It is now well established that people with psychotic disorders have higher rates of cannabis use compared with the general population (Reference Regier, Farmer and RaeRegier et al, 1990; Reference Degenhardt and HallDegenhardt & Hall, 2001), which, in turn, is associated with poorer functional and clinical outcomes (Reference Jablensky, Sartorius and ErnbergJablensky et al, 1991). Cannabis use is strongly associated with greater psychotic symptom severity; with such effects found up to 4 years later (Reference Linszen, Dingemans and LeniorLinszen et al, 1994; Reference van Os, Bak and Hanssenvan Os et al, 2002; Reference Sorbara, Liraud and AssensSorbara et al, 2003; Reference Grech, van Os and JonesGrech et al, 2005; Reference Henquet, Krabbendam and SpauwenHenquet et al, 2005). Cannabis misuse has also been associated with up to four times the risk of psychotic relapse (Reference Linszen, Dingemans and NugterLinszen et al, 1997) and has emerged as the strongest predictor of relapse over 12 months compared with a range of other risk factors, including medication adherence, duration of untreated psychosis, chronic and acute stress, and expressed emotion (Reference Linszen, Dingemans and LeniorLinszen et al, 1994; Reference Martinez-Arevalo, Calcedo-Ordonez and Varo-PrietoMartinez-Arevalo et al, 1994; Reference Linszen, Dingemans and NugterLinszen et al, 1997).
The high rates of cannabis use among people with psychosis may be related to attempts to self-medicate distressing symptoms or the side-effects of antipsychotic medications (Reference Verdoux, Tournier and CougnardVerdoux et al, 2005). However, there has been little empirical investigation or evidence for this hypothesis to date (Reference Hamera, Schneider and DevineyHamera et al, 1995; Reference Verdoux, Gindre and SorboraVerdoux et al, 2003; Reference Henquet, Krabbendam and SpauwenHenquet et al, 2005). In addition, little information is available on key variables associated with relapse to cannabis use among individuals with psychosis.
This study examines the relative influence of cannabis use on psychotic relapse, after controlling for other established predictors of relapse (specifically duration of untreated psychosis, medication adherence, subjective life stress and the family environment) (Reference Nuechterlein, Dawson and GitlinNuechterlein et al, 1992; Reference Pallanti, Quercioli and PazzagliPallanti et al, 1997; Reference Marshall, Lewis and LockwoodMarshall et al, 2005; Reference Pourmand, Kavanagh and VaughanPourmand et al, 2005). A further aim was to determine whether an increase in psychotic symptoms was followed by a substantial increase in cannabis use, referred to in the current study as cannabis relapse.
METHOD
Participants
Participants were required to have a current DSM-IV (American Psychiatric Association, 1994) diagnosis of a psychotic disorder (schizophreniform or schizoaffective disorder, schizophrenia, delusional disorder, substance-induced psychosis, depressive, bipolar or mixed episode with psychotic features), to be aged 16 years or over, to have had no more than two previous psychotic episodes and to be within 3 years of initial diagnosis. Individuals with non-psychotic affective disorders, brief psychotic disorders associated with medical conditions or intellectual disability were excluded.
Over a 7-month period from March to October 2000, 121 patients consecutively admitted to three acute psychiatric wards in Brisbane, Australia, met inclusion criteria for the study. Of these, 96 (79%) were approached for inclusion in the study, after 14 were discharged before recruitment and a further 11 were either away without leave or too unwell to be approached. In all, 84 (88%) in-patients agreed to participate in the baseline assessment, 81 (96%) of whom agreed to participate in the 6-month follow-up study.
Measures
Diagnostic status for a current psychotic disorder was confirmed using the Operational Criteria Checklist (OPCRIT; Reference McGuffin, Farmer and HarveyMcGuffin et al, 1991), a 90-item checklist of signs and symptoms of mental illness. The age at onset of first psychotic symptoms (delusions, hallucinations or suspiciousness) was obtained using the Interview for the Retrospective Assessment of the Onset of Schizophrenia (IRAOS), a valid and reliable semi-structured interview for assessing the first appearance of symptoms of schizophrenia (Reference Hafner, Riecher Rossler and HambrechtHafner et al, 1992). Psychiatric symptoms were monitored using the Brief Psychiatric Rating Scale (BPRS; Reference Overall and GorhamOverall & Gorham, 1962) at baseline assessment and throughout the 6-month follow-up; BPRS positive, negative and depression-anxiety symptom scores were derived from the sub-scales identified by Ventura et al (Reference Ventura, Nuechterlein and Subotnik2000). Only BPRS items that did not require interviewer observation were included in the telephone interviews during follow-up.
Diagnostic information on substance misuse and dependence in the 12 months before admission was obtained using Section L of the Composite International Diagnostic Interview (CIDI; World Health Organization, 1997). Baseline cannabis and other substance use in the 6 weeks before admission was retrospectively assessed using the Timeline Followback procedure (TLFB; Reference Sobell, Sobell, Litten and AllenSobell & Sobell, 1992). This calendar-based method has well established reliability and validity and obtains precise information on the frequency (days) of substance use, by anchoring substance use against key life events to assist recall (Reference Sobell, Sobell, Litten and AllenSobell & Sobell, 1992; Reference Fals Stewart, O'Farrell and FreitasFals Stewart et al, 2000). Key life events were defined according to the Psychiatric Epidemiological Interview-Life Events Scale (PERI-LES; Reference Dohrenwend, Krasnoff and AskenasyDohrenwend et al, 1978). The TLFB was also used to monitor the frequency (days) of cannabis and other substance use, stressful life events, life stress (subjectively rated from 0 to 10) and the number of days of medication adherence for each week over the 6-month follow-up period.
A number of measures of key constructs previously related to psychotic symptom severity and relapse were included at baseline only. The conflict, expressiveness, cohesion and control sub-scales of the Family Environment Scale (FES; Reference Moos and MoosMoos & Moos, 1994) were used to provide a measure of current family functioning for individuals in regular contact with their family or partners. Participants' objective quality of life and global well-being in the past 12 months was assessed using the Quality of Life Interview-Brief Version (QOLIBV; Reference LehmanLehman, 1995). Premorbid adjustment in the 6 months preceding first admission to a psychiatric hospital was assessed using the 21-item Premorbid Adjustment Scale (PAS; Reference Cannon-Spoor, Potkin and WyattCannon-Spoor et al, 1982).
Urinary drug screening was performed either at 6 months or while in hospital, to corroborate self-reports of recent substance use and antipsychotic medication adherence. Urine was screened using a cannabis immunoassay and gas chromatography/mass spectrometry.
The criteria used to define psychotic symptom stabilisation, exacerbation and relapse were drawn from those proposed by Nuechterlein and colleagues (Reference Nuechterlein, Snyder and Dawson1986) using BPRS scores (Table 1). Participants who met criteria for psychotic relapse or symptom exacerbation (including criteria for unremitting symptoms) were considered to have relapsed. Cannabis relapse was defined as an increase to at least 5 days of cannabis use within a 1-week period following stabilisation of both psychotic symptoms and cannabis use (Table 1).
Table 1 Psychotic and cannabis relapse criteria
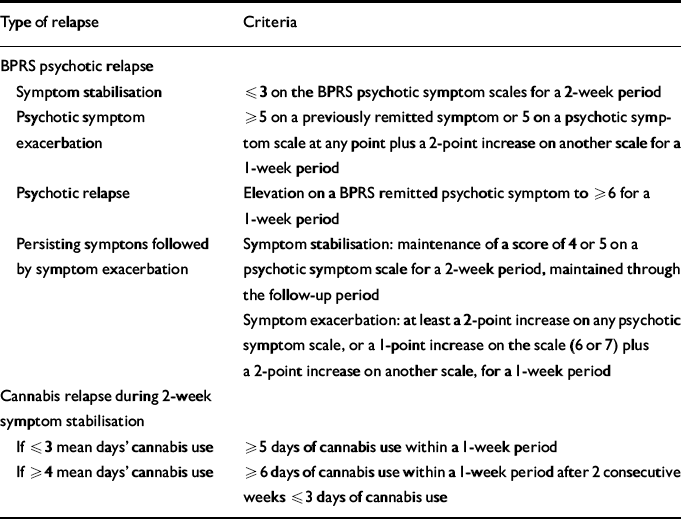
| Type of relapse | Criteria |
|---|---|
| BPRS psychotic relapse | |
| Symptom stabilisation | ⩽3 on the BPRS psychotic symptom scales for a 2-week period |
| Psychotic symptom exacerbation | ⩾5 on a previously remitted symptom or 5 on a psychotic symptom scale at any point plus a 2-point increase on another scale for a 1-week period |
| Psychotic relapse | Elevation on a BPRS remitted psychotic symptom to ⩾6 for a 1-week period |
| Persisting symptons followed by symptom exacerbation | Symptom stabilisation: maintenance of a score of 4 or 5 on a psychotic symptom scale for a 2-week period, maintained through the follow-up period |
| Symptom exacerbation: at least a 2-point increase on any psychotic symptom scale, or a 1-point increase on the scale (6 or 7) plus a 2-point increase on another scale, for a 1-week period | |
| Cannabis relapse during 2-week symptom stabilisation | |
| If ⩽3 mean days' cannabis use | ⩾5 days of cannabis use within a 1-week period |
| If ⩾4 mean days' cannabis use | ⩾6 days of cannabis use within a 1-week period after 2 consecutive weeks ⩽3 days of cannabis use |
BPRS, Brief Psychiatric Rating Scale
Procedure
Consenting participants took part in a baseline assessment of psychopathology, substance use, and clinical and functional variables. Those who agreed to remain in the study were followed up on a weekly basis for 3 months, and then fortnightly for the remaining 3 months, making a total of 18 contacts. The first interview was conducted within 1 week of the baseline assessment. Monthly face-to-face interviews were conducted in participants' homes or in another setting convenient to them. The remaining interviews were conducted by telephone. The BPRS symptom ratings segment of the telephone interview was audiotaped for interrater reliability purposes for 70% of participants. Participants were reimbursed Australian $10 for their time and travel expenses for each face-to-face follow-up interview. Ethical approval to conduct the study was granted by the Griffith University Research Ethics Committee, and participating hospital institutional ethics committee.
Statistical analysis
Data were analysed with the Statistical Package for the Social Sciences (SPSS for Windows 12.0; SPSS, Chicago, IL). Several variables had skewed distributions and required transformation. However, in accordance with guidelines suggested by Tabachnick & Fidell (Reference Tabachnick and Fidell2001), results using untransformed data are reported as there was no difference between results using transformed and untransformed data.
Cox regression survival analyses were performed to assess the relative contribution of cannabis use (days of cannabis use per week) on psychotic relapse after adjusting for other predictors of outcome. Cannabis use was first entered into the analysis to determine its individual effect on psychotic relapse. Cannabis use was then paired with a range of other variables to determine if it still had an individual effect on relapse after adjusting for these variables. These included: demographic variables, functioning (including PAS total score and QOLI-BV sub-scales), other substance use (days of alcohol and amphetamine use), family environment stress (including subjective life stress and stressful life events) and clinical variables (including BPRS psychotic, depression-anxiety and negative symptom severity), obtained at baseline (subsequently identified with the prefix baseline) and during follow up. This was done to determine its individual effect on relapse after adjusting for these variables. A Cox regression analysis was then conducted to determine the independent influence of cannabis use (entered at step 2) on psychotic relapse, after controlling for significant predictors of relapse. These comprised predictors identified in the previous analyses, as well as other established predictors of psychotic relapse described in the clinical research literature, including: duration of untreated psychosis (time period between the first signs of psychotic symptoms and first contact with psychiatric services); subjective life stress (rated 0-10 per week); antipsychotic medication medication adherence (days of medication per week); and other substance use (days of alcohol and amphetamine use per week) (entered at step 1). All prospective variables were lagged segmented time-dependent covariates based on the time from symptom stabilisation (week 1) to the week before relapse.
Cox regression survival analyses were also performed to determine the influence of psychotic symptom severity as measured by the BPRS scales of unusual thought content, hallucinations and conceptual disorganisation (psychotic symptom total per week) on cannabis relapse relative to other predictors of outcome. Psychotic symptom severity was first entered into the analysis to determine its individual effect on cannabis relapse, and then paired with demographic, functioning, substance use, stress, family and clinical variables to assess whether it still had an individual effect on cannabis relapse after adjusting for each of these variables. The independent influence of psychotic symptom severity (entered at step 2) on cannabis relapse was then evaluated, after adjusting for the significant predictors of relapse identified above as well as other key predictors of outcome, including: age at onset of regular cannabis use (age at onset of the most frequent cannabis use in the previous 12 months); subjective life stress; medication adherence; and other substance use (entered at step 1). As in the previous analysis, all prospective variables entered into the analyses were lagged segmented time-dependent covariates based on the time from symptom stabilisation (week 1) to the week before cannabis relapse. The Wald test was used to determine the significance of the influence of covariates on time to relapse in all Cox regression analyses.
RESULTS
Participant characteristics
The sample was predominantly male (n=59, 72.8%) with a mean age of 24.49 (s.d.=5.29) years. The majority were single (n=64, 79.0%), on disability/unemployment benefits (n=62, 76.5%), and lived with either their family or partner (n=58, 71.6%). Mean duration of education was 10.81 (s.d.=2.06) years, and 72 participants (88.9%) were Caucasian, 4 (4.9%) were Asian and 5 (6.2%) were indigenous Australians. Using the OPCRIT, 58 participants (71.6%) met DSM-IV criteria for a psychotic disorder and the remaining participants met criteria for affective disorders with psychotic features. Of the 81 participants, 28 (34.6%) were first admissions, and 36 (44.4%) were experiencing their first psychotic episode. The mean duration of untreated psychosis was 117.90 (s.d.=241.20) days. Before baseline admission 36 participants (44.4%) received primarily antipsychotic medication (77.8% atypical agents); 3 (3.7%) received antidepressants and 3 participants (3.7%) received mood stabilisers. Only nine of these participants (11.1%) adhered to their prescribed medication for over half of the 6 weeks before admission. All participants on discharge were receiving antipsychotic medication (82.7% atypical agents), 17 (21.0%) were also receiving antidepressants, 23 (28.4%) anti-anxiety medication and 9 (11.1%) anticholinergics. Table 2 displays information on other clinical, family and functional variables.
Table 2 Clinical and functional variables at admission
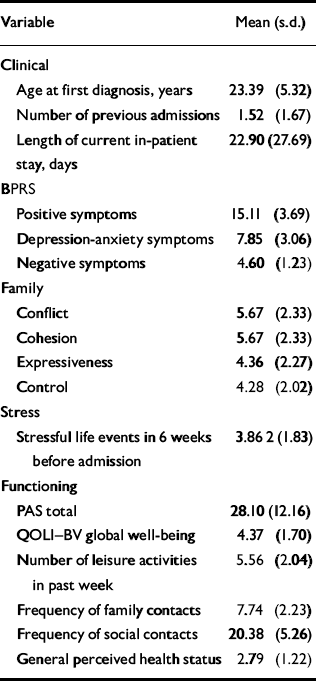
| Variable | Mean (s.d.) |
|---|---|
| Clinical | |
| Age at first diagnosis, years | 23.39 (5.32) |
| Number of previous admissions | 1.52 (1.67) |
| Length of current in-patient stay, days | 22.90 (27.69) |
| BPRS | |
| Positive symptoms | 15.11 (3.69) |
| Depression-anxiety symptoms | 7.85 (3.06) |
| Negative symptoms | 4.60 (1.23) |
| Family | |
| Conflict | 5.67 (2.33) |
| Cohesion | 5.67 (2.33) |
| Expressiveness | 4.36 (2.27) |
| Control | 4.28 (2.02) |
| Stress | |
| Stressful life events in 6 weeks before admission | 3.86 2 (1.83) |
| Functioning | |
| PAS total | 28.10 (12.16) |
| QOLI–BV global well-being | 4.37 (1.70) |
| Number of leisure activities in past week | 5.56 (2.04) |
| Frequency of family contacts | 7.74 (2.23) |
| Frequency of social contacts | 20.38 (5.26) |
| General perceived health status | 2.79 (1.22) |
BPRS, Brief Psychiatric Rating Scale; PAS, Premorbid Adjustment Scale; QOLI–BV, Quality of Life Interview–Brief Version
Cannabis was the most commonly used substance, with 57 participants (70.4%) meeting DSM-IV criteria for cannabis dependence in the 12 months preceding baseline assessment. Furthermore, 25 participants (30.9%) met criteria for amphetamine dependence and 20 (24.7%) met criteria for both cannabis and amphetamine dependence. There were low levels of heroin (n=4, 4.9%) and hallucinogen (n=1, 1.2%) dependence. Only 12 participants (14.8%) had not used any illicit substance in the previous 12 months. Mean age at first cannabis use was 15.16 (s.d.=3.24) years and mean age at onset of regular cannabis use was 17.48 (s.d.=3.96) years. Participants had used cannabis for a mean of 17.43 (s.d.=16.32, minimum 0, maximum 42) days in the 6 weeks before admission. There was a lower level of other substance use in the 6 weeks before admission, with a mean of 8.84 (s.d.=11.56, minimum 0, maximum 42) days of alcohol and amphetamine use combined.
Psychotic and cannabis relapse during follow-up
There were no significant differences between participants recruited to the study and those who were discharged before assessment or who refused to participate on the grounds of age or gender. Of the original 81 participants, 56 (69.1%) were retained in the study for 6 months, and a further 3 (72.8% total) were available until a psychotic relapse that occurred before 6 months; 63 (77.7%) were retained for 6 months or until a cannabis relapse. Dropout typically occurred early in the follow-up period, with 19 participants withdrawing within the first 8 weeks of the study. Of these, 11 were lost to contact immediately following discharge. Two participants died by suicide (2.5%). Participants who were lost to follow-up before 6 months and who did not experience a psychotic or cannabis relapse were retained for a median of 4.50 (minimum 1, maximum 17) weeks and 4.00 (minimum 1, maximum 13) weeks respectively. There were no significant differences between those retained to 6 months and those who were lost to follow-up on any demographic, symptom or substance use variables at admission, with the exception of living arrangements. Those retained were significantly more likely to live at home with their parents or partners/families (χ2(1)=9.91, P<0.01).
Reliability analysis
Of the 57 participants (87.7%), 50 approached consented to auditotaping of the BPRS symptom-rating segment of a telephone interview. An interrater reliability coefficient of 0.84 (Cohen's kappa) was obtained for the BPRS psychotic symptom total.
A total of 49 urine drug screens (60.5%) were performed to corroborate self-reported medication adherence and recent substance use with 41 (83.7%) samples collected at 6 months' follow-up and 8 (16.3%) collected during the baseline hospital admission. Using a detection time of 2 weeks for cannabis use (Reference Vandevenne, Vandenbussche and VerstraeteVandevenne et al, 2000), there was a high level of agreement (Cohen's kappa=0.90) between participants' self-reported cannabis use and urinalysis.
There was substantial agreement between participants' reported antipsychotic medication adherence (Cohen's kappa 0.72) and amphetamine use (Cohen's kappa 0.65) in the last week with the urine drug results.
Cannabis use as a predictor of psychotic relapse
The relative contribution of cannabis use and other established predictors of outcome to time to psychotic relapse was determined using a Cox regression survival analysis. The number of days of cannabis use was a significant predictor of time to psychotic relapse (P=0.001) when entered individually into the analysis, and remained a significant predictor after adjusting for a range of demographic, functioning, substance use, stress, family and clinical variables. The severity of BPRS positive psychotic (P=0.017) and depression-anxiety (P=0.001) symptoms at baseline were significant predictors of relapse independently of cannabis use.
In all, 69 patients were entered into the principal analysis, with 42 censored at 180 days and 12 excluded (i.e. 6 participants with less than 3 weeks of data from symptom stabilisation, and 6 participants whose symptoms did not stabilise before dropout). Table 3 displays the regression coefficients, standard error, Wald statistics, degrees of freedom, P values and hazard ratios for each covariate. Using the Wald test, the number of days of cannabis use significantly predicted time to psychotic relapse after adjusting for the six covariates, with each additional day of cannabis use within a 1-week period increasing psychotic relapse risk by approximately 6.4%. Depression-anxiety symptoms at baseline were also predictive; each point of increase in symptom severity increased relapse risk by 26.3%. Excluding participants with an initial clinical diagnosis of a substance-induced psychotic disorder did not alter the results of the analysis.
Table 3 Cox regression survival analysis on BPRS psychotic relapse with cannabis use and other predictor variables in one model
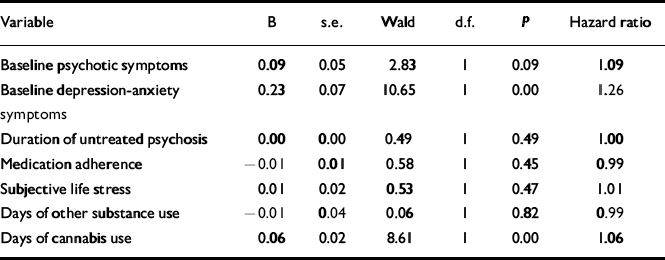
| Variable | B | s.e. | Wald | d.f. | P | Hazard ratio |
|---|---|---|---|---|---|---|
| Baseline psychotic symptoms | 0.09 | 0.05 | 2.83 | 1 | 0.09 | 1.09 |
| Baseline depression-anxiety | 0.23 | 0.07 | 10.65 | 1 | 0.00 | 1.26 |
| symptoms | ||||||
| Duration of untreated psychosis | 0.00 | 0.00 | 0.49 | 1 | 0.49 | 1.00 |
| Medication adherence | –0.01 | 0.01 | 0.58 | 1 | 0.45 | 0.99 |
| Subjective life stress | 0.01 | 0.02 | 0.53 | 1 | 0.47 | 1.01 |
| Days of other substance use | –0.01 | 0.04 | 0.06 | 1 | 0.82 | 0.99 |
| Days of cannabis use | 0.06 | 0.02 | 8.61 | 1 | 0.00 | 1.06 |
Psychotic symptom severity as a predictor of cannabis relapse
The relative influence of psychotic symptom severity on cannabis relapse was then determined using a Cox regression survival analysis. Psychotic symptom severity was a significant predictor of cannabis relapse (P=0.001), and remained a significant predictor after adjusting for a range of demographic, functioning, substance use and clinical variables. Baseline cannabis use (P=0.004) in the 6 weeks before admission, and also medication adherence (P=0.006), were other significant predictors of cannabis relapse in addition to psychotic symptom severity.
The influence of BPRS psychotic symptom severity on cannabis relapse was then examined relative to the age at onset of regular cannabis use, medication adherence, life stress, other substance use and baseline cannabis use. A total of 67 patients were entered into the analysis, with 25 censored at 180 days and 14 excluded (an additional 2 were excluded from this analysis because of missing data). Table 4 displays the regression coefficients, standard error, Wald statistics, degrees of freedom, P values and hazard ratios for each covariate. After adjusting for the five covariates, psychotic symptom severity significantly predicted time to cannabis relapse, with each point of increase in symptom severity in a 1-week period increasing relapse risk by approximately 2.5%. Medication adherence was also predictive, with each additional day of medication adherence in a 1-week period decreasing relapse risk by 1.5%. Excluding participants with an initial clinical diagnosis of a substance-induced psychotic disorder did not alter the results of the analysis.
Table 4 Cox regression survival analysis on cannabis relapse with psychotic symptom severity and other predictor variables in one model

| Variables | B | s.e. | Wald | d.f. | P | Hazard ratio |
|---|---|---|---|---|---|---|
| Baseline cannabis use | 0.02 | 0.01 | 2.40 | 1 | 0.12 | 1.02 |
| Age at onset of regular cannabis use | 0.03 | 0.04 | 0.42 | 1 | 0.52 | 1.03 |
| Medication adherence | –0.02 | 0.01 | 4.21 | 1 | 0.04 | 0.99 |
| Subjective life stress | 0.00 | 0.01 | 0.05 | 1 | 0.82 | 1.00 |
| Days of other substance use | 0.02 | 0.01 | 1.64 | 1 | 0.20 | 1.02 |
| Psychotic symptom severity | 0.03 | 0.01 | 8.02 | 1 | 0.00 | 1.03 |
DISCUSSION
This prospective study explored the influence of cannabis use on psychotic relapse in a sample of young people with recent-onset psychosis. The frequency of cannabis use emerged as a strong predictor of time to psychotic relapse over a 6-month period. This was independent of other key predictors of poor outcome, including medication adherence, stress and duration of untreated psychosis. The risk of psychotic relapse increased by approximately 6.4% with each additional day of cannabis use within a 1-week period. These results are consistent with those of Linszen et al (Reference Linszen, Dingemans and Nugter1997) who identified an association between cannabis misuse and BPRS psychotic relapse over 12 months, independent of the influence of gender, expressed emotion and age at onset of first psychotic episode. Results are also consistent with the finding of Martinez-Arevalo et al (Reference Martinez-Arevalo, Calcedo-Ordonez and Varo-Prieto1994) that cannabis use at baseline and during follow up (at least twice weekly) was the strongest predictor of DSM-III psychotic relapse, followed by non-adherence to treatment, stress and baseline cannabis use only. However, no previous study has demonstrated an association between cannabis use and psychotic relapse over a 6-month period incorporating highly sensitive and standardised measures (TFLB, BPRS) and frequent follow-up. The use of a repeated-measures design to obtain a detailed picture of symptoms, medication, stress and substance use provides the best evidence to date for the presence of a strong association between cannabis use and psychotic relapse.
The predictive effects from cannabis use in the current study - strong as they were - may however remain an underestimation of its true impact. Previous research has demonstrated that the distal effects of cannabis use over 3 or 4 years are more strongly associated with the onset of psychosis than cannabis use in the past 6-12 months (Reference van Os, Bak and Hanssenvan Os et al, 2002). Future replications of the current study should include previous cannabis use as a predictor, to see if this further increases the predictive impact.
The severity of BPRS depression-anxiety symptoms at baseline also emerged as a significant predictor of time to psychotic relapse, with each point of increase in symptom severity increasing relapse risk by 26.3%. However, this finding requires replication, as neither the severity of depression-anxiety symptoms during follow-up nor the presence of an affective-type psychosis at admission were predictors of relapse. The numbers of previous psychotic episodes or hospital admissions were also not predictive of relapse, thus providing some indication that this finding was not related to the individual's adjustment to an index episode or admission. In addition, depression-anxiety symptoms (at baseline and during follow-up) were not predictive of a relapse in cannabis use, indicating there may be a specific relationship between depression-anxiety symptoms and psychotic relapse, which requires further investigation.
A number of variables previously identified as predictors of psychotic relapse, including duration of untreated psychosis, life stress, medication adherence and the family environment, did not emerge as predictor variables in the current study. The most marked inconsistency with previous studies was in relation to life stress, as neither subjective life stress nor stressful life events was predictive of relapse. However, this was the first study to examine the influence of subjective life stress on relapse during cannabis use, since previous studies in which stressful life events were found to be associated with relapse excluded participants with substance use disorders (Reference Nuechterlein, Dawson and GitlinNuechterlein et al, 1992; Reference Pallanti, Quercioli and PazzagliPallanti et al, 1997).
Although a recent meta-analysis found evidence that duration of untreated psychosis is associated with a poorer course and outcome of first-episode psychosis (Reference Marshall, Lewis and LockwoodMarshall et al, 2005), the majority of studies did not assess concurrent substance misuse. The current findings are consistent with Linszen and colleagues' (Reference Linszen, Dingemans and Nugter1997) results using the same rigorous definition of relapse based on BPRS criteria. Furthermore, it should be noted that the relatively small size of the group of participants who were able to report on family environment made it difficult to determine whether this had a direct influence on relapse. None the less, although further research is clearly needed, as this point it would appear that when compared with the effect of cannabis use, other risk factors have less impact on the relapse process.
In order to add to the existing literature, the influence of psychotic symptom severity on relapse in cannabis use during the 6-month follow-up was also examined. There was a high rate of cannabis relapse, with 60.9% of participants increasing their use of cannabis to a level that fitted with the definition of a cannabis relapse. After controlling for medication adherence, life stress, other substance use and the age at onset of regular cannabis use, psychotic symptom severity was predictive of a cannabis relapse, with each point of increase in psychotic symptom severity in a 1-week period increasing risk of cannabis relapse by 2.5%. In contrast, each additional day of medication adherence within a 1-week period reduced risk of cannabis relapse by 1.5%. Thus it would appear that, whereas an increase in psychotic symptoms results in an increase in the number of days of cannabis use, medication adherence has a relatively small protective effect in decreasing the number of days of use. As this study is one of the first to examine the influence of psychotic symptom severity on cannabis relapse among regular cannabis users with an established psychotic disorder, replication is needed. However, the results are consistent with the reports of participants that cannabis use is one way of coping with an increase in positive psychotic symptoms (Reference Test, Wallisch and AllnessTest et al, 1989; Reference Mueser, Pallavi and TracyMueser et al, 1995).
On balance, these data indicate that the relationship between cannabis use and psychosis may be bidirectional. The high attrition rate (30.9%) in the current study should be noted, although data from all 69 (85.2%) participants whose symptoms stabilised were included in the principal Cox regression analyses. Furthermore, the only baseline difference between those who remained in the study for the full 6 months and those who dropped out was a greater likelihood of living at home. In relation to relapse, 39.1% of participants met criteria for psychotic relapse, a rate that is higher than in previous studies of recent-onset or first-episode groups (range 24-28%; Reference Nuechterlein, Dawson and GitlinNuechterlein et al, 1992; Reference Linszen, Dingemans and LeniorLinszen et al, 1994). However, these studies excluded participants with substance use disorders and/or those who did not live at home with their families, and the participants are likely to represent a less chaotic and troubled group of young people. The current sample was notable for the high rates of cannabis dependence, young age, short-duration of psychosis, almost total reliance on government benefits and lack of a stable home environment, characteristics typical of young people with a first-episode or recent-onset psychosis in Australia (Reference Lambert, Conus and LubmanLambert et al, 2005; Reference Wade, Harrigan and EdwardsWade et al, 2005).
Participants with an initial clinical diagnosis of substance-induced psychosis were included in the current study. It is possible that the role of cannabis in relapse may differ between those with and those without a substance-induced psychosis. Notably, the results of the analyses did not differ when the substance-induced psychosis group was excluded. This is not to say, however, that the influence of cannabis is identical across the two groups, as diagnostic status is often unclear in recent-onset psychosis. Further studies need to look at the stability of diagnoses over a longer time period and ascertain the impact of substance use and other relapse variables when there is greater diagnostic certainty. Finally, the reliability of self-report measures to accurately assess substance use is often questioned (Reference Cook, Bernstein and ArringtonCook et al, 1995). However, there was a high level of agreement between participants' self-reported cannabis use and urine drug screening, and there is growing evidence that self-reported cannabis use is more sensitive than collateral reports, laboratory tests (blood, urine, hair and saliva) and medical examinations across a range of populations, including first-episode patients with comorbid substance use disorders (Reference McPhillips, Kelly and BarnesMcPhillips et al, 1997; Reference Wolford, Rosenberg and DrakeWolford et al, 1999; Reference Selten, Bosman and de BoerSelten et al, 2002). None the less, future research could benefit from more frequent screening substance use with serum drug screens that allow for quantitative analysis.
This is the first prospective study to systematically explore the relationship between cannabis use and psychotic symptoms and relapse, relative to other key predictors of outcome over a 6-month period using highly sensitive measures and frequent follow-up. More frequent cannabis use was associated with a higher risk of psychotic relapse, and more severe psychotic symptoms were associated with increased risk of cannabis relapse. By indicating that the relationship between cannabis use and psychosis is bidirectional, these findings provide some support for the stress-vulnerability coping model of psychosis, and highlight the need for early intervention programmes to target both cannabis use and psychotic symptom severity in this population. In addition, common psychological (e.g. personality traits), genetic (e.g. COMT gene polymorphism) and neurobiological factors (e.g. increased density of cannabinoid receptors) may underlie the association between cannabis use and psychosis and require future exploration.
Acknowledgements
We thank Hok Pan Yuen for his advice and assistance with the statistical analyses. The study was supported by a grant from the Australian Research Council.







eLetters
No eLetters have been published for this article.