7) | 145.25 (189.8) | 54.4 (115.4)f,g |
| Controls (21) | No DSH (14) | <3 DSH (13) | > 3 DSH (13) | |
| PRL (dFEN — PBO) AUC | 167.8 (173.7) | 142.38 (178.5) | 222.1 (213.1) | 19.05 (121.1)*f,h |
Prolactin response in controls and patients with/without an illicit-drug history
Of the 23 patients with a previous history of drug misuse (minimum abstinence of 5 years), the majority had used cannabis and only four were cocaine users. There were no significant differences in dFEN metabolism or PRL response to dFEN in controls (non-drug-users) and patients with and without drug use (F(2,84)=1.26, P=0.28) (see Table 3). Covarying for fenfluramine level did not alter the findings.
Prolactin response in controls and patients with/without an alcohol history
Of the 28 patients with a prior history of alcohol misuse, the mean abstinence period was 5 years (range 1.6-15 years). Drug metabolism (dFEN) did not differ in patients with and without a history of alcohol misuse. Differences in PRL response to dFEN were observed between controls and patients with and without a history of alcohol misuse (F(2,84)=4.36, P<0.01). Mean PRL responses were lower in patients with a history of alcohol misuse than in patients without a history of alcohol misuse (t=2.8, d.f.=58, P<0.01) and controls (t=2.22, d.f.=53, P<0.05) (see Table 3). Differences in PRL responses between controls and SHAPS psychopaths and non-psychopaths still showed a trend towards significance (F(2,36)=2.79, P=0.06) when patients with a history of alcohol misuse were excluded. These differences reached significance when dFEN drug levels were used as covariates (F(3,55)=3.59, P<0.05).
Dimensional relationships between variables
Psychometric data: intercorrelations
Composite impulsivity and aggression measures tended to correlate highly with each other (r=0.83, P<0.001). In addition, impulsivity tended to correlate with BDI score (r=0.28, P<0.01), trait anxiety (r=0.38, P<0.001) and frequency of self-harm (r=0.61, P<0.001). Composite aggression scores also showed significant correlations with self-harm frequency (r=0.65, P<0.001), BDI score (r=0.28, P<0.01) and trait anxiety (r=0.39, P<0.001).
Biochemical measures: intercorrelations
Baseline (+60 min) CORT and PRL levels did not correlate significantly (r=0.16, P=0.12), whereas the PRL and CORT responses to dFEN did correlate significantly (r=0.45, P<0.001). Testosterone levels did not correlate with either measure. Baseline CORT correlated negatively with the CORT response to dFEN (r=-0.45, P<0.001) and baseline PRL with PRL response to dFEN (r=-0.20, P=0.06).
Impulsivity/aggression and 5-HT
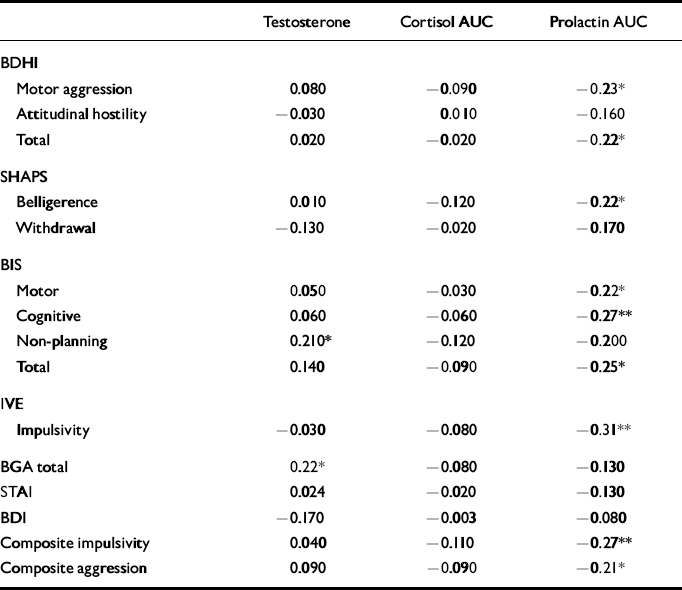
Correlations between hormonal data and psychometric measures are shown in Table 4. There were significant negative correlations between PRL response and composite impulsivity and aggression scores, which were largely attributable to significant individual associations between PRL response and IVE impulsivity, BIS scores (see Table 4) and BDHI indirect aggression scores (r=-0.25, P<0.05). A trend towards a significant inverse relationship with BDHI irritability (r=-0.20, P=0.06) was also noted. Analysis of the correlations in the larger patient sample alone gave the same results. Although BDI score correlated with impulsivity, there was no significant correlation between depression score and PRL response.
Table 4 Correlations between hormonal data and psychometric measures
| Testosterone | Cortisol AUC | Prolactin AUC | |
|---|---|---|---|
| BDHI | |||
| Motor aggression | 0.080 | -0.090 | -0.23* |
| Attitudinal hostility | -0.030 | 0.010 | -0.160 |
| Total | 0.020 | -0.020 | -0.22* |
| SHAPS | |||
| Belligerence | 0.010 | -0.120 | -0.22* |
| Withdrawal | -0.130 | -0.020 | -0.170 |
| BIS | |||
| Motor | 0.050 | -0.030 | -0.22* |
| Cognitive | 0.060 | -0.060 | -0.27** |
| Non-planning | 0.210* | -0.120 | -0.200 |
| Total | 0.140 | -0.090 | -0.25* |
| IVE | |||
| Impulsivity | -0.030 | -0.080 | -0.31** |
| BGA total | 0.22* | -0.080 | -0.130 |
| STAI | 0.024 | -0.020 | -0.130 |
| BDI | -0.170 | -0.003 | -0.080 |
| Composite impulsivity | 0.040 | -0.110 | -0.27** |
| Composite aggression | 0.090 | -0.090 | -0.21* |
The CORT AUC did not correlate significantly with any of the psychometric measures, whereas testosterone showed a positive correlation with BGA and the non-planning sub-scale on the BIS, which reflects a failure to plan ahead.
Impulsivity, aggression, alcohol misuse and 5-HT
To clarify further the relative contribution of impulsivity, aggression and alcohol misuse to PRL response, these variables were entered into a stepwise multiple regression analysis with PRL response as the dependent variable. Impulsivity was entered in step 1 (multiple R=0.28, F(1,85)=7.78, P=0.006) and alcohol misuse in step 2 (multiple R=0.33, F(2,84)=6.21, P=0.003). Aggression score was not accepted in the equation. Standardised beta (β) weights for impulsivity (β=0.39, P=0.0008) were higher than for alcohol misuse (β=0.23, P=0.04), indicating that impulsivity makes a greater contribution than a history of alcohol misuse to PRL response.
DISCUSSION
The main finding from this study is a dimensional relationship between 5-HT function and impulsivity. At the impulsive end of the spectrum, lower PRL responses to dFEN tended to occur in those defined as SHAPS psychopaths and those with DSM-III-R Axis II Cluster B disorders, particularly borderline personality disorders. At the low impulsive, or ‘inhibited’ end of the spectrum, the aggressive offenders categorised as SHAPS non-psychopaths and those with DSM-III-R Cluster A/C diagnosis (e.g. schizoid/obsessional personality disorders) had increased PRL responses to dFEN compared with controls. Measures of aggression also showed a significant negative correlation with 5-HT function but the relative importance of impulsivity as opposed to aggression in relationship to 5-HT function is extremely difficult to tease out, given the high correlation between impulsivity and aggression measures. The regression analysis, however, suggests that impulsivity makes a greater contribution to 5-HT function than composite aggression scores. Impulsivity as a dimension of extraversion (IVE) showed the strongest correlation with 5-HT function. Other findings of interest were the positive correlation between plasma testosterone and aggressive acts and the lower plasma cortisol concentration immediately after cannulation in SHAPS psychopaths, which may be a reflection of lower arousal in response to aversive stimulation in this group.
Methodological issues
Recruiting from maximum-security hospitals allowed us to investigate a population with personality disorders who were ‘pharmacologically clean’ in terms of recent illicit drug/alcohol misuse and prescribed psychotropic medication, thus minimising these confounding factors. Sampling patients and controls from the same institution also allowed some degree of control for the effects of environment, but the findings may not be truly representative of other samples of offenders with personality disorders or of controls. The fact that five staff controls had high scores on measures designed to tap anti-authoritarian attitudes and aggressive traits (SHAPS) suggests that some ‘normal’ subjects may be more aggressive than the general population. In terms of the overall findings of the study, however, it is possible that a comparison with a community sample of controls would have resulted in even greater observed differences between controls and psychopathic patients on measures of 5-HT function. The psychometric measures used in this study were selected so that comparisons could be drawn with previous studies in the field. The SHAPS was chosen for patient group assignment because it was validated in forensic populations and allowed categorisation of subjects on the basis of impulsivity and aggression (belligerence) dimensions, overcoming some of the problems of comorbid Axis II pathology in patients.
Although the PRL response to challenge by the dFEN is a dynamic index of 5-HT function, it reflects 5-HT synaptic activity in the hypothalamus and may not give a picture of 5-HT function in other parts of the brain, such as the frontal lobes, believed to be relevant to impulse control. At present, there are no established techniques for assessing regional 5-HT function in humans, although it is of interest that Siever et al (Reference Siever, Buchsbaum and New1999) reported reduced cerebral glucose utilisation using positron emission tomography in the frontal lobes of six subjects with impulsive aggressive behaviour compared with controls following dFEN administration, suggesting that 5-HT function also may be reduced in this region.
Relationship between impulsivity and aggression
In this study, impulsivity and aggression measures tended to correlate highly with each other. The SHAPS non-psychopathic offenders scored similar to controls on the SHAPS aggression sub-scale but higher than controls on other measures of aggression (BDHI assault and the BGA), consistent with their offences. The SHAPS sub-scale of aggression measures irritable/angry aggression rather than assault, which may account for these findings, but alternatively it may be less likely to suffer from ceiling effects because it was designed for use in forensic populations. The SHAPS non-psychopaths scored near control values on impulsivity measures, with non-significantly lower scores on the IVE than controls. This suggests that low impulsivity as part of the dimension of extraversion (IVE) may be an important component in determining group membership. The impulsivity measures may, however, have demonstrated floor effects and not been able to detect ‘overcontrol’ or ‘inhibition’ in this small group who had strikingly large PRL responses.
Impulsivity tended to correlate with anxiety and depression scores. Similar findings have been reported by others (Reference Apter, Van Praag and PlutchukApter et al, 1990), who suggest that these variables represent a serotonergically linked cluster. The association of anxiety measures with impulsivity and aggression argues against a simple model of impulsive aggression being due to low fear, as has been suggested for primary psychopaths (Reference FowlesFowles, 1980), but does not exclude subgroups of individuals for whom this might be true.
Serotonin function in relation to impulsivity and aggression
Our findings confirm previous reports (Reference O'Keane, Moloney and O'NeillO'Keane et al, 1992; Reference Coccaro, Kavoussi and HaugerCoccaro et al, 1998) that subjects characterised by high levels of impulsivity and aggression have reduced central 5-HT function revealed using the 5-HT specific challenge agent dFEN. That SHAPS psychopaths and those with borderline personality disorders have blunted PRL responses to dFEN is in line with Coccaro et al's (Reference Coccaro, Siever and Klar1989) findings using d,l-fenfluramine and points to impulsivity as a significant correlate of 5-HT function. The overlap between diagnoses of personality disorders makes it unlikely that low 5-HT function is a characteristic linked to specific personality disorders. In contrast to previous research in this area, we identified a small subgroup of offenders with elevated 5-HT function. It has been reported, however, that subjects with obsessive-compulsive disorder (Reference Insel, Mueller and AltermanInsel et al, 1985) and those with schizoid/autistic traits (Reference Sedvall, Fyro and GullbergSedvall et al, 1980; Reference McBride, Anderson and HertzigMcBride et al, 1989) have elevated 5-HT function. This suggests that 5-HT may influence a behavioural dimension with aspects of inhibition overcontrol at one end and impulsivity at the other.
In this study, PRL response to dFEN did not correlate with anxiety or depression in either controls or patients. Rydin et al (Reference Rydin, Schalling and Asberg1982) reported high anxiety and hostility scores in subjects with low CSF 5-HIAA, which would concur with our findings in SHAPS secondary psychopaths but subjects with impulsive aggressive behaviour without elevated anxiety also showed evidence of reduced 5-HT function. The relationship between anxiety, impulsivity/aggression and 5-HT remains unclear and it is likely that it is much more complex than can be illuminated using a single global measure of 5-HT function such as in this study. For example, there is evidence for differing roles for 5-HT depending on the type of anxiety and site of action in the brain (Reference Deakin and GraeffDeakin & Graeff, 1991). The PRL responses to dFEN are believed to be mediated through 5-HT2 receptors and it is known that other receptors such as the 5-HT1A receptor also play a role in anxiety (Reference HollisterHollister, 1994) and aggression (see Reference Mak, De Koning, Olivier, Hollander and SteinMak et al, 1995).
In line with previous reports, we found that alcohol misuse is related to a low 5-HT state and this may be a significant confounding factor in studies of 5-HT and aggression (see Reference Markowitz, Coccaro, Hollander and SteinMarkowitz & Coccaro, 1995, for a review). Our findings, however, suggest that alcohol misuse does not satisfactorily account for the observed relationships between impulsivity and 5-HT function.
As in Coccaro et al's (Reference Coccaro, Siever and Klar1989) study, we were unable to report an association between a history of drug misuse and 5-HT function. Previous studies addressing comorbid impulsivity/aggression and drug misuse (Reference Fishbein, Lozovsky and JaffeFishbein et al, 1989; Reference Moss, Yao and PanzakMoss et al, 1990) have produced conflicting findings. The discrepancies observed may relate to the sample characteristics, but also to the types of drugs used.
We observed a tendency to lower PRL responses in patients with a history of repetitive self-harming behaviour. The frequency of self-harm correlated positively with impulsivity and aggression measures and with anxiety and depression scores. However, the lack of relationship between mood measures and 5-HT function suggests that the dimension of impulsivity/aggression is more important in determining self-harming behaviour.
We did not find significant CORT responses to dFEN, which is likely to explain the lack of relationship between CORT measures and impulsivity. The PRL and CORT responses to 5-HT challenge are probably mediated by different serotonergic receptors (Reference CoccaroCoccaro, 1992) and there is some evidence that CORT responses to FEN may be mediated by non-5-HT mechanisms (Reference Van de Klar and BetheaVan de Klar & Bethea, 1982). We observed a significantly lower post-cannulation plasma CORT concentration in SHAPS psychopaths, particularly primary psychopaths, than controls, possibly suggesting a lower than normal physiological stress response at the prospect of the invasive procedure. This finding concurs with previous reports that ‘criminal psychopaths’, particularly ‘primary psychopaths’, have reduced levels of stress prior to anxiety-provoking situations and display electrodermal hyporesponsiveness when anticipating aversive stimulation (Reference HareHare, 1978).
Testosterone and impulsivity/aggression
Although plasma-free or salivary testosterone is known to correlate better with aggressiveness (Reference ArcherArcher, 1991), our finding that plasma testosterone correlated positively with life-time aggression and that mean testosterone levels were higher in SHAPS impulsive aggressive psychopaths, compared with controls, supports early reports that an early onset and a repetitive pattern of aggressiveness are associated with elevated testosterone concentrations (Reference Virkkunen, Rawlings and TakolaVirkkunen et al, 1994). A suggested mechanism for this link has been a reduction in CORT's usual inhibition of luteinising hormone (LH) secretion due to low plasma CORT resulting in greater stimulation of testosterone secretion by LH (Reference Mason, Giller and KostenMason et al, 1988). We found a tendency towards higher testosterone and lower post-cannulation CORT concentrations, but baseline CORT values did not differ between groups and there was no inverse correlation between hormones, giving no direct support to this theory. Differences in the measures of testosterone employed in each study may account for the discrepancies.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ This study of aggressive offenders highlights the heterogeneity of diagnosis of personality disorders within this population.
-
▪ Serotonin (5-HT) function may particularly relate to the dimension of behaviour control (inhibition-impulsivity) and suggest that serotonergic agents may have a therapeutic role for some offenders with personality disorders.
-
▪ In offenders with personality disorders who have high impulsivity but low anxiety levels, testosterone may also play a role in aggression control.
LIMITATIONS
-
▪ The study sample may not be representative of offenders with aggressive personality disorders in other non-hospital settings.
-
▪ The biological measures used only provide an indirect measure of brain function and hormonal influences.
-
▪ The correlations between impulsivity, aggression and 5-HT function are relatively weak and emphasise the need to investigate its relationship to other important determinants of impulsive aggressive behaviour, such as cognitive function.
ACKNOWLEDGEMENTS
We thank the staff and patients at Ashworth and Broadmoor hospitals for their cooperation with this project, and Professors P. Taylor and R. Blackburn for their support and assistance. We also thank Dr M. Franklin and staff at the Psychopharmacology Unit, Littlemore Hospital, Oxford.








eLetters
No eLetters have been published for this article.