Effective treatment of the first episode of schizophrenia has lately come to be seen as crucial to improving long-term outcomes. However, the evidence base for optimal first-episode treatments is not strong. The mainstay of management for acute symptoms of schizophrenia continues to be antipsychotic drug treatment and there is evidence that young people in their first episode are sensitive to both its therapeutic and adverse effects (Reference Remington, Kapur and ZipurskyRemington et al, 1998). Direct psychological approaches have not been studied. Four separate randomised controlled trials have reported the effectiveness of cognitive—behavioural therapy (CBT) as an adjunct to usual treatment in treating persistent symptoms of chronic schizophrenia (Tarrier et al, Reference Tarrier, Beckett and Harwood1993, Reference Tarrier, Yusupoff and Kinney1998; Reference Kuipers, Garety and FowlerKuipers et al, 1997; Reference Sensky, Turkington and KingdonSensky et al, 2000). One trial has shown efficacy in acute relapse (Drury et al, Reference Drury, Birchwood and Cochrane1996a , Reference Drury, Birchwood and Cochrane b ).
Our hypothesis for this study was that CBT in addition to routine care would accelerate remission from acute symptoms in first- and second-episode schizophrenia and prevent future relapse. The trial was designed to recruit a large, representative subject sample, using independent, concealed randomisation, a well-specified intervention with objective measures of fidelity, controls for non-specific effects, blind assessments and intention-to-treat analysis. The acute-phase outcome data are reported here.
METHOD
The design of the study (SoCRATES: Study of Cognitive Reality Alignment Therapy in Early Schizophrenia) was a prospective, rater-blind, randomised controlled trial. The feasibility of the planned intervention in patients in an early, acute stage of schizophrenia was tested and confirmed in a pilot study in a separate sample of 35 patients (Reference Haddock, Tarrier and MorrisonHaddock et al, 1999c ).
A power calculation group showed that a sample size of 118 per treatment arm would give: 90% power to detect a 50% drop in Positive and Negative Syndrome Scale (PANSS) scores by the end of the treatment phase in 70% of cases in the experimental treatment group v. 50% of the routine care group; and 85% power to detect a reduction in mean length of index admission in the treatment group of 25%. We assumed that 40% of eligible subjects would decline to enter the study and there would be 10% attrition because of withdrawals and other losses.
Participants
Subjects were recruited over 26 months from the 11 mental health units serving 3 geographically defined catchment areas, Manchester/Salford, Liverpool and north Nottinghamshire, in England with a combined population of 2 150 000.
Inclusion criteria for subjects to enter the trial were: (a) either first or second admission (within 2 years of a first admission) to in-patient or day patient unit for treatment of psychosis; (b) DSM—IV criteria for schizophrenia, schizophreniform disorder, schizoaffective disorder or delusional disorder (American Psychiatric Association, 1994); (c) positive psychotic symptoms for 4 weeks or more; (d) score of 4 or more (moderate or severe) on the PANSS (Reference Kay, Opler and LindenmayerKay et al, 1989) target item either for delusions (P1) or hallucinations (P3); (e) neither substance misuse nor organic disorder judged to be the major cause of psychotic symptoms. Patients legally detained in hospital were eligible. Potentially eligible patients were screened within 14 days of hospital admission by a research psychiatrist. Following written consent, baseline assessments were done, including demographic data. Diagnostic assessments at baseline were confirmed by raters on chart review at 12-week follow-up.
Outcome measures
Two measures of symptoms at baseline and follow-up were used as primary outcome measures: the PANSS total and positive scale scores and the Psychotic Symptom Rating Scales (PSYRATS; Reference Haddock, McCarron and TarrierHaddock et al, 1999a ). The PSYRATS scales were developed to measure dimensions of delusional beliefs (Delusions Scale, DS) and auditory hallucinations (Auditory Hallucination Scale, AHS) and have been shown to have good reliability and validity with sensitivity to change (Reference Haddock, McCarron and TarrierHaddock et al, 1999a ). Good reliability between the three raters was established using videotaped interviews. The main planned analysis was a regression analysis by treatment group and the main goal of outcome assessments was to achieve a minimum of at least one outcome assessment point on the primary outcome measures for every subject during the acute-phase follow-up period from baseline to 70 days. Five post-baseline assessment visits were scheduled: at 14, 21, 28 and 35 days and the final acute-phase assessment between 42 and 70 days.
Intervention groups: CBT and supportive counselling
The interventions were carried out independently of clinical staff, who were kept unaware of treatment allocation. Direct family interventions were not undertaken. Procedures to standardise routine clinical care, including drug treatment, were not used.
The CBT was manual-based and conducted by one of five therapists trained in CBT in psychosis, supervised by experienced cognitive therapists. The design of the delivery was to aim for 15-20 hours within a 5-week treatment envelope, plus ‘booster’ sessions at a further 2 weeks and 1, 2 and 3 months. Details of the treatment are given in Haddock et al (Reference Haddock, Morrison and Hopkins1999b ). In summary, treatment was conducted in four stages. The first stage was engagement and a detailed assessment of mental state and symptom dimensions (psychotic and nonpsychotic) to allow a cognitive—behavioural analysis of how symptoms might relate to cognitions, behaviour and coping strategies. Education about the nature and treatment of psychosis, using a stress vulnerability model to link biological and psychological mechanisms, was used to help engagement. Second, a problem list was generated collaboratively with the patient. This was then prioritised according to the degree of distress attached, feasibility and, where relevant, clinical risk involved. Prioritised problems were assessed in detail and a formulation was agreed which included such issues as trigger situations and cognitions. Third and fourth stages were intervention and monitoring. Interventions particularly addressed positive psychotic symptoms of delusions and hallucinations, generating alternative hypotheses for abnormal beliefs and hallucinations, identifying precipitating and alleviating factors and reducing associated distress.
Supportive counselling was used as a comparison intervention to control for non-specific elements of therapist exposure. It was delivered in the same 5-week format with three boosters, with the aim of matching the duration of total therapist contact time to that in the CBT arm. The supportive counselling was also manual-based and supervised by an experienced counsellor. The same five research therapists administered both CBT and supportive counselling interventions, according to randomisation.
Interventions were started within 3 days of randomisation. Patients were seen in hospital settings, family practitioner surgeries and in their own homes for treatment sessions. All treatment sessions, both for CBT and supportive counselling, were audiotaped where consent was given. After the acute phase of the study was completed, a random selection of 50 tapes were rated blindly by two independent raters, who were asked to classify them as CBT or supportive counselling sessions and to rate the quality of therapy on the Cognitive Therapy Scale for Psychosis (CTS—Psy; Reference Haddock, Devane and BradshawHaddock et al, 2001). Raters correctly classified 49 of the 50 tapes to the appropriate therapy and the quality was assessed as high compared with accepted criteria.
Statistical analysis
Analyses were conducted on an intention-to-treat basis, with patients analysed in the treatment group to which they were randomised. All randomised patients were included with the exception of those in whom diagnostic inclusion criteria were violated within 1 week. The acute phase for the purposes of the regression analysis was preset at 70 days, because all CBT and supportive counselling treatment envelopes had been completed by this time, as had the acute-phase assessments. Primary outcomes were PANSS total and positive sub-scale scores and PSYRATS—DS and PSYRATS—AHS scores. The analysis of PSYRATS—AHS was confined to those patients with auditory hallucinations (i.e. PSYRATS—AHS scores >0) at baseline.
An initial exploratory analysis involved calculation of means and standard deviations of the various outcome scores after first grouping the assessment times to the closest assessment visit schedule in the trial protocol.
The repeated measures (up to 70 days) were analysed through the use of the Stata xtreg procedure (Stata Corporation, 1997) with the stratifying variables as covariates, linear and quadratic effects of the assessment time (in weeks), together with a contrast measuring the difference linear rate of change between treated patients (CBT and supportive counselling combined) and the controls (routine care) and another contrast comparing the linear rate of change in the two treated groups (i.e. CBT v. supportive counselling). The default fitting method (generalised least squares) was used for all models. Note that in this model the mean symptom scores at baseline are constrained to be equal for the treatment groups within strata (an implication of randomisation). A negative trend corresponds to an improvement in symptoms. A negative contrast implies that the treated are improving more quickly than the untreated (contrast 1) and that the patients undergoing CBT are doing better than those with supportive counselling (contrast 2).
Assignment
Independent, concealed randomisation of individuals with minimisation was then performed by a trial administrator at each centre. Stratification was undertaken with the following variables: first or second admission; in-patient or day patient admission; male or female; with the first-episode cases further stratified for duration of symptoms of more or less than 6 months.
All outcome assessments were made blind to treatment allocation. Extensive steps were taken to maintain blindness of raters. In all cases, randomisation was carried out by a trial administrator independently of rater or therapist. Therapist and rater were not to communicate details about individual patients to each other. Office space and data storage were kept separate and secure. Clinical staff were instructed not to divulge details of therapist contacts to the raters.
RESULTS
Participant flow and follow-up
Initial case note review led to 433 patients being screened at interview. Of these, 370 met eligibility criteria for study entry. Of these, 315 gave written consent to participate in the study, after 10 were judged incapable of giving informed consent and a further 45 declined (Fig. 1). Patients were recruited at a median of 6 days after hospital admission and all randomised within 3 days of consent. There were no significant differences between consenting and non-consenting subjects in age, gender, ethnicity, first versus second admission and day versus in-patient treatment. One subject was randomised twice by error (once on first and once on second admission); the second randomisation was disregarded in the analysis. Six patients were excluded from the study and the analysis after it became clear on assessment by 1 week that they met diagnostic exclusion criteria (three organic psychosis; two bipolar disorder; four factitious psychosis).
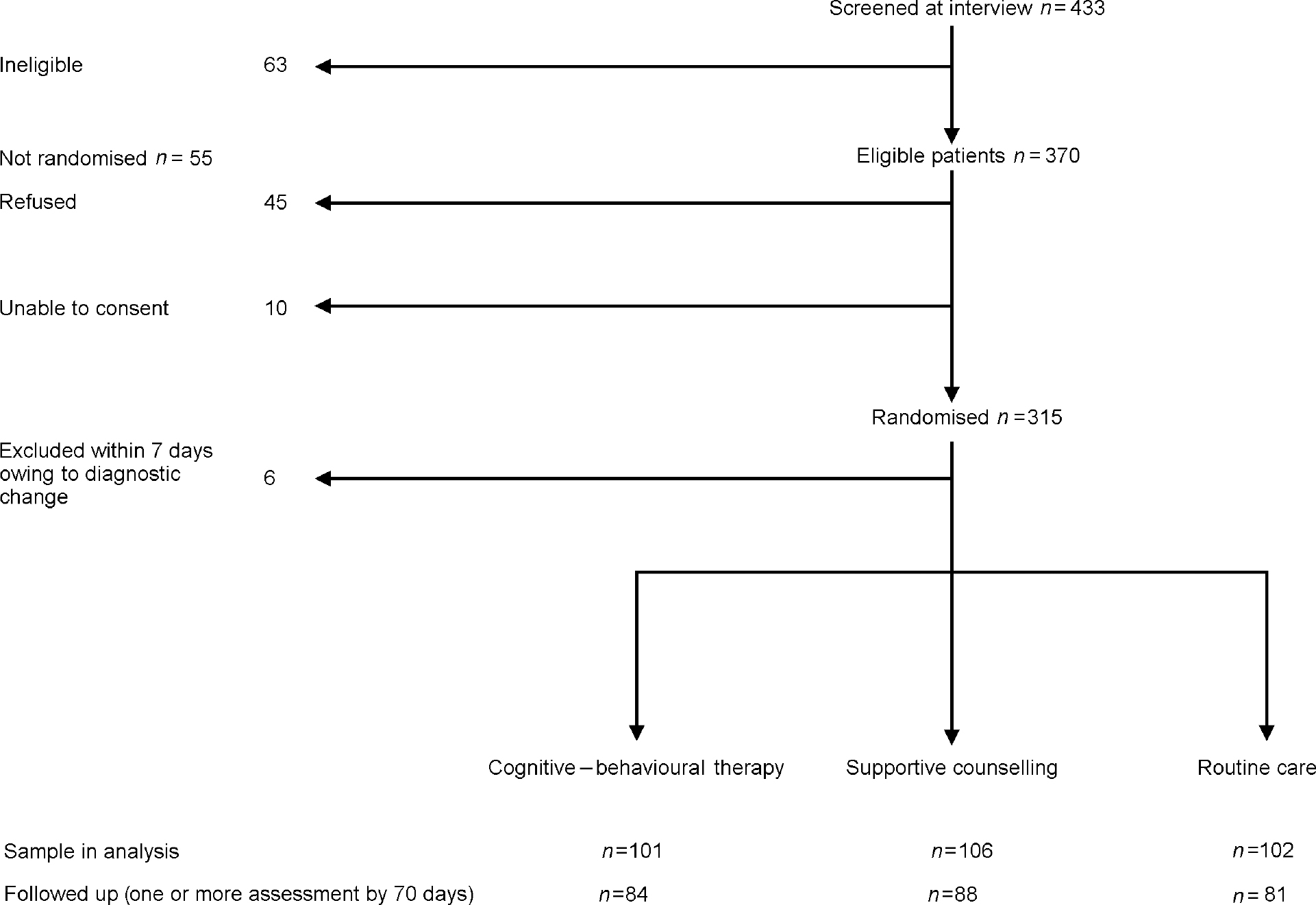
Fig. 1 Trial profile
Thus the total sample for analysis was 309: CBT 101; supportive counselling 106; routine care 102 (Table 1). Fifteen patients (CBT 4; supportive counselling 4; routine care 7) withdrew consent to participate during the follow-up period, but are included in the analysis prior to their withdrawal; 13 of these withdrawals occurred during the first 2 weeks. One patient died during the follow-up period (in the supportive counselling group). Of the sample for analysis, 253 (82%) subjects had outcome data at one or more follow-up assessments over 70 days and contributed to the main outcome analysis. The subjects with one or more assessments at follow-up did not differ significantly from the unassessed subjects on age, gender or ethnicity.
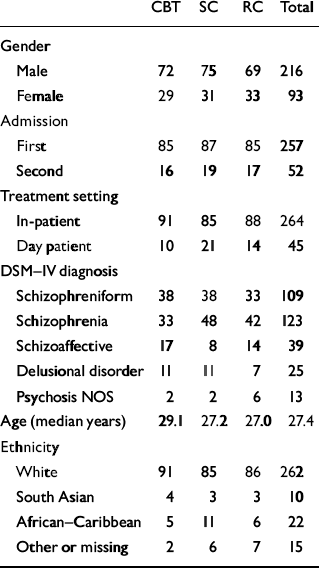
Table 1 Demographic and clinical characteristics of participants in analysis (n=309)
| CBT | SC | RC | Total | |
|---|---|---|---|---|
| Gender | ||||
| Male | 72 | 75 | 69 | 216 |
| Female | 29 | 31 | 33 | 93 |
| Admission | ||||
| First | 85 | 87 | 85 | 257 |
| Second | 16 | 19 | 17 | 52 |
| Treatment setting | ||||
| In-patient | 91 | 85 | 88 | 264 |
| Day patient | 10 | 21 | 14 | 45 |
| DSM—IV diagnosis | ||||
| Schizophreniform | 38 | 38 | 33 | 109 |
| Schizophrenia | 33 | 48 | 42 | 123 |
| Schizoaffective | 17 | 8 | 14 | 39 |
| Delusional disorder | 11 | 11 | 7 | 25 |
| Psychosis NOS | 2 | 2 | 6 | 13 |
| Age (median years) | 29.1 | 27.2 | 27.0 | 27.4 |
| Ethnicity | ||||
| White | 91 | 85 | 86 | 262 |
| South Asian | 4 | 3 | 3 | 10 |
| African—Caribbean | 5 | 11 | 6 | 22 |
| Other or missing | 2 | 6 | 7 | 15 |
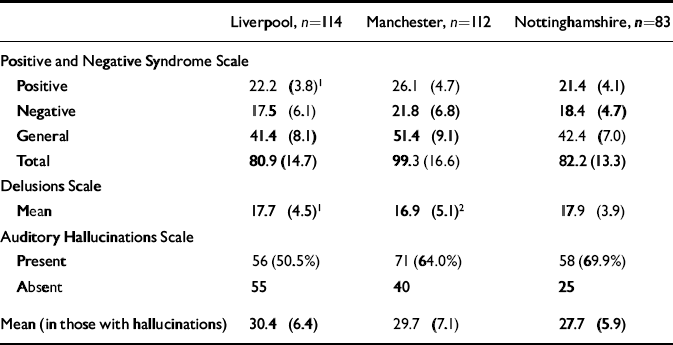
Overall, 70% of the sample were male and 83% were first admissions. Of the total sample, 38% were detained under the Mental Health Act during the 70-day period, reflecting the fact that this was a severely ill sample. Baseline symptom characteristics by centre are shown in Table 2.
Table 2 Baseline symptom scores by centre (mean (s.d.))
| Liverpool, n=114 | Manchester, n=112 | Nottinghamshire, n=83 | |
|---|---|---|---|
| Positive and Negative Syndrome Scale | |||
| Positive | 22.2 (3.8)1 | 26.1 (4.7) | 21.4 (4.1) |
| Negative | 17.5 (6.1) | 21.8 (6.8) | 18.4 (4.7) |
| General | 41.4 (8.1) | 51.4 (9.1) | 42.4 (7.0) |
| Total | 80.9 (14.7) | 99.3 (16.6) | 82.2 (13.3) |
| Delusions Scale | |||
| Mean | 17.7 (4.5)1 | 16.9 (5.1)2 | 17.9 (3.9) |
| Auditory Hallucinations Scale | |||
| Present | 56 (50.5%) | 71 (64.0%) | 58 (69.9%) |
| Absent | 55 | 40 | 25 |
| Mean (in those with hallucinations) | 30.4 (6.4) | 29.7 (7.1) | 27.7 (5.9) |
Treatment exposure and fidelity
In terms of exposure to treatment, mean number of therapy sessions was similar in the CBT group (mean 16.1 sessions, 95% CI 15.2-17.1) and the supportive counselling group (mean 15.7 sessions, 95% CI 14.7-16.7). The CBT group did receive more total therapy time (mean 8.6 hours, 95% CI 7.6-9.63) than the supportive counselling group (mean 7.1 hours, 95% CI 6.3-7.9). Four subjects allocated to CBT and 6 allocated to supportive counselling attended no treatment sessions. For the rating of treatment fidelity, agreement between the two independent blind raters was good (intraclass correlation on Cognitive Therapy Scale of 0.93). Quality of CBT was assessed as good, with the ‘Cognitive techniques’ sub-scale score of the CTS-Psy confirming the specificity of cognitive—behavioural techniques to the CBT group (mean sub-scale score 20.7; 95% CI 18.2-23.2) and their absence in the supportive counselling group (mean sub-scale score 2.7; 95% CI 1.9-3.6). Raters correctly classified 49 of the 50 tapes to the appropriate therapy.
Outcomes
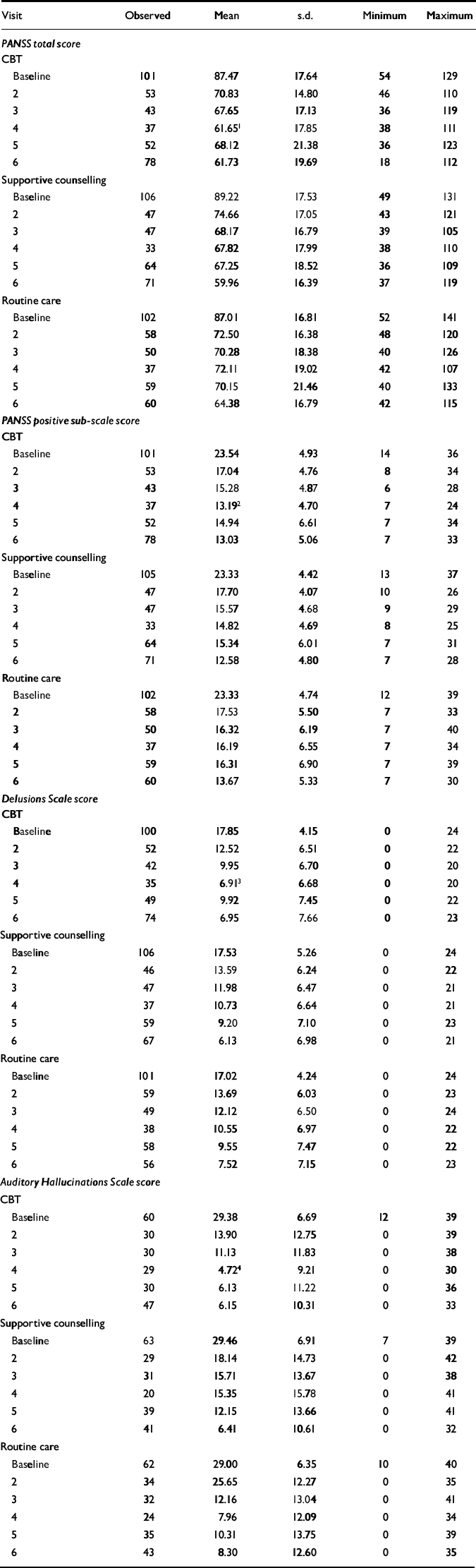
Summary statistics, showing mean outcome scores by scheduled visit, are shown in Table 3. Results of the main intention-to-treat regression analysis for the data at 70 days are shown in Table 4. The main effect, attributable to the routine care shared by all three treatment groups, is very large over the 70-day period. For each of the four main outcomes, PANSS total and positive, delusion and hallucinations scores, there was a trend for the CBT group to improve fastest of the three treatment groups. Inspection of the means for the assessment visits (Table 3) suggests this effect to be greatest at 5 weeks from baseline (visit 4). At 5 weeks, the differences in mean PANSS total and positive sub-scale scores and PSYRATS-DS score between CBT and routine care groups is statistically significant (Table 3), but these data should be treated with caution because they are uncorrected for multiple comparisons and were analysed post hoc.
Table 3 Observed mean scores for primary outcome variables by treatment group
| Visit | Observed | Mean | s.d. | Minimum | Maximum |
|---|---|---|---|---|---|
| PANSS total score | |||||
| CBT | |||||
| Baseline | 101 | 87.47 | 17.64 | 54 | 129 |
| 2 | 53 | 70.83 | 14.80 | 46 | 110 |
| 3 | 43 | 67.65 | 17.13 | 36 | 119 |
| 4 | 37 | 61.651 | 17.85 | 38 | 111 |
| 5 | 52 | 68.12 | 21.38 | 36 | 123 |
| 6 | 78 | 61.73 | 19.69 | 18 | 112 |
| Supportive counselling | |||||
| Baseline | 106 | 89.22 | 17.53 | 49 | 131 |
| 2 | 47 | 74.66 | 17.05 | 43 | 121 |
| 3 | 47 | 68.17 | 16.79 | 39 | 105 |
| 4 | 33 | 67.82 | 17.99 | 38 | 110 |
| 5 | 64 | 67.25 | 18.52 | 36 | 109 |
| 6 | 71 | 59.96 | 16.39 | 37 | 119 |
| Routine care | |||||
| Baseline | 102 | 87.01 | 16.81 | 52 | 141 |
| 2 | 58 | 72.50 | 16.38 | 48 | 120 |
| 3 | 50 | 70.28 | 18.38 | 40 | 126 |
| 4 | 37 | 72.11 | 19.02 | 42 | 107 |
| 5 | 59 | 70.15 | 21.46 | 40 | 133 |
| 6 | 60 | 64.38 | 16.79 | 42 | 115 |
| PANSS positive sub-scale score | |||||
| CBT | |||||
| Baseline | 101 | 23.54 | 4.93 | 14 | 36 |
| 2 | 53 | 17.04 | 4.76 | 8 | 34 |
| 3 | 43 | 15.28 | 4.87 | 6 | 28 |
| 4 | 37 | 13.192 | 4.70 | 7 | 24 |
| 5 | 52 | 14.94 | 6.61 | 7 | 34 |
| 6 | 78 | 13.03 | 5.06 | 7 | 33 |
| Supportive counselling | |||||
| Baseline | 105 | 23.33 | 4.42 | 13 | 37 |
| 2 | 47 | 17.70 | 4.07 | 10 | 26 |
| 3 | 47 | 15.57 | 4.68 | 9 | 29 |
| 4 | 33 | 14.82 | 4.69 | 8 | 25 |
| 5 | 64 | 15.34 | 6.01 | 7 | 31 |
| 6 | 71 | 12.58 | 4.80 | 7 | 28 |
| Routine care | |||||
| Baseline | 102 | 23.33 | 4.74 | 12 | 39 |
| 2 | 58 | 17.53 | 5.50 | 7 | 33 |
| 3 | 50 | 16.32 | 6.19 | 7 | 40 |
| 4 | 37 | 16.19 | 6.55 | 7 | 34 |
| 5 | 59 | 16.31 | 6.90 | 7 | 39 |
| 6 | 60 | 13.67 | 5.33 | 7 | 30 |
| Delusions Scale score | |||||
| CBT | |||||
| Baseline | 100 | 17.85 | 4.15 | 0 | 24 |
| 2 | 52 | 12.52 | 6.51 | 0 | 22 |
| 3 | 42 | 9.95 | 6.70 | 0 | 20 |
| 4 | 35 | 6.913 | 6.68 | 0 | 20 |
| 5 | 49 | 9.92 | 7.45 | 0 | 22 |
| 6 | 74 | 6.95 | 7.66 | 0 | 23 |
| Supportive counselling | |||||
| Baseline | 106 | 17.53 | 5.26 | 0 | 24 |
| 2 | 46 | 13.59 | 6.24 | 0 | 22 |
| 3 | 47 | 11.98 | 6.47 | 0 | 21 |
| 4 | 37 | 10.73 | 6.64 | 0 | 21 |
| 5 | 59 | 9.20 | 7.10 | 0 | 23 |
| 6 | 67 | 6.13 | 6.98 | 0 | 21 |
| Routine care | |||||
| Baseline | 101 | 17.02 | 4.24 | 0 | 24 |
| 2 | 59 | 13.69 | 6.03 | 0 | 23 |
| 3 | 49 | 12.12 | 6.50 | 0 | 24 |
| 4 | 38 | 10.55 | 6.97 | 0 | 22 |
| 5 | 58 | 9.55 | 7.47 | 0 | 22 |
| 6 | 56 | 7.52 | 7.15 | 0 | 23 |
| Auditory Hallucinations Scale score | |||||
| CBT | |||||
| Baseline | 60 | 29.38 | 6.69 | 12 | 39 |
| 2 | 30 | 13.90 | 12.75 | 0 | 39 |
| 3 | 30 | 11.13 | 11.83 | 0 | 38 |
| 4 | 29 | 4.724 | 9.21 | 0 | 30 |
| 5 | 30 | 6.13 | 11.22 | 0 | 36 |
| 6 | 47 | 6.15 | 10.31 | 0 | 33 |
| Supportive counselling | |||||
| Baseline | 63 | 29.46 | 6.91 | 7 | 39 |
| 2 | 29 | 18.14 | 14.73 | 0 | 42 |
| 3 | 31 | 15.71 | 13.67 | 0 | 38 |
| 4 | 20 | 15.35 | 15.78 | 0 | 41 |
| 5 | 39 | 12.15 | 13.66 | 0 | 41 |
| 6 | 41 | 6.41 | 10.61 | 0 | 32 |
| Routine care | |||||
| Baseline | 62 | 29.00 | 6.35 | 10 | 40 |
| 2 | 34 | 25.65 | 12.27 | 0 | 35 |
| 3 | 32 | 12.16 | 13.04 | 0 | 41 |
| 4 | 24 | 7.96 | 12.09 | 0 | 34 |
| 5 | 35 | 10.31 | 13.75 | 0 | 39 |
| 6 | 43 | 8.30 | 12.60 | 0 | 35 |
Table 4 Rate of change of symptom scores
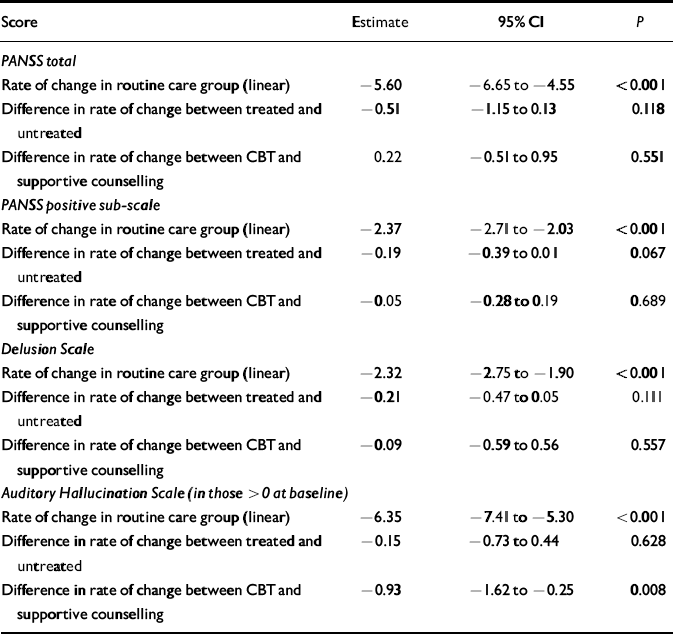
| Score | Estimate | 95% CI | P |
|---|---|---|---|
| PANSS total | |||
| Rate of change in routine care group (linear) | -5.60 | -6.65 to -4.55 | <0.001 |
| Difference in rate of change between treated and untreated | -0.51 | -1.15 to 0.13 | 0.118 |
| Difference in rate of change between CBT and supportive counselling | 0.22 | -0.51 to 0.95 | 0.551 |
| PANSS positive sub-scale | |||
| Rate of change in routine care group (linear) | -2.37 | -2.71 to -2.03 | <0.001 |
| Difference in rate of change between treated and untreated | -0.19 | -0.39 to 0.01 | 0.067 |
| Difference in rate of change between CBT and supportive counselling | -0.05 | -0.28 to 0.19 | 0.689 |
| Delusion Scale | |||
| Rate of change in routine care group (linear) | -2.32 | -2.75 to -1.90 | <0.001 |
| Difference in rate of change between treated and untreated | -0.21 | -0.47 to 0.05 | 0.111 |
| Difference in rate of change between CBT and supportive counselling | -0.09 | -0.59 to 0.56 | 0.557 |
| Auditory Hallucination Scale (in those > 0 at baseline) | |||
| Rate of change in routine care group (linear) | -6.35 | -7.41 to - 5.30 | <0.001 |
| Difference in rate of change between treated and untreated | -0.15 | -0.73 to 0.44 | 0.628 |
| Difference in rate of change between CBT and supportive counselling | -0.93 | -1.62 to -0.25 | 0.008 |
In the linear regression, faster resolution of symptoms in the groups allocated to either psychological treatment condition was seen, compared with routine care alone, but not at statistically significant levels. For auditory hallucinations, present at baseline in 60% of the sample, resolution was faster in the CBT group than in the supportive counselling group (parameter estimate -0.93 with 95% CI -1.62 to -0.25). As a result of this statistically significant finding, the contrast between the trend in the combined treatment groups and that with routine care is difficult to interpret and should be ignored. Instead, the model was re-parameterised to allow direct contrasts of trend for CBT versus routine care, and supportive counselling versus routine care. The resulting parameter estimates were -0.61 (95% CI -1.30 to 0.07; P=0.08) and 0.32 (95% CI -0.36 to -1.00; P=0.36). Note that the difference between -0.61 and 0.32 is the estimate of the difference between CBT and supportive counselling (i.e. -0.93) obtained in the original model. In summary, for auditory hallucinations, CBT is an improvement on routine care (but the effect is not statistically significant at the α =0.05 level) whereas patients receiving supportive counselling do slightly worse than under routine care (but the effect is not statistically significant). In view of the skewed nature of the PSYRATS—AHS scores, the robustness of the difference between CBT and supportive counselling was checked by dichotomising the outcome score (<10 v. ≥ 10) and rerunning the repeated measures analysis using logistic regression (allowing for patient ID as a clustering variable and requesting robust standard errors). The results (not shown) confirmed those already obtained.
A secondary analysis was performed for length of index hospital admission. There was no difference between the treatment groups, with median lengths of stay of 48 days for CBT, 53 for supportive counselling and 47 for routine care.
DISCUSSION
We aimed to test the effectiveness of a package of CBT in accelerating remission from acute symptoms in early schizophrenia and related disorders. We recruited a geographically defined sample presenting to day or in-patient services for their first or second admission. Of those eligible, 85% consented to trial entry, of whom 83% were first admissions. Serial blinded assessments up to 70 days showed that all treatment groups improved markedly on the four primary outcome measures. In addition, patients treated with CBT showed a trend towards faster weekly improvement over the 70-day treatment period for total and positive symptom score on the PANSS. Uncorrected secondary analyses showed statistically significant improvements in three of the four main outcome measures with CBT compared with routine care, at week 4, which did not persist to the final acute-phase assessment. One interpretation is that CBT leads to a level of remission at 4 weeks which is achieved at 6 weeks with routine care.
Limitations
Patients treated acutely in out-patient or community services were not included, for logistic reasons. The preponderance of males in our sample echoes that found in other service-based first-episode samples (Reference Power, Elkins and AdlardPower et al, 1998). There was no attempt to standardise ‘routine care’, including drug treatment, in the sample as a whole. This means that the content of ‘routine care’ is not specifiable, except that it always included day or in-patient treatment and included antipsychotic drugs. This would reduce the likelihood of showing an experimental effect, but increases the generalisability of the findings.
Pointers to future research
Choice of control groups is important in trials such as this. Supportive counselling was chosen to control for non-specific effects of exposure to an empathic individual, allowing us to test for predicted, specific effects of CBT. As with the CBT, the supportive counselling intervention was derived from that used by Tarrier et al (Reference Tarrier, Yusupoff and Kinney1998) and was manual-based and supervised. In that study, a supportive counselling intervention showed outcomes intermediate between CBT and routine care. Sensky et al (Reference Sensky, Turkington and Kingdon2000) used a ‘befriending’ control in their trial of CBT in persistent symptoms and found an unexpected benefit immediately post-treatment in this group, although this effect was not sustained at follow-up, in contrast to the gains with CBT.
Auditory hallucinations were present at baseline in 60% of participants. These scores improved significantly faster in the CBT group than the supportive counselling group. Inspection of the observed means suggests that part of this effect appeared to be a slowing of resolution of these symptoms in the supportive counselling group, compared with routine care alone. This was unexpected and suggests that some element of supportive counselling may be detrimental with respect to auditory hallucinations. This echoes a similar finding recently in a separate cohort of patients with chronic schizophrenia (Tarrier et al, Reference Tarrier, Yusupoff and Kinney1998, Reference Tarrier, Kinney and McCarthy2001) and deserves further study. It could be that undirected encouragement to discuss these psychotic symptoms increases their intensity or the associated distress.
Cognitive—behavioural therapy is effective for persistent symptoms in chronic schizophrenia (Reference Jones, Cormac and MotaJones et al, 1998). In the current study in early schizophrenia, it was found to accelerate resolution from positive symptoms but, in the intent-to-treat analysis, other effects were non-significant. The size of the main effect of routine care in first-episode psychosis is large, with over 85% of cases in a first-episode of schizophrenia achieving remission with drug treatment, at a median in one study of 11 weeks (Reference Lieberman, Jody and GeislerLieberman et al, 1993). It is difficult to demonstrate efficacy of an adjunctive treatment if routine care alone usually results in swift remission.
The absence of large benefits immediately post-intervention does not discount longer-term benefits, as was the case in the trial of Sensky et al (Reference Sensky, Turkington and Kingdon2000). This possibility will be examined in the 18-month follow-up, in terms of residual symptoms and time to relapse. The package of CBT was of just 5 weeks' duration, with most participants receiving less, and the raw data suggest that, although improvements were seen by week 5, these were not maintained by 70 days. The issue of ‘dose—response’ in the treatment group will be explored in a planned observed-case analysis.
CLINICAL IMPLICATIONS
-
▪ Cognitive—behavioural therapy (CBT) appears to have some effect in acute, early schizophrenia, but overall the effect is transient.
-
▪ Psychological treatments are deliverable to people with acute psychosis.
-
▪ Auditory hallucinations respond significantly better to CBT than to supportive counselling.
LIMITATIONS
-
▪ Remission with routine care in first-episode psychosis is swift so the effectiveness of added treatments is difficult to show.
-
▪ The psychological treatment package was brief.
-
▪ Final results await the 18-month follow-up.
Acknowledgements
We thank independent members of the Trial Steering Committee: Professors E. Paykel (Cambridge) Chair, Peter Tyrer, Til Wykes; and the Data Monitoring and Ethics Committee: Peter Diggle (Lancaster) Chair, David Clarke and Steven Hirsch. The trial was funded as follows: UK Medical Research Council (41%); Northwest England NHSE Office (27%); Trent NHSE Office (7%); the following health authorities: Manchester (8%); Salford and Trafford (2%); Liverpool (3%); Sefton (3%); St Helens and Knowsley (3%); North Nottinghamshire (6%).








eLetters
No eLetters have been published for this article.