Dementia occurs in 5% of people over 65 years old, rising to 30% of people over 90 years old (Reference Hofman, Rocca and BrayneHofman et al, 1991). Almost three-quarters of people in residential homes have dementia (Reference Macdonald, Carpenter and BoxMacdonald et al, 2002), but little is known about these individuals’ quality of life. Although studies in dementia have used patient ratings, proxy ratings or both, patients’ subjective ratings may be the gold standard for measuring quality of life in dementia; however, observational ratings may still be useful in those with the most severe dementia (Reference Brod, Stewart and SandsBrod et al, 1999; Reference Whitehouse, Patterson and SamiWhitehouse et al, 2003). In studies that have compared patient and proxy ratings for people with mild to moderate dementia, quality of life has been consistently rated lower by the caregivers (Reference Selai, Trimble and RossorSelai et al, 2001; Reference Logsdon, Gibbons and McCurryLogsdon et al, 2002). Logsdon et al (Reference Logsdon, Gibbons and McCurry2002) found that differences between patient and caregiver reports were due to varying perceptions of the patient's quality of life rather than the reliability of the assessment scale, and were associated with family caregiver depression and burden. Thorgrimsen et al (Reference Thorgrimsen, Selwood and Spector2003) found that depression in people with dementia was strongly associated with self-rated quality of life.
The aim of our study was to compare the views of residents with dementia and staff within residential homes about each resident's quality of life, and to identify factors associated with the resident's quality of life as rated by the staff and the person with dementia. We predicted that depression in the person with dementia would be the main factor associated with both subjectively rated and staff-rated quality of life.
METHOD
Sample
This investigation was undertaken as part of a larger project examining the needs of older people with dementia living in residential homes (Reference Hancock, Woods and ChallisHancock et al, 2006). In total 238 persons with dementia, over 60 years of age, were recruited to the study from 24 residential homes in London, Manchester and North Wales. Residents who were permanently placed and had lived in the home for at least 1 month with possible or definite memory problems had a case-note review and diagnostic interview to identify dementia according to DSM–IV criteria (American Psychiatric Association, 1994). All participants were asked for written consent or assent depending on their level of cognitive abilities. Residents were excluded if they were likely to be leaving the home in the immediate future. Trained mental health researchers from nursing and clinical psychology conducted all the interviews. The interview was stopped if the resident asked to withdraw or showed distress.
Procedure
The interviews were undertaken with participants at the residential home and the instruments were administered by a clinical research team. Information was obtained through interview, observation and a review of the care home documentation. The residents and staff members were interviewed separately and the investigator applied an overall clinical rating where relevant, based on all the information obtained.
Instruments
Quality of Life in Alzheimer's Disease
The Quality of Life in Alzheimer's Disease scale (QoL–AD; Reference Logsdon, Gibbons and McCurryLogsdon et al, 1999) measures quality of life in dementia and can be completed by both patient and caregivers. It contains 13 items, which include domains relevant to physical and mental health, personal relationships, finances and overall life quality. Higher scores indicate better quality of life. The QoL–AD scale has been found to have good reliability and validity and can be used with people with mild, moderate and severe dementia (Reference Thorgrimsen, Selwood and SpectorThorgrimsen et al, 2003; Reference Hoe, Katona and RocheHoe et al, 2005).
Mini-Mental State Examination
The Mini-Mental State Examination (MMSE; Reference Folstein, Folstein and McHughFolstein et al, 1975) is a brief test of cognitive function that measures orientation, memory and attention and is sensitive to change.
Clinical Dementia Rating
The Clinical Dementia Rating (CDR; Reference Hughes, Berg and DanzigerHughes et al, 1982) is an investigator-rated global score of severity of dementia graded from 0 for mild to 3 for severe dementia. It comprises six domains: memory; orientation; judgement and problem-solving; community affairs; home and hobbies; and personal care.
Cornell Scale for Depression in Dementia
The Cornell Scale for Depression in Dementia (Reference Alexopolous, Abrams and YoungAlexopolous et al, 1988) assesses depression in people with dementia by means of 19 items rated on a three-point scale, with a total score of 8 or over indicating significant depressive symptoms.
Rating Anxiety in Dementia
The Rating Anxiety in Dementia (RAID; Reference Shankar, Walker and FrostShankar et al, 1999) is a brief screening measure comprising 18 items, rated on a three-point scale. A total score of 11 or over indicates significant anxiety symptoms.
Camberwell Assessment of Need for the Elderly
The Camberwell Assessment of Need for the Elderly (CANE; Reference Orrell and HancockOrrell & Hancock, 2004) is a comprehensive measure of need in older people and has high levels of reliability and validity (Reference Reynolds, Thornicroft and AbasReynolds et al, 2000; Reference Orrell and HancockOrrell & Hancock, 2004). It includes mental and physical health, social and environmental needs, and identifies whether needs are met or unmet. Information is collected from patients, carers and professionals. The investigator makes an overall rating of need.
Challenging Behaviour Scale
The Challenging Behaviour Scale (CBS; Reference Moniz-Cook, Woods and GardinerMoniz-Cook et al, 2001) is a 25-item checklist that measures and rates the frequency and severity of challenging behaviour presented by older people with dementia.
Clifton Assessment Procedures for the Elderly–Behaviour Rating Scale
The Clifton Assessment Procedures for the Elderly – Behaviour Rating Scale (CAPE–BRS; Reference Pattie and GilleardPattie & Gilleard, 1979) measures behaviour problems and functional ability and can be used to rate dependency.
Barthel Index
The Barthel Index of Activities of Daily Living (Reference Mahoney and BartheLMahoney & Barthel, 1965) is designed to measure the individual's ability to complete various activities of daily living. The scale provides an indication of low to high dependency, with higher scores indicating better functional ability.
Analysis
We analysed the data and report descriptive data, relevant associations and correlations of clinical and demographic data with quality of life ratings. Where one or two items were missing mean QoL–AD scores were inserted, because for multidimensional data, person mean methods of imputation give better results with respect to measures of discrepancy (Reference Bernaards and SijtsmaBernaards & Sijtsma, 2000). A multivariate regression analysis was undertaken to determine predictors of quality of life, as rated by the QoL–AD, using individual and staff perceptions of residents’ quality of life.
RESULTS
In total 238 residents with dementia participated in the study. Their mean age was 86.5 years (s.d.=7.4, range 60–104) and they were predominantly female (192, 80.7%) and White (197, 82.8%). In this sample 177 (74.4%) were widowed or divorced, 50 (21.0%) were single and 11 (4.6%) were married; 104 lived in London (43.7%), 57 in Manchester (23.9%) and 77 in Wales (32.4%). The mean length of stay in the residential home was 33.5 months (s.d.=30.0, range 1–180).
The mean CDR score was 2.0 (s.d.=0.8). The mean score on the Barthel Index was 63.8 (s.d.=18.5), on the CAPE–BRS it was 16.8 (s.d.=5.2) and on the CBS it was 26.8 (s.d.=30.2). The mean Cornell Scale score was 5.8 (s.d.=5.0) and mean RAID score was 6.1 (s.d.=6.0). The residents had a mean of 12.1 met needs (s.d.=2.6) and 4.4 unmet needs (s.d.=2.6). Only 186 residents had a completed MMSE (mean score 8.7, s.d.=7.8); the rest were either too impaired or refused to complete it.
Quality of life
Overall 123 (52%) residents and 224 (94%) staff were able to complete the QoL–AD (Table 1). The residents’ mean QoL–AD score was 33.1 (s.d.=6.9; n=123) and the staff-rated mean score was 29.9 (s.d.=6.3; n=224). Where one or two items were missing mean QoL–AD scores were inserted; this was done for 54 (23%) resident-completed scales and for 132 (56%) staff-completed scales (Reference Logsdon, Gibbons and McCurryLogsdon et al, 2002). Ratings by a further 7 residents (3%) and 14 staff (6%) had three or more items missing and so these QoL–AD scales were excluded. Of the 108 (45%) residents who were unable to rate any items of the QoL–AD scale, 3 residents scored above 10 on the MMSE (3%) and 15 residents scored between 1 and 10 on the MMSE (14%). The remaining 90 residents either had an unrecorded score (n=40; 37%) or scored 0 on the MMSE (n=50; 46%).
Table 1 Participants’ completion of the Quality of Life in Alzheimer's Disease (QoL–AD) scale

| Residents (n=238) n (%) | Staff (n=238) n (%) | |
|---|---|---|
| Completed QoL—AD scale | 123 (51.7) | 224 (94.1) |
| Partly completed QoL—AD scale (> 2 items missing) | 7 (2.9) | 14 (5.9) |
| Did not complete QoL—AD scale | 108 (45.4) | 0 |
| Matched resident and staff completed QoL—AD scales | 119 (50) | 119 (50) |
Factors associated with individual- and staff-rated quality of life
The initial analyses only included residents (n=119) for whom both staff- and self-completed QoL–AD scales were available (Table 2). Resident ratings of higher quality of life were significantly correlated with less depressed mood and less anxiety, fewer unmet needs and more cognitive impairment. The correlations were then repeated using only the corresponding staff-completed QoL–AD scales (n=119) (Table 2). Higher staff-rated QoL–AD scores were significantly associated with less physical disability, less cognitive impairment, fewer neuropsychiatric symptoms, lower levels of depression and anxiety symptoms and fewer unmet needs. An additional analysis was undertaken using all the available staff-rated QoL–AD scales (n=224). Again, there were highly significant correlations with the CAPE–BRS (–0.47, P<0.001), Barthel (0.36, P<0.001), CDR (–0.32, P<0.001), Cornell Scale (–0.32, P<0.001), CBS (–0.28, P<0.001), MMSE (0.27, P<0.001), RAID (–0.25, P<0.001) and unmet needs (–0.30, P<0.001). This highlighted the strong association between staff perception of residents’ quality of life and level of dependency.
Table 2 Correlations of resident- and staff-completed Quality of Life in Alzheimer's Disease (QoL–AD) scale scores with other assessment ratings
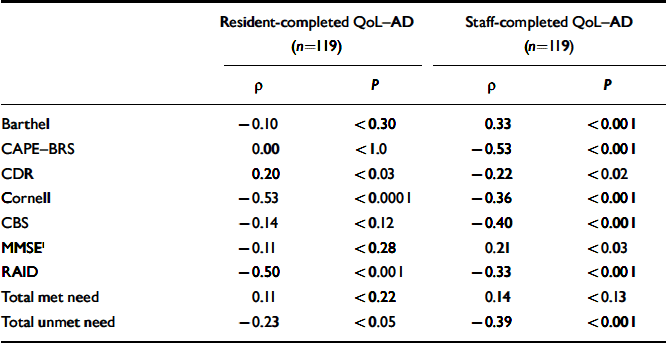
| Resident-completed QoL—AD (n=119) | Staff-completed QoL—AD (n=119) | |||
|---|---|---|---|---|
| ρ | P | ρ | P | |
| Barthel | -0.10 | <0.30 | 0.33 | <0.001 |
| CAPE—BRS | 0.00 | <1.0 | -0.53 | <0.001 |
| CDR | 0.20 | <0.03 | -0.22 | <0.02 |
| Cornell | -0.53 | <0.0001 | -0.36 | <0.001 |
| CBS | -0.14 | <0.12 | -0.40 | <0.001 |
| MMSE1 | -0.11 | <0.28 | 0.21 | <0.03 |
| RAID | -0.50 | <0.001 | -0.33 | <0.001 |
| Total met need | 0.11 | <0.22 | 0.14 | <0.13 |
| Total unmet need | -0.23 | <0.05 | -0.39 | <0.001 |
Associations between individual and staff perceptions of residents’ quality of life
The QoL–AD scores for residents were compared using only the matched resident- and staff-completed scales (n=119). The resident-completed QoL–AD mean score was 33.1 (s.d.=7.0) and the staff-completed mean score was 30.8 (s.d.=6.4) (Table 3). The total QoL–AD scores for individual and staff perceptions of residents’ quality of life were significantly correlated (ρ=0.27, P<0.005). An item-by-item correlation was then calculated for the resident- and staff-completed QoL–AD scales but significant correlations were observed only for the items ‘family’, ‘marriage’, ‘friends’, ‘ability to do things for fun’ and ‘life as a whole’ (Table 3). Although there were similar mean scores for both residents and staff, for all items the overall level of agreement between ratings measured by the inter-item correlations (ρ< 0.4) and kappa (<0.4) was low, indicating a clear discrepancy between staff and resident ratings of each item.
Table 3 Quality of Life in Alzheimer's Disease (QoL–AD) scale item-by-item mean correlation and κ coefficients

| QoL—AD item | Resident-completed QoL—AD (n=119) | Staff-completed QoL—AD (n=119) | Correlation | ||
|---|---|---|---|---|---|
| Mean (s.d.) | Mean (s.d.) | ρ | P | κ | |
| Physical health | 2.7 (0.84) | 2.5 (0.81) | -0.02 | NS | 0.0 |
| Energy | 2.4 (0.83) | 2.4 (0.83) | 0.16 | NS | 0.13 |
| Mood | 2.6 (0.86) | 2.5 (0.72) | -0.01 | NS | -0.02 |
| Living situation | 2.8 (0.83) | 3.0 (0.59) | 0.16 | NS | 0.15 |
| Memory | 2.4 (0.87) | 1.9 (0.75) | 0.15 | NS | 0.06 |
| Family | 2.8 (0.90) | 2.6 (1.1) | 0.36 | ≤0.001 | 0.13 |
| Marriage | 2.9 (0.87) | 2.6 (0.92) | 0.31 | ≤0.001 | 0.02 |
| Friends | 2.5 (0.98) | 2.2 (1.0) | 0.23 | ≤0.012 | 0.07 |
| Self as a whole | 2.6 (0.87) | 2.6 (0.73) | 0.15 | NS | 0.20 |
| Ability to do chores | 2.2 (0.95) | 1.7 (0.91) | 0.11 | NS | 0.11 |
| Ability to do things for fun | 2.2 (0.80) | 2.1 (0.95) | 0.26 | ≤0.005 | 0.14 |
| Money | 2.3 (0.94) | 2.1 (0.99) | 0.06 | NS | 0.06 |
| Life as a whole | 2.6 (0.83) | 2.6 (0.71) | 0.21 | ≤0.020 | 0.02 |
| Total score | 33.1 (7.0) | 30.8 (6.3) | 0.27 | ≤0.005 | 0.29 |
Regression analysis
A multiple linear regression analysis was undertaken to determine which scales were the best predictors of quality of life. Completed resident- and staff-rated QoL–AD scores were each used as the dependent variables. The multiple independent variables included all completed scales for the Barthel, CAPE–BRS, CBS, Cornell, MMSE, RAID, met need and unmet need. Any scales with missing items were excluded. Residents’ perception of their quality of life was significantly predicted by the Cornell (β=-0.40, P<0.005) and the RAID (β=-0.32 P<0.05) instruments only. This model accounted for 34% of the variance (F=6.3, P<0.001; adjusted R 2=0.28). For the staff-rated quality of life, the QoL–AD score was only associated with the CAPE–BRS (β=-0.59, P<0.001). This model accounted for 43% of the variance (F=9.5, P<0.001; adjusted R 2=0.39).
DISCUSSION
Our study has shown that in a sample of residential homes the QoL–AD can be used to measure the quality of life of many people with dementia. These homes were considered representative of the care homes available nationally as they covered different areas of the UK (London, northern England and Wales: inner-city, urban, suburban and rural), and therefore the results may be generalisable within the care home population. Both individual and staff perceptions of residents’ quality of life were measured, and in keeping with earlier studies the caregivers’ ratings were lower (Reference Logsdon, Gibbons and McCurryLogsdon et al, 1999; Reference Selai, Trimble and RossorSelai et al, 2001).
In this study, assuming that only correlations of 0.4 and above can be considered clinically significant (Reference Dunn and EverittDunn & Everitt, 1995), only 5 of 13 QoL–AD items of the resident and staff ratings were correlated, none at the level of clinical significance. The kappa coefficients also showed that none of the QoL–AD items was consistently rated the same by both staff and residents. This suggests that staff ratings cannot be assumed to be a suitable proxy for quality of life from the viewpoint of a person with dementia.
The residents’ ratings of their own quality of life were highly associated with symptoms of both depression and anxiety. In contrast, ratings of the residents’ quality of life by staff were clearly associated with level of dependency and behaviour problems. This suggests that staff perception of the residents’ quality of life was most strongly influenced by levels of dependency. Perhaps staff are less likely to see the residents in terms of the resident's subjective experiences (e.g. mood, pleasant and unpleasant experiences) and more likely to see them as people whose quality of life is determined by disability. The multivariate analysis also showed that the Cornell Scale and the RAID were the only predictors of quality of life as rated by residents. In contrast, the CAPE–BRS was the only predictor for staff ratings of residents’ quality of life. This further suggests that residents’ perception of quality of life is influenced by mood and the staff perception of it is influenced by functional ability.
A number of other studies have investigated the potential predictors of quality of life in people with dementia. Lower levels of depression and higher levels of functional ability, educational level, social contact and activity were found to be related to higher quality of life in dementia (Reference Logsdon, Gibbons and McCurryLogsdon et al, 1999; Reference Burgener and TwiggBurgener & Twigg, 2002). Conversely, low quality of life was linked to poor physical health and memory, loss of role, increased boredom and loneliness (Reference Ready, Ott and GraceReady et al, 2002; Reference Thorgrimsen, Selwood and SpectorThorgrimsen et al, 2003). More recent studies have suggested that quality of life in dementia is influenced by mood and environmental factors independent of dementia severity (Reference Thorgrimsen, Selwood and SpectorThorgrimsen et al, 2003; Reference Hoe, Katona and RocheHoe et al, 2005).
There have, however, been contradictory findings in studies that used only staff proxy ratings of quality of life when those living in the community and in long-term care institutions were compared. The long-term residents experienced poorer quality of life than community patients (Reference Leon, Neumann and HermannLeon et al, 1998), and low ratings of quality of life by staff were associated with orientation disturbances, physical dependence and anxiolytic treatment (Reference Gonzalez-Salvador, Lyketsos and BakerGonzalez-Salvador et al, 2000). The need for privacy and enjoyment has proved difficult to measure reliably in the more cognitively impaired residents (Reference Kane, Kling and BershadskyKane et al, 2003).
In studies that have investigated carer and individual perceptions of quality of life, the ratings were strongly influenced by the individual's mood and the caregiver's experience of caring (Reference Karlawish, Casarett and KlocinskiKarlawish et al, 2001; Reference Logsdon, Gibbons and McCurryLogsdon et al, 2002; Reference Sands, Ferreira and StewartSands et al, 2004). These studies investigated people with mild to moderate dementia living in the community and found that lower ratings of quality of life by the person with dementia were predicted by the presence of depressive symptoms, whereas lower ratings by carers were associated with caregiver depression and burden. A further study that investigated caregiver, staff and individual perceptions of quality of life for people with dementia in institutional care found poor agreement between patient and proxy ratings other than for observable measures of function such as physical health and disability (Reference Novella, Jochum and MorroneNovella et al, 2001). The spouse and qualified nursing staff were in closer agreement with the patient's ratings of quality of life than other family and staff members. Coucill et al (Reference Coucill, Bryan and Bentham2001) also investigated the quality of life of people with mild to moderate dementia using a modified version of the EuroQol EQ–5D instrument (http://www.euroqol.org) and compared these scores with caregiver and physician ratings. The study found there were differences between the two proxy ratings, and it was unclear who the most appropriate proxy was. Although Coucill concluded that the EQ–5D is suitable for use with this patient population, concerns were raised about the validity of patient self-rating because 91% of self-rated responses accounted for all ceiling responses (Reference Coucill, Bryan and BenthamCoucill et al, 2001). Similarly, Thorgrimsen et al (Reference Thorgrimsen, Selwood and Spector2003) found that most people did not report problems in the five domains of the EQ–5D and many found the visual analogue scale difficult to complete; these authors concluded that the QoL–AD was the preferable scale for this patient population.
Limitations of the study were that staff perceptions of residents’ quality of life might have been influenced by the nature of their relationship to the resident, their knowledge of the resident, their knowledge of and attitudes to dementia and staff factors such as stress and job satisfaction. Where possible the keyworker was interviewed, then information was corroborated if necessary by asking a senior care worker or the home manager. By using this method we attempted to obtain a staff rating of the resident's quality of life from staff who knew the resident well. It is interesting to note that some staff felt unable to complete the QoL–A, finding fewer problems with the other scales. Just over half of the residents in the total sample were able to complete the QoL–AD; many of these had severe dementia. Previous studies have shown that some people with dementia who have an MMSE score as low as 3 can rate the QoL–AD (Reference Thorgrimsen, Selwood and SpectorThorgrimsen et al, 2003; Reference Hoe, Katona and RocheHoe et al, 2005). In our study, where there were two or fewer items missing, mean scores were inserted for these items on the QoL–AD; these were predominantly for the items involving family relationships and money. This was usually the result of the resident having no known spouse and family, or lack of knowledge about the resident's financial circumstances. Of the residents who could not complete the QoL–AD, most had severe dementia, and it may not be the case that these residents would feel the same as residents who could complete this measure.
In conclusion, the QoL–AD was an effective measure of quality of life for many people with dementia in residential homes and was able to reflect perceptions of individuals and their well-being. Future research should consider how the individual's quality of life changes as the dementia process progresses. It would also be of interest to look at quality of life of residents with dementia compared with residents without dementia who live in the same care homes. As both objective and subjective ratings were included in the scale, further qualitative research could also explore in more depth which factors influence a person with dementia's quality of life and why such people regard it more positively than their caregivers do.
Despite most having severe dementia, residents’ views of their own quality of life were strongly linked to their mood, suggesting that improving mood would increase quality of life. In contrast, staff related quality of life to dependency and behaviour problems, suggesting that they considered disability to be the most important factor. Care staff and health professionals should be aware that the quality of life of people with dementia in residential homes might primarily relate to their mood in terms of both anxiety and depression. Maximising their enjoyment and enhancing well-being along with the identification and treatment of mood disorders should therefore be prioritised in care plans.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ Ratings of quality of life by people with dementia living in residential homes were influenced most strongly by their mood.
-
▪ Ratings by staff caring for people with dementia living in residential homes of the latter's quality of life was influenced most strongly by levels of dependency and challenging behaviour.
-
▪ Staff and individual ratings of quality of life for residents with dementia living in care homes had poor agreement. This suggests that staff ratings cannot be assumed to provide a suitable proxy for the person with dementia.
LIMITATIONS
-
▪ Just under half of the residents included in the study could not complete the quality of Life – Alzheimer's Disease (QoL–AD) scale, most of whom had severe dementia.
-
▪ A small number of staff found it difficult to comment on the residents'quality of life.
-
▪ We do not know whether family carers differ from staff in rating the quality of life of people with dementia using the QoL–AD.
Acknowledgements
We thank the Wellcome Trust for funding the study and the research nurses who assisted with the data collection (Joanne Baker, Claire O'Donoghue and Bridie Bains). We also thank all the people with memory problems and their families, friends and other carers who participated in the study, together with all the staff of the residential homes who provided us with support and help. Professors Martin Knapp, Bob Woods and David Challis were also grant holders.






eLetters
No eLetters have been published for this article.