Rapid eye movement (REM) sleep behaviour disorder (American Sleep Disorders Association, 1990) is an REM-stage parasomnia characterised by violent motor and verbal activity, in contrast to the usual paralysis in REM sleep. Polysomnography demonstrates the absence of the normal atonia of REM. Violence may relate to vivid dream content and be directed at others, resulting in injury to a bed partner; the dreams are typically recalled (Reference Schenck, Bundlie and EttingerSchenck et al, 1986). It is most commonly reported in men aged about 60. While ‘idiopathic’ in about half of reported cases (Reference Schenck, Bundlie and MahowaldSchenck et al, 1996a ), in the remainder it has been described in association with a wide range of neurological disorders, including 11 cases with Parkinson's disease, two patients with diffuse Lewy body disease (one of them having dementia), one with ‘atypical’ dementia, one with an unspecified type of dementia and one with Alzheimer's disease (Schenck et al, Reference Schenck, Bundlie and Ettinger1986, Reference Schenck, Bundlie and Patterson1987, Reference Schenck, Bundlie and Mahowald1996a ,Reference Schenck, García-Rill and Skinner1996b ; Reference Uchiyama, Isse and TanakaUchiyama et al, 1995; Reference Turner, Chervin and FreyTurner et al, 1997). Non-progressive cognitive dysfunction has been described in one patient, a former alcoholic with subarachnoid haemorrhage (Reference Schenck, Bundlie and EttingerSchenck et al, 1986). Other case reports have linked the condition with Guillain-Barré syndrome, head injury, multiple system atrophy, pontine lesions and post-traumatic stress, and as a side-effect of drugs including alcohol, fluoxetine and clomiprimine (Reference Bental, Lavie and SharfBental et al, 1979; Reference Schenck, Bundlie and PattersonSchenck et al, 1987; Reference Nofzinger and ReynoldsNofzinger & Reynolds, 1994; Reference Tison, Wenning and QuinnTison et al, 1995). The condition, still little known to many clinicians, may easily be treated with clonazepam (Reference Schenck, Bundlie and PattersonSchenck et al, 1987).
CASE HISTORY
Presenting complaint
A 74-year-old man first attended St Thomas' Hospital, London in 1993, with a four-year history of low mood with increasing irritability and anhedonia (particularly for previously enjoyable hobbies such as driving), associated with apathy, withdrawal from social activities (such as seeing old friends) and reduced daytime physical activity. The onset of affective symptoms was precipitated by moving family home, and exacerbated by the subsequent divorce of his daughter. Never an emotionally demonstrative man, he had not shown excessive tearfulness, or hopelessness. He had found his relative inactivity in retirement hard to tolerate.
Collateral history
The patient's wife stated that he had become more vague, and that his previously very good memory had deteriorated, over four years. On further questioning, she complained bitterly of a change in his sleep behaviour, which had become increasingly disturbed over the same period. One-and-a-half to two hours after falling asleep, the patient would thrash his arms and legs about, talk or shout unintelligibly while sitting up, and exhibit increasingly violent behaviour towards his wife. On occasion, he knocked or threw bedside furniture towards her. His snoring had increased greatly, and he reported ‘menacing’ dreams. This behaviour occurred for prolonged periods between the hours of 01.30 and 04.30. The subject was difficult to rouse during these episodes; on awaking he had no dream recall, appeared confused and was unaware of what had happened. At no time did he describe to his wife a dream of a violent nature that could be directly related to his sleeping behaviour. The patient complained of increased waking during the night.
Personal history
Apart from some night terrors in childhood, there was no significant personal or family history of psychiatric disorder. An electrical engineer, the subject retired in 1984 as a director of a multinational company. He remarried at the age of 40; his wife had never noticed sleep-talking, sleep-walking, abnormal nocturnal movements, or bruxism until the recent symptoms. A non-smoker, he drank moderately. He lost consciousness for one hour in a road traffic accident in 1960, but there was no skull fracture.
CLINICAL ASSESSMENT, INVESTIGATIONS AND PROGRESS
Mental state
When first seen by us, the subject's social manner and dress were normal. He had somewhat circumlocutary speech. He described his mood state as mildly ‘worried’. Generally pleasant and charming within the clinic, he was occasionally tense and more withdrawn, and he often became irritable with his wife. On clinical cognitive testing, he was fully oriented in time, place and person, with good attention and performance on basic screening tests.
Neuropsychology
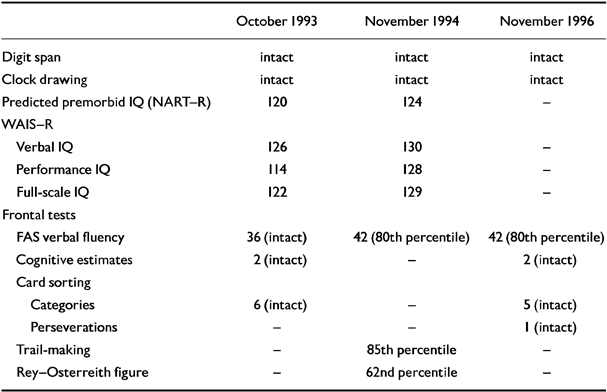
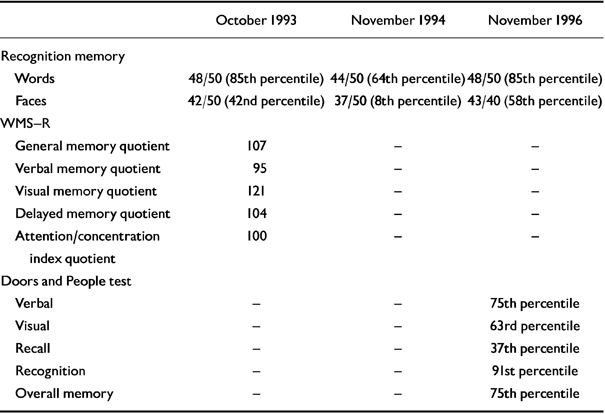
Neuropsychological testing at this time showed a high verbal and full-scale IQ consistent with the patient's predicted premorbid IQ (as measured by using the National Adult Reading Test-Revised (NART-R; Reference Nelson and WillisonNelson & Willison, 1991), with a 12-point verbal-performance IQ difference (see Tables 1 and 2). Although frontal/executive test scores were within the normal range, one might expect the subject's FAS verbal fluency score (Reference BentonBenton, 1968) to have been higher, given his premorbid educational achievement. The visual memory quotient from the Wechsler Memory Scale - Revised (WMS-R; Reference WechslerWechsler, 1987) and the score on the words component of the Recognition Memory Test (RMT; Reference WarringtonWarrington, 1984) were well above average (Table 2). However, the verbal memory quotient and the delayed recall and attention/concentration quotients on the WMS-R were some 20-30 points below what would be expected on the basis of the subject's IQ, raising suspicions of an early dementia.
Table 1 General cognitive tests
| October 1993 | November 1994 | November 1996 | |
|---|---|---|---|
| Digit span | intact | intact | intact |
| Clock drawing | intact | intact | intact |
| Predicted premorbid IQ (NART-R) | 120 | 124 | - |
| WAIS-R | |||
| Verbal IQ | 126 | 130 | - |
| Performance IQ | 114 | 128 | - |
| Full-scale IQ | 122 | 129 | - |
| Frontal tests | |||
| FAS verbal fluency | 36 (intact) | 42 (80th percentile) | 42 (80th percentile) |
| Cognitive estimates | 2 (intact) | - | 2 (intact) |
| Card sorting | |||
| Categories | 6 (intact) | - | 5 (intact) |
| Preseverations | - | - | 1 (intact) |
| Trail-making | - | 85th percentile | - |
| Rey-Osterreith figure | - | 62nd percentile | - |
Table 2 Anterograde memory tests
| October 1993 | November 1994 | November 1996 | |
|---|---|---|---|
| Recognition memory | |||
| Words | 48/50 (85th percentile) | 44/50 (64th percentile) | 48/50 (85th percentile) |
| Faces | 42/50 (42nd percentile) | 37/50 (8th percentile) | 43/40 (58th percentile) |
| WMS-R | |||
| General memory quotient | 107 | - | - |
| Verbal memory quotient | 95 | - | - |
| Visual memory quotient | 121 | - | - |
| Delayed memory quotient | 104 | - | - |
| Attention/concentration index quotient | 100 | - | - |
| Doors and People test | |||
| Verbal | - | - | 75th percentile |
| Visual | - | - | 63rd percentile |
| Recall | - | - | 37th percentile |
| Recognition | - | - | 91st percentile |
| Overall memory | - | - | 75th percentile |
Investigations
The results of a routine blood screen were normal, as were those of a chest X-ray, 24-hour electrocardiogram (ECG), daytime and sleep electroencephalograms (EEGs), pulse oximetry, and fluorodeoxyglucose brain positron emission tomography (PET). Brain magnetic resonance imaging (MRI) showed mild cortical atrophy and a slight degree of ventricular dilatation.
Initial treatment
Before referral to the clinic, dothiepin 150 mg at night had failed to improve the subject's symptoms. We replaced this treatment with sertraline, titrated to 150 mg daily. This produced clinical improvement in mood, and his wife reported that the subject became less withdrawn and irritable. The new treatment also helped his sleep disturbance, reducing the frequency and severity of the sleep symptoms. This response was found to be dose-dependent: the symptoms of thrashing, shouting and disturbed sleep returned within a few days of the sertraline dose being reduced to 100 mg daily, and improved again within days of reinstitution of the 150 mg dose.
Further sleep investigation and treatment
After some months, the sleep and behaviour disturbance worsened again despite the treatment with sertraline, at which time nocturnal polysomnography was performed, using a conventional montage of EEG (C1A2, C2A3), sub-mental electromyelogram (EMG), ECG, respiratory inductance plethysmography, measurement of oronasal airflow by thermistor, tibial EMG and pulse oximetry. The important findings were periods of REM without atonia (Fig. 1), frequent bursts of periodic leg movements associated with arousal, and normal respiration throughout without apnoeas or hypopnoeas. Clonazepam 0.5 mg at night was prescribed and produced a period of asymptomatic sleep, but intermittent motor activity returned, unresolved by an increase in dose to 0.75 mg. At about this time, the subject developed loud snoring: repeat nocturnal polysomnography (on treatment) now demonstrated moderate obstructive sleep apnoea, in addition to the features previously seen; this was effectively controlled by nasal continuous positive airways pressure. The latter treatment quickly appeared to improve the patient's sleep pattern, and in the daytime gave him ‘more energy’, a renewed interest in his hobbies, improved alertness and reduced irritability. These changes have persisted. He has remained on continuous pharmacotherapy (sertraline and clonazepam) and continuous positive airways pressure.

Fig. 1 Two-minute epoch of rapid eye movement sleep (note electro-oculogram (EOG) activity) without atonia (note chin electromyelogram (EMG) activity) and with bursts of increased chin EMG associated with limb movements tibialis (LTIB) EMG. EEG, electroencephalogram; MIC, microphone; FLOW, airflow; THOR, thoracic effort; ABD, abdominal effort.
Further cognitive examination
In-patient investigations in November 1994 and November 1996 included repeat neuro-psychological testing, a blood screen for dementia, and neuroimaging. Although the subject showed slight improvements in his IQ and FAS verbal fluency scores in November 1994 (which were maintained two years later), there was evidence of decline on the word and face recognition memory test. By November 1996, the results of this test had also returned to normal (Tables 1 and 2). On the latter occasion, the patient's ‘overall memory’ score on the Doors and People test (Reference Baddeley, Emslie and Nimmo-SmithBaddeley et al, 1994) was at the 75th percentile, equivalent to a memory quotient greater than 115 (i.e. close to his estimated premorbid IQ score). The second brain MRI showed very minimally increased general cortical atrophy. Results of the repeat blood dementia screen were normal.
The patient's erythrocyte sedimentation rate, initially normal, rose to 89 at one point in association with symptoms of polymyalgia rheumatica. It responded fully to low-dose prednisolone, which was gradually withdrawn.
Outcome
No further sleep, mood or behavioural abnormalities were observed during two inpatient assessments with clonazepam and sertraline therapy. By February 1997, the subject's depression had improved and he was off medication (at his insistence); his memory and frontal test scores had not declined (several of these scores are well above the population means), while the verbal-performance IQ discrepancy had narrowed to only two points.
DISCUSSION
The present case involved an unusual combination of three problems — depression, mild cognitive impairment and sleep disorder. It was only after detailed questioning that we elicited the last. Sleep laboratory investigation confirmed REM sleep behaviour disorder, which responded temporarily to sertraline, then briefly to clonazepam. Later, when sleep apnoea had been identified in addition, a combination of these drugs with nasal continuous positive airways pressure proved effective.
Sleep disorder diagnosis
The diagnosis of REM sleep behaviour disorder was made on the basis of the patient's history and the findings from polysomnography. The violent motor jerks and apparently coordinated actions and gestures, occurring with cries and speech in the middle and late stages of the night, when REM sleep was indicated on sleep staging, clearly indicated this diagnosis. The transient confusion on waking from this state, the lack of dream recall and the detection of additional sleep apnoea do not accord with the original description of the condition by Schenck et al (Reference Schenck, Bundlie and Ettinger1986). However, similar absence of dream recall, with associated sleep apnoea and daytime ‘confusion’, has been reported by Turner et al (Reference Turner, Chervin and Frey1997) in a case of REM sleep behaviour disorder with clinical diffuse Lewy body disease. Although our patient may represent an interesting parallel, he did not have symptoms sufficient to make a diagnosis of diffuse Lewy body disease (Reference McKeith, Fairbairn and PerryMcKeith et al, 1994). The only sleep pathology in depression, reduction in REM latency, is unlikely to have contributed to the loss of REM atonia. Our patient did not sleepwalk, and showed no other symptoms suggestive of a partial arousal disorder. His history of infantile night terrors may represent a vulnerability to sleep disorders.
Non-progressive cognitive impairment
The subject's cognitive deterioration, which was initially interpreted as early dementia, showed no further progress. In contrast, many of the later tests (e.g. FAS verbal fluency) showed ‘normal’ or improved scores. This leads us to attribute his initial cognitive decline to normal ageing aggravated by a combination of depression and REM sleep behaviour disorder — a variant of ‘depressive pseudodementia’. This contrasts with the ‘organic’ central nervous system pathology found to be present in up to half the known cases of REM sleep behaviour disorder to date (Reference Schenck, Bundlie and MahowaldSchenck et al, 1996a ).
Neurochemistry of REM sleep behaviour disorder and depression
It has been suggested that loss of noradrenergic locus ceruleus neurons may cause REM sleep behaviour disorder in humans by removing a gate mechanism responsible for inhibiting the cholinergic mesopontine neurons. The latter are involved in the reticular activating system, and may ‘drive’ REM sleep (Reference Schenck, García-Rill and SkinnerSchenck et al, 1996b ). The ‘release’ of our patient's REM sleep behaviour disorder in association with depression, and the subsequent response of both conditions to a selective serotonin reuptake inhibitor (SSRI) antidepressant, may further implicate adrenergic (and serotonergic) brain dysfunction in the aetiology of REM sleep behaviour disorder.
Drug treatment of the sleep disorder
This case is the first reported instance of a serotonergic antidepressant drug (transiently) suppressing REM sleep behaviour disorder. Reduction in duration of REM sleep by SSRIs provides a possible explanation for such an effect (Reference Sharpley and CowenSharpley & Cowen, 1995), but the cellular mechanism for this is not understood. With the use of a benzodiazepine for REM sleep behaviour disorder, the development of obstructive sleep apnoea could possibly have been predicted. In this age group, more than 31% of men have more than five apnoeas per hour of sleep (Reference Young, Palta and DempseyYoung et al, 1993). The clonazepam might therefore have precipitated clinically significant sleep apnoea — and, with this, the recurrence of thrashing in bed, a recognised feature of the disorder (Reference GuilleminaultGuilleminault, 1989). Nasal continuous positive airways pressure is uniformly effective in treating sleep apnoea and allows the continued use of sedatives. Alleviation of the sleep disruption caused by obstructive sleep apnoea may also have contributed to the relief of our patient's depression.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ REM sleep behaviour disorder is often overlooked as a diagnosis. It can be determined from the clinical history and specialist sleep investigations.
-
▪ REM sleep behaviour disorder is a treatable condition (with clonazepam).
-
▪ REM sleep behaviour disorder may occur against a background of treatable depression.
LIMITATIONS
-
▪ This paper describes a single case only.
-
▪ Lack of current understanding of the neuronal basis of REM sleep behaviour disorder limited the interpretation of the mechanism(s) by which the treatments used had their effect.
-
▪ The complex interactions between REM sleep behaviour disorder, depression and cognitive impairment sometimes made interpretation of symptoms difficult.
ACKNOWLEDGEMENTS
The authors thank Drs Hana Laing and Eli Jaldow for their help with cognitive testing, and Simone deLacy for her help with polysomnography.






eLetters
No eLetters have been published for this article.