Quality of life (QoL) is becoming an increasingly important outcome of healthcare for several reasons. Subjective valuations, autonomy and the needs of patients are increasingly respected. Generic measures are needed to directly compare the burden of different conditions and treatments. Health policy and economic evaluations favour quality-adjusted life-years (QALYs) Reference Rawlins and Culyer1 that can be based on health-related quality of life (HRQoL) measures. With psychotic disorders, deinstitutionalisation has further emphasised the importance of optimising functional status. Positive psychotic symptoms, the traditional target of antipsychotic drug treatment, are not strong determinants of either ability to function or QoL. Reference Becker, Diamond, Katschnig, Freeman and Sartorius2–Reference Narvaez, Twamley, McKibbin, Heaton and Patterson5
Psychotic disorders are a heterogeneous group, and comparisons of disorders using generic QoL/HRQoL are scarce. Most studies on QoL/HRQoL and psychotic disorders have investigated selected clinical samples, so information on the severity of these disorders in the general population is lacking. Which symptoms determine QoL/HRQoL is also poorly known. Using a representative population sample, we investigated:
-
(a) the comparative burden of different functional psychotic disorders on QoL/HRQoL;
-
(b) how different disorders decrease subjective QoL relative to utility-based HRQoL, and which dimensions of HRQoL are influenced;
-
(c) the correlation between different psychotic symptoms, depression, clinician-assessed outcome and QoL/HRQoL.
Method
Health 2000 survey
The data come from the Health 2000 survey, a representative study of the Finnish population aged 30 and over, and its substudy – Psychoses in Finland. The methods and basic results of the Health 2000 survey have been published previously Reference Aromaa and Koskinen6 (available at www.terveys2000.fi/indexe.html). Briefly, the survey had a two-stage, stratified cluster sampling design. The original sample included 8028 people, with double-sampling of people over 80 years of age, giving a response rate of 93% for any part of the survey. The survey consisted of a health interview, a thorough health examination, laboratory tests, a structured mental health interview (the Munich version of the Composite International Diagnostic Interview, CIDI) Reference Wittchen, Lachner, Wunderlich and Pfister7 and several self-report questionnaires. The data were collected between August 2000 and July 2001.
Psychiatric diagnostics
The Psychoses in Finland study methodology has been described in detail previously. Reference Perälä, Suvisaari, Saarni, Kuoppasalmi, Isometsä and Pirkola8 People from the Health 2000 survey were included in the Psychoses in Finland study if they reported ever having had a psychotic disorder, were diagnosed by the physician conducting the health examination to have a definite or probable psychotic disorder, or had a lifetime history of psychotic or manic symptoms in the CIDI interview. Register-based screening was also used, covering hospital treatments in Finland for psychotic disorders, free antipsychotic medication, mood-stabilising medication use without diagnosis of a relevant somatic disorder or disability pension because of a psychotic disorder. As CIDI is not reliable in diagnosing psychotic disorders, people identified using CIDI were re-interviewed with the Research Version of the Structured Clinical Interview for DSM–IV (SCID–I). Reference First, Anthony, Tepper and Dryman9 All case notes from hospital and out-patient treatments were collected, even from those who were not interviewed. The ethics committees of the National Institute for Health and Welfare (former National Public Health Institute) and the Hospital District of Helsinki and Uusimaa approved the studies. Participants provided written informed consent. Three experienced clinicians (J.P., J.S. and S.I.S.) made the final best-estimate lifetime DSM–IV diagnoses based on a systematic evaluation of all available data. The Kappa values between the raters ranged from 0.74 to 0.97 for different psychotic disorders, and were either good or excellent regardless of whether the diagnosis was based on both the SCID–I interview and case records or on case records alone. Reference Perälä, Suvisaari, Saarni, Kuoppasalmi, Isometsä and Pirkola8
Lifetime diagnoses of psychotic disorders were classified into schizophrenia, other non-affective psychosis (including schizoaffective disorder, schizophreniform disorder, delusional disorder, brief psychotic disorder and psychotic disorder not otherwise specified) and affective psychosis (major depressive disorder with psychotic features and bipolar I disorder). The main focus of this study was on these groups, but we also examined separately the most common individual psychotic disorders (Table 1). This study considers lifetime disorders that had their onset before participants completed the QoL/HRQoL questionnaires as part of the Health 2000 study.
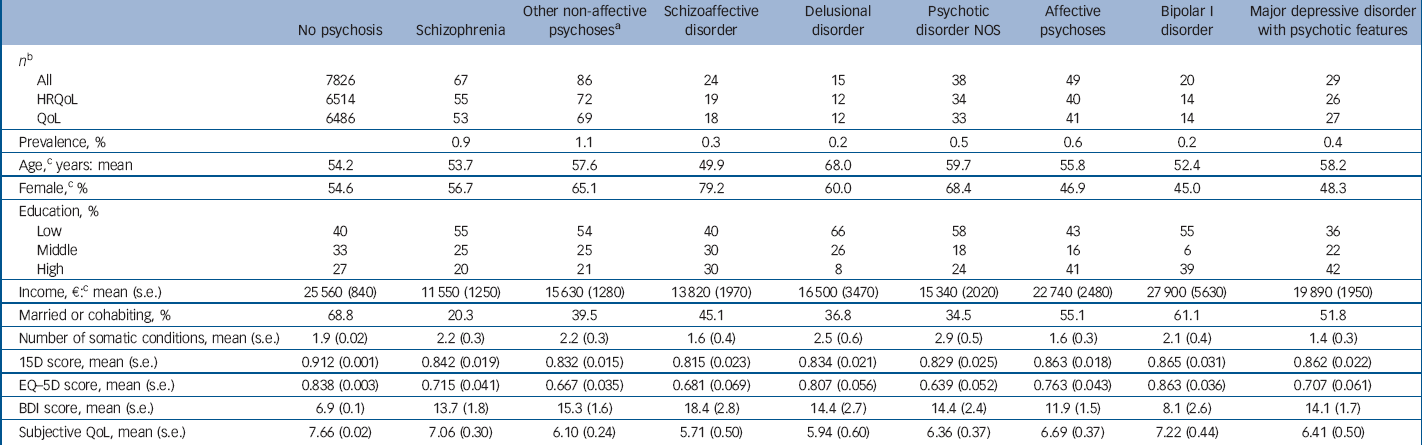
Table 1 Prevalence of psychotic disorders, age, somatic and psychiatric comorbidity and health-related quality of life (HRQoL) scores of respondents
| No psychosis | Schizophrenia | Other non-affective psychosesa | Schizoaffective disorder | Delusional disorder | Psychotic disorder NOS | Affective psychoses | Bipolar I disorder | Major depressive disorder with psychotic features | |
|---|---|---|---|---|---|---|---|---|---|
| n b | |||||||||
| All | 7826 | 67 | 86 | 24 | 15 | 38 | 49 | 20 | 29 |
| HRQoL | 6514 | 55 | 72 | 19 | 12 | 34 | 40 | 14 | 26 |
| QoL | 6486 | 53 | 69 | 18 | 12 | 33 | 41 | 14 | 27 |
| Prevalence, % | 0.9 | 1.1 | 0.3 | 0.2 | 0.5 | 0.6 | 0.2 | 0.4 | |
| Age,c years: mean | 54.2 | 53.7 | 57.6 | 49.9 | 68.0 | 59.7 | 55.8 | 52.4 | 58.2 |
| Female,c % | 54.6 | 56.7 | 65.1 | 79.2 | 60.0 | 68.4 | 46.9 | 45.0 | 48.3 |
| Education, % | |||||||||
| Low | 40 | 55 | 54 | 40 | 66 | 58 | 43 | 55 | 36 |
| Middle | 33 | 25 | 25 | 30 | 26 | 18 | 16 | 6 | 22 |
| High | 27 | 20 | 21 | 30 | 8 | 24 | 41 | 39 | 42 |
| Income, €:c mean (s.e.) | 25 560 (840) | 11 550 (1250) | 15 630 (1280) | 13 820 (1970) | 16 500 (3470) | 15 340 (2020) | 22 740 (2480) | 27 900 (5630) | 19 890 (1950) |
| Married or cohabiting, % | 68.8 | 20.3 | 39.5 | 45.1 | 36.8 | 34.5 | 55.1 | 61.1 | 51.8 |
| Number of somatic conditions, mean (s.e.) | 1.9 (0.02) | 2.2 (0.3) | 2.2 (0.3) | 1.6 (0.4) | 2.5 (0.6) | 2.9 (0.5) | 1.6 (0.3) | 2.1 (0.4) | 1.4 (0.3) |
| 15D score, mean (s.e.) | 0.912 (0.001) | 0.842 (0.019) | 0.832 (0.015) | 0.815 (0.023) | 0.834 (0.021) | 0.829 (0.025) | 0.863 (0.018) | 0.865 (0.031) | 0.862 (0.022) |
| EQ–5D score, mean (s.e.) | 0.838 (0.003) | 0.715 (0.041) | 0.667 (0.035) | 0.681 (0.069) | 0.807 (0.056) | 0.639 (0.052) | 0.763 (0.043) | 0.863 (0.036) | 0.707 (0.061) |
| BDI score, mean (s.e.) | 6.9 (0.1) | 13.7 (1.8) | 15.3 (1.6) | 18.4 (2.8) | 14.4 (2.7) | 14.4 (2.4) | 11.9 (1.5) | 8.1 (2.6) | 14.1 (1.7) |
| Subjective QoL, mean (s.e.) | 7.66 (0.02) | 7.06 (0.30) | 6.10 (0.24) | 5.71 (0.50) | 5.94 (0.60) | 6.36 (0.37) | 6.69 (0.37) | 7.22 (0.44) | 6.41 (0.50) |
Health-related and subjective quality-of-life measurement
Health-related quality of life is the part of QoL that can potentially be influenced by health and healthcare. As there is no gold standard for HRQoL measurement, Reference Tengs and Wallace10,Reference Saarni, Härkänen, Sintonen, Suvisaari, Koskinen and Aromaa11 we used two well-established, generic self-report preference-based HRQoL measures: the EQ–5D 12 and the 15D. Reference Sintonen13
Preference-based HRQoL questionnaires include several dimensions that can be summarised as a single score using utility theory and preferences elicited from the population. The result is a quantitative measure of the severity of health states as people value them, referred to as health utility. Health utilities range from 0, being equal to death, and 1, which is perfect health. They form the quality-component of QALYs, which combine length and quality of life into a single metric. Reference Dolan, Culyer and Newhouse14 For example, the National Institute for Health and Clinical Excellence uses QALYs as their preferred outcome measure in cost-effectiveness analyses. Reference Rawlins and Culyer1
The 15D Reference Sintonen13,Reference Sintonen15,Reference Sintonen16 has 15 dimensions with five categories of severity: mobility, vision, hearing, breathing, sleeping, eating, speech, elimination, usual activities, mental function, discomfort and symptoms, depression, distress, vitality and sexual activity (www.15d-instrument.net). The 15D utility index Reference Sintonen15 ranges between 1 (full health) and 0 (death). We included participants with 12 or more completed 15D dimensions, imputing missing values as recommended. Reference Sintonen13 Changes of over 0.02–0.03 points on the 15D are considered clinically important. Reference Sintonen16 The EQ–5D 12,Reference Brooks17,Reference Rabin and de Charro18 has five dimensions with three categories of severity: mobility, self-care, usual activities, pain or discomfort, and anxiety or depression (www.euroqol.org). The EQ–5D UK time trade-off index Reference Kind, Hardman and Macran19 ranges between 1 (full health) and –0.59 (0 is death). A time trade-off index is based on hypothetical trade-offs between length of life and symptoms. Only participants who fully completed the EQ–5D questionnaire were included. Although there is no unequivocally agreed threshold for a minimum clinically important change on the EQ–5D, thresholds of 0.07 points have been observed. Reference Walters and Brazier20
Both the 15D and EQ–5D enquire about the health state of the respondent ‘today’. The 15D compares favourably with similar HRQoL instruments in most of the important properties. Reference Sintonen13,Reference Sintonen15,Reference Sintonen16,Reference Hawthorne, Richardson and Day21,Reference Stavem, Bjornaes and Lossius22 Although EQ–5D is among the most extensively evaluated HRQoL measure, Reference Garratt, Schmidt, Mackintosh and Fitzpatrick23 it is problematic in general population samples due to its low sensitivity in detecting deviations from full health. Reference Saarni, Härkänen, Sintonen, Suvisaari, Koskinen and Aromaa11
Whereas the HRQoL instruments used require answers to a predefined set of questions concerning symptoms and functioning, the subjective QoL here means global life satisfaction as defined by the respondent. Subjective QoL was measured by asking the respondents to rate their current QoL as a whole, over the past 30 days, on a visual-analogue scale (VAS) from 0 to 10, anchored at best and worst possible QoL.
Sociodemographic variables, somatic conditions and depressed mood
Data on sociodemographic variables and chronic somatic diseases were collected using structured interviews at home or institution. As the relationship between HRQoL and age is not linear, age was categorised as 30–44, 45–54, 55–64, 65–74, 75–85 and over 85 years. Education was categorised as basic, secondary or higher. Household income, derived from registers on taxes and welfare benefits, was adjusted for family size 24 and divided into quintiles. Marital status was classified into two categories: married or cohabiting, and others. Chronic somatic conditions were diagnosed by asking, separately for each condition, whether the participant had ever been diagnosed by a physician with one of the 25 included conditions. Reference Saarni, Härkänen, Sintonen, Suvisaari, Koskinen and Aromaa11 Depressed mood was assessed with the Beck Depression Inventory (BDI). Reference Beck, Ward, Mendelson, Mock and Erbaugh25
Symptom measures
For lifetime symptoms of psychotic disorders, we used the Major Symptoms of Schizophrenia Scale (MSSS) Reference Kendler, Karkowski and Walsh26,Reference Kendler, McGuire, Gruenberg, O'Hare, Spellman and Walsh27 and some global ratings from the Scale for the Assessment of Positive Symptoms (SAPS) Reference Andreasen28 and from the Scale for the Assessment of Negative Symptoms (SANS). Reference Andreasen29 These were completed based on the SCID–I interview and all medical records on a lifetime basis. Reference Suvisaari, Perälä, Saarni, Juvonen, Tuulio-Henriksson and Lönnqvist30
The symptoms in the MSSS are rated from 1, clearly not present, to 5, severe; the symptoms in the SANS and SAPS are coded on a six-point scale, ranging from 0, not at all, to 5, severe. The following symptom and course ratings were used in the analysis: hallucinations, delusions, positive formal thought disorder, catatonia, affective deterioration, negative formal thought disorder, depression, mania, course and outcome. From these ratings, we formed summary scores for positive symptoms (delusions and hallucinations), disorganised symptoms (positive thought disorder and bizarre behaviour) and negative symptoms (negative thought disorder, avolition–apathy, anhedonia–asociality and affective deterioration).
Statistical methods
To analyse non-response, we investigated whether people with psychotic disorders who completed the QoL/HRQoL measures differed from non-responders using information from the ratings for psychotic symptoms. The following symptom and course ratings were used in the analysis: hallucinations, delusions, positive formal thought disorder, catatonia, affective deterioration, negative formal thought disorder, depression, mania, course and outcome. Differences between responders and non-responders were tested with the Kruskal–Wallis test. The following groups were investigated: schizophrenia, other non-affective psychosis and affective psychoses. From the latter two groups, schizoaffective disorder, delusional disorder, bipolar I disorder and major depressive disorder with psychotic features were investigated separately.
To estimate the association between different disorders and loss of HRQoL or QoL, we created separate multiple regression models using each of the instruments (15D, EQ–D, QoL) as dependent variables. To estimate the effects covariates, we created three sets of regression models in a stepwise manner: the first model controlled for age and gender, the second added education, income and marital status, and the third the 25 somatic conditions. To estimate the effect of depression, we added a fourth model with BDI. The covariates were entered as dummy variables except for BDI, which was entered as a continuous variable. Linear regression for survey data was used to analyse subjective QoL. As the HRQoL measures have a ceiling effect (55.4% of respondents scored full health on EQ–D and 17.3% on 15D) we used a Tobit model to account for this censoring. Reference Saarni, Härkänen, Sintonen, Suvisaari, Koskinen and Aromaa11,Reference Austin, Escobar and Kopec31,Reference Tobin32 We report the marginal effects of the unconditional expected value of the HRQoL score, valued at the means of the explanatory variables. Reference Cong33 These marginal effects are interpreted comparably to beta-coefficients of linear regression, i.e. as the change in HRQoL score (health utility) associated with each psychotic disorder (adjusted for age or age and other covariates), compared with people without the disorder.
To investigate which dimensions of HRQoL were affected by schizophrenia, schizoaffective disorder and bipolar I disorder, we created 15D profiles using linear regression to adjust the losses on each 15D dimension for age and gender. As the 15D preference-based scoring system scales all dimensions between 0 and 1, the losses are comparable. To investigate the correlations between QoL/HRQoL ratings and clinician-rated symptom severity and outcome, we calculated Spearman rank-order correlations as partial correlations, adjusting for age and gender. Regression analyses were conducted using Stata 8.2 for Windows, and the other analyses on SAS 9.1.3 on Windows. Analyses accounted for the two-stage sampling design. Post-stratification weights were used to correct for non-response (based on register information on the whole sample) and for oversampling of people aged over 80 years. Reference Aromaa and Koskinen6
Results
Background variables
The sociodemographic characteristics of the sample, somatic conditions and BDI scores are presented in Table 1. People with non-affective psychoses were more often living alone, had lower education and less income than people with affective psychoses or the general population. Mean BDI scores were elevated (about 14–18) for all disorders except bipolar I disorder, where they were close to that of the general population.
Analysis of non-response
Response rates are presented in Table 1 (see online supplement for symptom-specific details). Briefly, QoL information was available for 85.1%, EQ–5D for 78.5% and 15D for 80.2% of the sample.
Loss of QoL/HRQoL in different disorders
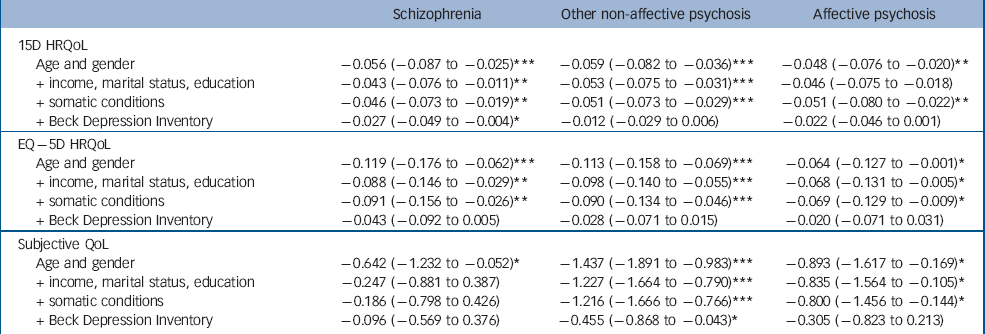
Unadjusted HRQoL and QoL scores are presented in Table 1, and adjusted scores in Tables 2 and 3. The results of only two models are presented in Table 3, as the results of the omitted models were essentially similar to the first model controlling only for age and gender.
Table 2 Health-related quality of life (HRQoL) (15D and EQ–5D) and subjective quality of life (QoL) decrements associated with main groups of psychotic disorders, adjusted stepwise for age, gender, income, marital status, education, somatic conditions and Beck Depression Inventory score, reporting marginal effects for HRQoL and beta coefficients for QoL (95% CI)
| Schizophrenia | Other non-affective psychosis | Affective psychosis | |
|---|---|---|---|
| 15D HRQoL | |||
| Age and gender | –0.056 (–0.087 to –0.025)*** | –0.059 (–0.082 to –0.036)*** | –0.048 (–0.076 to –0.020)** |
| + income, marital status, education | –0.043 (–0.076 to –0.011)** | –0.053 (–0.075 to –0.031)*** | –0.046 (–0.075 to –0.018) |
| + somatic conditions | –0.046 (–0.073 to –0.019)** | –0.051 (–0.073 to –0.029)*** | –0.051 (–0.080 to –0.022)** |
| + Beck Depression Inventory | –0.027 (–0.049 to –0.004)* | –0.012 (–0.029 to 0.006) | –0.022 (–0.046 to 0.001) |
| EQ–5D HRQoL | |||
| Age and gender | –0.119 (–0.176 to –0.062)*** | –0.113 (–0.158 to –0.069)*** | –0.064 (–0.127 to –0.001)* |
| + income, marital status, education | –0.088 (–0.146 to –0.029)** | –0.098 (–0.140 to –0.055)*** | –0.068 (–0.131 to –0.005)* |
| + somatic conditions | –0.091 (–0.156 to –0.026)** | –0.090 (–0.134 to –0.046)*** | –0.069 (–0.129 to –0.009)* |
| + Beck Depression Inventory | –0.043 (–0.092 to 0.005) | –0.028 (–0.071 to 0.015) | –0.020 (–0.071 to 0.031) |
| Subjective QoL | |||
| Age and gender | –0.642 (–1.232 to –0.052)* | –1.437 (–1.891 to –0.983)*** | –0.893 (–1.617 to –0.169)* |
| + income, marital status, education | –0.247 (–0.881 to 0.387) | –1.227 (–1.664 to –0.790)*** | –0.835 (–1.564 to –0.105)* |
| + somatic conditions | –0.186 (–0.798 to 0.426) | –1.216 (–1.666 to –0.766)*** | –0.800 (–1.456 to –0.144)* |
| + Beck Depression Inventory | –0.096 (–0.569 to 0.376) | –0.455 (–0.868 to –0.043)* | –0.305 (–0.823 to 0.213) |
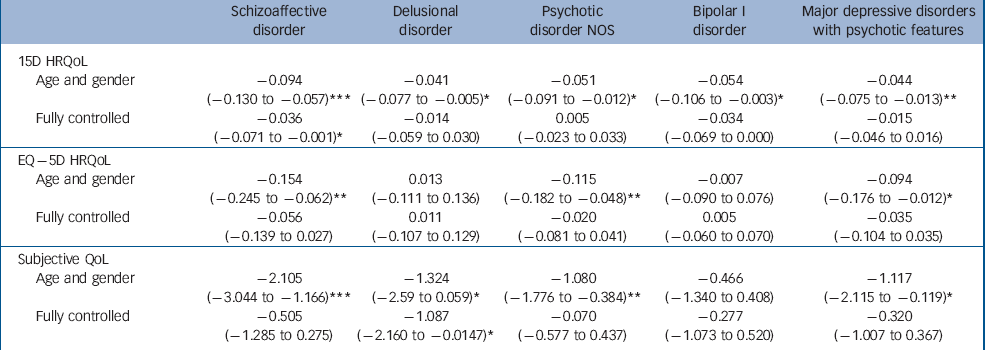
Table 3 Health-related quality of life (HRQoL) (15D and EQ–5D) and subjective quality of life (QoL) decrements associated with different psychotic disorders, adjusted stepwise for age and gender, or age, gender, income, marital status, education, somatic conditions and Beck Depression Inventory (BDI) score, reporting marginal effects for HRQoL and beta-coefficients for QoL (95% CI)
| Schizoaffective disorder | Delusional disorder | Psychotic disorder NOS | Bipolar I disorder | Major depressive disorders with psychotic features | |
|---|---|---|---|---|---|
| 15D HRQoL | |||||
| Age and gender | –0.094 (–0.130 to –0.057)*** | –0.041 (–0.077 to –0.005)* | –0.051 (–0.091 to –0.012)* | –0.054 (–0.106 to –0.003)* | –0.044 (–0.075 to –0.013)** |
| Fully controlled | –0.036 (–0.071 to –0.001)* | –0.014 (–0.059 to 0.030) | 0.005 (–0.023 to 0.033) | –0.034 (–0.069 to 0.000) | –0.015 (–0.046 to 0.016) |
| EQ–5D HRQoL | |||||
| Age and gender | –0.154 (–0.245 to –0.062)** | 0.013 (–0.111 to 0.136) | –0.115 (–0.182 to –0.048)** | –0.007 (–0.090 to 0.076) | –0.094 (–0.176 to –0.012)* |
| Fully controlled | –0.056 (–0.139 to 0.027) | 0.011 (–0.107 to 0.129) | –0.020 (–0.081 to 0.041) | 0.005 (–0.060 to 0.070) | –0.035 (–0.104 to 0.035) |
| Subjective QoL | |||||
| Age and gender | –2.105 (–3.044 to –1.166)*** | –1.324 (–2.59 to 0.059)* | –1.080 (–1.776 to –0.384)** | –0.466 (–1.340 to 0.408) | –1.117 (–2.115 to –0.119)* |
| Fully controlled | –0.505 (–1.285 to 0.275) | –1.087 (–2.160 to –0.0147)* | –0.070 (–0.577 to 0.437) | –0.277 (–1.073 to 0.520) | –0.320 (–1.007 to 0.367) |
Participants with non-affective psychoses other than schizophrenia had the lowest unadjusted HRQoL scores; their mean 15D score was 0.08 and EQ–5D score 0.17 points lower than the mean of the population without psychosis. For schizophrenia these differences were 0.07 and 0.12 respectively. When controlling for age and gender, schizophrenia, other non-affective psychosis and affective psychoses were all associated with decreases of 0.05–0.06 points on the 15D. On the EQ–5D, the decreases were 0.12 for schizophrenia, 0.11 for other non-affective psychosis and 0.06 for affective psychoses. Considering individual disorders, people with schizoaffective disorder had the largest age- and gender-adjusted losses of HRQoL: –0.09 on the 15D and –0.15 on the EQ–5D. Whereas all disorders were associated with statistically significant and clinically important decreases on the 15D, the EQ–5D did not detect any HRQoL losses for delusional or bipolar I disorders.
The other non-affective psychosis group also had the lowest unadjusted QoL scores: their mean QoL was 1.6 points below the population without psychosis, whereas this difference was 0.6 for schizophrenia. People in the other non-affective psychosis group also had the largest reductions of QoL in all the regression models. The schizophrenia group had statistically significant reductions of QoL when controlling for age and gender only, whereas the affective psychoses group fell between the other groups. Of individual disorders, people with schizoaffective disorder had the worst QoL, whereas those with bipolar I disorder did not have a statistically significant reduction of QoL.
Effect of current depression
The stepwise entering of socioeconomic variables and somatic conditions into regression models changed the HRQoL results relatively little. However, adding BDI score into the models mostly diminished the impact of disorders on HRQoL. Only schizophrenia and schizoaffective disorder were associated with statistically significant reductions of the 15D after controlling for the BDI, whereas the EQ–5D detected no statistically significant effects of diagnoses after controlling for the BDI.
For QoL the picture was similar and the BDI explained most of the observed decreases, with two exceptions: for schizophrenia, socioeconomic variables explained most of the reduction of QoL. Delusional disorder was the only disorder where QoL was still statistically significantly reduced after controlling for the BDI.
Dimensions of HRQoL influenced by different disorders
The HRQoL profiles are presented in Fig. 1, in the form of age- and gender-adjusted decreases from population averages. The decreases were widespread for schizophrenia (11 of 15 dimensions statistically significantly decreased) and schizoaffective disorder (10 of 15) but less so for bipolar I disorder (3 of 15).

Fig. 1 Age- and gender-adjusted losses on different health-related quality of life (15D) dimensions with 95% CI. (a) Schizophrenia, (b) schizoaffective disorder, (c) bipolar I disorder.
The 15 dimensions: Move, mobility; See, vision; Hear, hearing; Breath, breathing; Sleep, sleeping; Eat, eating; Speech, speaking; Elim, elimination; Uact, usual activity; Mental, mental function; Disco, discomfort and symptoms; Depr, depression; Distr, distress; Vital, vitality; Sex, sexuality.
Clinician-rated psychotic symptoms, outcome and QoL/HRQoL
Online Table DS1 presents the Spearman correlations between QoL/HRQoL and lifetime ratings of positive, negative, disorganisation, depressive symptoms, course and outcome.
For all psychotic disorders combined, depressive and negative symptoms had small (0.1–0.3) correlations with QoL/HRQoL. Positive symptoms were not correlated with QoL/HRQoL. There was a trend for small positive correlations between mania, disorganisation symptoms and QoL/HRQoL, although this was statistically significant only for QoL and disorganisation. Clinician-rated course and outcome variables had small correlations with all QoL/HRQoL measures.
For schizophrenia, correlations were generally small and not statistically significant except for the correlation between depressive symptoms and QoL. For schizoaffective disorder, there were large (<–0.5) negative correlations between negative symptoms, course, outcome and QoL/HRQoL measures. For delusional disorders, none of the correlations were statistically significant, although the correlation coefficients between course and especially the EQ–5D and QoL were large. For bipolar I disorder, negative correlations between depressive symptoms and HRQoL measures were large, and moderate (–0.3 to –0.5) between depression and QoL. Correlations were positive – although not statistically significant – between lifetime mania ratings and QoL/HRQoL scores. Negative correlations between course, outcome and QoL/HRQoL ratings were mostly moderate or large. For major depressive disorders with psychotic features, negative correlations were large for negative symptoms and the EQ–5D and QoL. Negative correlations between course, outcome and QoL/HRQoL ratings were mostly moderate or large.
Discussion
Comparison with previous studies
Our results can be compared with several types of studies: those studying the impact of psychotic disorders on health utility or subjective QoL; those comparing the impact of different psychotic disorders using almost any global outcome measure; and those studying the correlation of global outcomes and different symptomatic measures. As the main strength of our data is general population representativeness, we will concentrate here on studies comparing the severity of different disorders.
Studies of health utility and psychotic disorders
Our previous study comparing HRQoL in 29 psychiatric and somatic disorders showed that people with self-reported psychotic disorders ranked third in severity, after Parkinson's disease and major depressive disorder. Reference Saarni, Härkänen, Sintonen, Suvisaari, Koskinen and Aromaa11 Further examination of non-psychotic disorders present within the past 12 months showed that HRQoL losses on the 15D (EQ–5D) were –0.04 (–0.07) for alcohol dependence, –0.07 (–0.13) for major depressive disorder and between –0.13 and –0.14 (–0.23 and –0.27) for dysthymia, social phobia, agoraphobia and generalised anxiety disorders. Reference Saarni, Suvisaari, Sintonen, Pirkola, Koskinen and Aromaa34 This puts the severity of lifetime schizoaffective disorder between 12-month major depressive disorder and anxiety disorders, and schizophrenia between 12-month major depressive disorder and alcohol dependence.
The Dutch Netherlands Mental Health Survey and Incidence Study (NEMESIS) originally showed that all 36-item Short Form Health Survey (SF–36) subscale scores for people with bipolar I disorder were lower than healthy controls. Reference ten Have, Vollebergh, Bijl and Nolen35 However, after a thorough diagnostic check (SCID–I), the remaining individuals with bipolar disorder did not differ from controls on the SF–36 or on the EQ–5D. Reference Hakkaart-van Roijen, Hoeijenbos, Regeer, ten Have, Nolen and Veraart36 This may have been as a result of the small sample size, as the EQ–5D score was 0.82 for the people with bipolar disorder and 0.88 for controls. Further, the response rate was only 47%. Still, an EQ–5D value of 0.82 is clearly lower than that observed in our study, even though our participants with bipolar I disorder were 10 years older.
Generalisations from clinical studies are difficult, as the inclusion criteria vary and adjusting for age would be essential given that HRQoL decreases with age. Reference Saarni, Härkänen, Sintonen, Suvisaari, Koskinen and Aromaa11 Participants commonly report low HRQoL values: EQ–5D scores have been between 0.52 and 0.57 in recent studies of schizophrenia. Reference Davies, Barnes, Jones, Lewis, Gaughran and Hayhurst37–Reference Prieto, Sacristan, Hormaechea, Casado, Badia and Gomez39 These values are much lower than our results. Studies with people not actively seeking treatment have yielded results closer to ours. A UK study selecting stable out-patients obtained a EQ–5D value of 0.86. Reference Briggs, Wild, Lees, Reaney, Dursun and Parry40 A German study of out-patients with non-affective psychoses (93% schizophrenia or schizoaffective disorder) found a mean EQ–5D value of 0.71. Reference Konig, Roick and Angermeyer41
In sum, this comparison emphasises the selected nature of participants seen in clinical trials that often recruit individuals who need some change of treatment. Care is needed when extrapolating from the results of clinical studies to population-level burden-of-disease estimations.
Studies comparing different psychotic disorders on any QoL/HRQoL measures
A review of the literature up to the year 2002 using a very wide conceptualisation of HRQoL (including, for example, Global Assessment of Functioning (GAF)) found 65 studies. Reference Dean, Gerner and Gerner42 Eight of the nine studies comparing bipolar disorder with schizophrenia suggested better HRQoL for people with bipolar disorder. Another review up to 2004 included 28 articles with five studies comparing bipolar disorder with other conditions. Reference Michalak, Yatham and Lam43 Most of these showed bipolar disorder to be comparable or milder than schizophrenia. Two studies suggested that the HRQoL of participants with bipolar disorder was equivalent to that of the general population.
In a US study on community-dwelling patients, no statistically significant differences were found between people with schizophrenia and bipolar I disorder on the Quality of Well-Being scale or SF–36. Reference Depp, Davis, Mittal, Patterson and Jeste44 Another study comparing treatment-seeking individuals with bipolar I disorder and schizoaffective disorder found the EQ–5D scores of participants with bipolar to be significantly higher than those of participants with schizoaffective disorder (0.77 and 0.67 respectively). Reference Kulkarni, Berk, Fitzgerald, de Castella, Montgomery and Kelin45 Participants with schizoaffective disorder had more depressive symptoms than the group with bipolar I disorder, in line with our results. A third study including out-patients with schizophrenia and schizoaffective disorder noted that those with schizoaffective disorder had significantly lower subjective QoL. Reference Narvaez, Twamley, McKibbin, Heaton and Patterson5
Our results fit with the previous literature in suggesting that, on average, schizoaffective disorder is associated with more severe HRQoL impairment than schizophrenia. Bipolar I disorder has been associated with a smaller or similar HRQoL impact to schizophrenia, depending on the sample and the measures used.
Studies of the association between symptoms, HRQoL and QoL
In line with our findings, two previous schizophrenia studies found current depressive Reference Narvaez, Twamley, McKibbin, Heaton and Patterson5 or depressive/anxiety Reference Meijer, Koeter, Sprangers and Schene4 symptoms to have the strongest correlation with QoL; correlations with positive, negative or disorganisation symptoms were not significant. One previous study investigating non-affective psychoses found EQ–5D to be correlated with positive and negative symptoms, but even then the strongest correlation was with depressive symptoms. Reference Konig, Roick and Angermeyer41
For bipolar disorder, previous reviews of different phases of bipolar disorder have generally concluded that the HRQoL or QoL of people with bipolar disorder is lowered even in a euthymic phase, although clearly less so than in a manic phase. Depressive or mixed episodes are generally considered to be the worst, and current depressive symptomatology is a major predictor of lowered HRQoL. Reference Namjoshi and Buesching46 Results concerning the impact of the longitudinal course of bipolar disorder on HRQoL of currently euthymic patients are variable, Reference Michalak, Yatham and Lam43,Reference Depp, Davis, Mittal, Patterson and Jeste44,Reference Ozer, Ulusahin, Batur, Kabakci and Saka47,Reference MacQueen, Young, Robb, Marriott, Cooke and Joffe48 but most studies have found that depressive symptoms are most strongly correlated with HRQoL and that they affect several domains of HRQoL. This fits well with our findings that the BDI score explains most of the lost HRQoL/QoL in bipolar disorder, and the lifetime severity of depressive symptoms strongly correlates with HRQoL.
Validity of self-reported QoL and HRQoL
Our study agrees with previous studies in showing that generic QoL/HRQoL instruments produce interesting but somewhat problematic results as outcome measures for psychotic disorders. They do not correlate well with all symptom dimensions or with clinician-assessed outcomes for all disorders, or with socioeconomic disadvantage. Reference Perala, Saarni, Ostamo, Pirkola, Haukka and Harkanen49 In contrast, current depression explains a major part of the loss in QoL/HRQoL. This poses a challenge for health-economic analyses of psychotic disorders: medical treatments commonly target positive symptom reduction, but their effect may go unnoticed when utility-based HRQoL is used as an outcome measure. The EQ–5D appears especially problematic. Disorder-specific measures should be used to complement generic HRQoL measures; in clinical use at least, depressive symptoms are likely to be better measured with depression scales than HRQoL instruments.
However, the alternative interpretation is also important to consider: as we used three different QoL/HRQoL measures, some construct validity of these instruments is likely. Previous studies converge in that depressive symptoms are extremely important for the subjective well-being of people with most psychiatric disorders. Our results show that people with schizophrenia have significant depressive symptoms that reduce their well-being substantially. Further, our results also offer some room for optimism concerning the QoL/HRQoL of people with bipolar disorder and schizophrenia. More general population studies are clearly needed.
The reliability of self-reports of people with psychotic disorders is sometimes questioned. Except for the most acute phases of illness, it has repeatedly been shown that people in stable phases of psychotic illnesses can assess their health states reasonably reliably and validly – even using cognitively demanding methods such as direct utility estimation. Reference Briggs, Wild, Lees, Reaney, Dursun and Parry40,Reference Voruganti, Awad, Oyewumi, Cortese, Zirul and Dhawan50 However, equally consistent is the finding that people with stable schizophrenia tend to rank their health utility higher than healthy people Reference Fan, Henderson, Chiang, Briggs, Freudenreich and Evins51 or professionals Reference Herrman, Hawthorne and Thomas3 would. So, although our results suggest that people with common anxiety disorders and dysthymia rate their HRQoL lower than people with schizophrenia, bipolar disorder or even schizoaffective disorder, this must be interpreted in the light of larger socioeconomic disadvantage and loss of functioning associated with psychotic disorders. Reference Perala, Saarni, Ostamo, Pirkola, Haukka and Harkanen49,Reference Viertio, Sainio, Koskinen, Perala, Saarni and Sihvonen52,Reference Pirkola, Isometsa, Suvisaari, Aro, Joukamaa and Poikolainen53
The aforementioned results – individuals reporting higher subjective well-being than professionals estimate or patients' actual functional status or socioeconomic circumstances suggests – provide caregivers with an ethical dilemma. If the higher valuations are not clearly ‘flawed’, i.e. caused by cognitive, affective or psychotic misunderstandings, they may still be caused by psychological adaptation to the condition and its limitations or lowered expectations. A theoretical key component to subjective QoL is the balance between expectations and opportunities. Reference Calman54 The ethical dilemma is to what extent the lowered expectations should be accepted? Taking at face value the high life satisfaction of people with psychoses appears problematic when objective indicators show great room for improvement. But excluding the subjective experience is also unacceptable.
Strengths and weaknesses
The significant strengths or our study are the use of a general population-based sample, the thorough diagnostics and the high participation rate. Our collection of case notes allowed us to compare the severity and symptoms of non-respondents with respondents. This revealed that people with more severe affective psychoses seemed to be somewhat underrepresented. On the other hand, people with the bipolar type of schizoaffective disorder were less likely to participate than those with the depressive type. These may have somewhat exaggerated our findings of the low burden of affective psychoses and the high burden of schizoaffective disorder, relative to schizophrenia. The group with delusional disorder was quite small. More general population studies that control for residual depressive symptoms are clearly needed.
It is important to emphasise that our results represent the individual disability and suffering of respondents. It thus underestimates the total burden of psychotic disorders because mortality and the burden on caregivers are not taken into account. A further possible source of underestimation is the age limit of our study. Psychotic disorders commonly have an onset before age 30, and the impact of psychoses may be relatively larger in young people when somatic conditions are rare and HRQoL usually high.
Depression had a large influence on HRQoL and QoL. Sociodemographic factors and chronic conditions had less influence than BDI score. However, as both the HRQoL measures used included questions relating to depression, this is also partly as a result of overlapping instruments. However, depression also affects subjective QoL and this supports the validity of this finding.
Implications
Schizoaffective disorder was associated with the lowest well-being in all measures used, followed by schizophrenia and bipolar I disorder. Schizophrenia and bipolar disorder were associated with a relatively larger loss of HRQoL than subjective QoL, whereas the contrary was true for delusional disorder and major depressive disorder with psychotic symptoms.
The results suggest that the subjective suffering reported by people with schizophrenia is smaller than the loss of objectively measured functioning. This discrepancy between self-reported well-being and functioning may be clinically relevant and also needs to be taken into account when HRQoL results are used for health policy or health economics purposes. The results also highlight the importance of minimising the side-effects of treatments, which may actually decrease health utility as much as the symptoms themselves. Reference Briggs, Wild, Lees, Reaney, Dursun and Parry40
Current depressive symptoms explained most of the loss of HRQoL and QoL found. In particular, EQ–5D seems to be insensitive and measures mostly current depressive symptoms. Although depressive symptoms are important with regard to the well-being of people with psychotic disorders, this questions the utility of utility-based HRQoL measurements as sole outcome measures in psychotic disorders.
Given that even participants with schizoaffective disorder had smaller HRQoL losses than people with dysthymia, generalised anxiety disorder, agoraphobia and social phobia, Reference Saarni, Suvisaari, Sintonen, Pirkola, Koskinen and Aromaa34 our results suggest some optimism concerning the subjective outcomes of psychotic disorders. However, although low QoL invites careful consideration of treatment alternatives, moderate subjective QoL does not warrant complacency in the treatment of psychotic disorders.







eLetters
No eLetters have been published for this article.