A second generation of schizophrenia and crime research – focusing upon aetiological processes predisposing to both conditions – may be advanced by examining the comorbidity between schizophrenia-spectrum personality disorders (paranoid, schizoid and schizotypal) and antisocial personality disorder, in an attempt to ascertain if people with this comorbidity differ in biological and/or behavioural ways beyond the additive effects of either condition alone. Although studies suggest that people with such comorbidity demonstrate skin conductance orienting response abnormalities (Reference Raine and RaineRaine, 2006a ) and are more antisocial than those with antisocial personality disorder alone (Reference Raine, Bihrle and VenablesRaine et al, 1999; Reference Moran and HodginsMoran & Hodgins, 2004), electrodermal arousal deficits in antisocial populations (Reference Fowles, Roy, Bouscein and FowlesFowles, 1993; Reference LorberLorber, 2004), and mixed arousal findings in samples of people with schizophrenia or schizotypal disorder (Reference ÖhmanÖhman, 1981; Reference Dawson, Nuechterlein and SchellDawson et al, 1994; Reference Raine, Venables and MednickRaine et al, 2002b ) indicate that ignoring comorbidity of schizophrenia-spectrum and antisocial personality disorders may obfuscate findings when examining each condition separately. This study sought to identify autonomic and behavioural characteristics of this comorbid group in the community, and tested the hypotheses that individuals with comorbidity of these disorders would demonstrate increased criminality and/or reduced skin conductance orienting and arousal when compared with individuals with either disorder alone and with controls.
METHOD
Participants
Adult men and women (n=101) were recruited from five temporary employment agencies in the greater Los Angeles area. Because participation in the larger study included magnetic resonance brain imaging (see Reference Raine, Lencz and BihrleRaine et al, 2000), participants were excluded if they were under 21 years or over 45 years of age; were not fluent in English; had claustrophobia; or had a pacemaker, a metal implant, or a history of epilepsy. Qualified participants were informed of the nature of the study and of its potential risks and benefits. After giving signed informed consent, participants were individually tested over 3 days at a University of Southern California research laboratory. All participants were paid. The study and all its procedures were approved by the university's institutional review board.
Diagnostic measures
Participants were administered the Structured Clinical Interviews for DSM-IV Axis I Disorders (SCID–I; Reference First, Spitzer and GibbonFirst et al, 1997b ) and Axis II Personality Disorders (SCID–II; Reference First, Gibbon and SpitzerFirst et al, 1997a ). The SCID–I and SCID–II were administered by a clinical PhD graduate student who had received systematised training in diagnostic assessment (Reference Ventura, Liberman and GreenVentura et al, 1998). The sample was classified into four groups, based upon SCID–II diagnoses and availability of self-report crime and skin conductance data (see below). The group with schizophrenia-spectrum personality disorder (SSPD) contained participants diagnosed with paranoid, schizoid and/or schizotypal paranoid personality disorders, with no comorbid antisocial personality disorder (n=9). The antisocial personality disorder (ASPD) group contained participants with an ASPD diagnosis but no comorbid SSPD (n=14). The comorbid SSPD/ASPD group contained participants who met diagnostic criteria for both SSPD and ASPD (n=8). The control group contained participants with no Axis II diagnosis (n=48). Participants with neither ASPD nor SSPD but with other Axis II psychopathology (n=22) were excluded from group assignment. Groups did not significantly differ on age, gender, ethnicity, IQ or socio-economic status (Hollingshead, 1979) (see Table 1).
Table 1 Demographic characteristics of the four diagnostic groups

| SSPD (n=9) | ASPD (n=14) | SSPD/ASPD (n=8) | Controls (n=48) | |
|---|---|---|---|---|
| Age, years: mean (s.d.) | 31.22 (5.33) | 32.36 (7.50) | 32.13 (6.08) | 30.06 (6.47) |
| Gender, n | ||||
| Male | 8 | 14 | 7 | 39 |
| Female | 1 | 0 | 1 | 9 |
| Ethnicity, n | ||||
| Asian | 0 | 0 | 0 | 2 |
| Black | 4 | 7 | 4 | 8 |
| Hispanic | 1 | 1 | 1 | 7 |
| White | 4 | 5 | 3 | 29 |
| Other | 0 | 1 | 0 | 2 |
| Full-scale IQ: mean (s.d.) | 93.56 (12.74) | 99.21 (11.23) | 94.00 (13.76) | 100.42 (15.94) |
| SES score: mean (s.d.) | 36.11 (13.43) | 35.29 (7.58) | 34.38 (11.05) | 35.32 (11.80) |
Criminal offending
Two forms of criminal offending data were collected. First, criminal records were searched and assessed for total numbers of arrests and convictions for each participant (Reference Raine, Lencz and BihrleRaine et al, 2000). Second, each participant was administered an adult extension (Reference Raine, Lencz and BihrleRaine et al, 2000) of the National Youth Survey self-report delinquency measure (Elliot et al, 1983). Self-report criminal offending was operationalised as the total number of property, violence and drug offences assessed by this 50-item instrument.
To encourage open reporting, a certificate of confidentiality was obtained from the Secretary of Health, pursuant to section 303(a) of Public Health Act 42. Participants were informed that any information they might provide about uninvestigated crimes could not be subpoenaed by any US federal, state or local court. Participants were reminded of confidentiality during administration of the measure and the limits to the confidentiality certificate.
Psychophysiological measures
Skin conductance was measured during both rest and orienting conditions.
Apparatus and recording procedures
Participants were tested in a temperature-controlled, light- and sound-attenuated psychophysiological recording laboratory. Skin conductance was recorded from the distal phalanges of the first and second fingers of both hands (to maximise responsivity; Reference Scerbo, Freedman and RaineScerbo et al, 1992) using Beckman silver–silver chloride electrodes (1 cm diameter) with sodium chloride 0.9% solution in Unibase as electrolyte, with the skin contact area delineated using double-sided adhesive masks with a hole of 1 cm diameter. Recordings were made using a Grass Model 7 polygraph (Grass Instruments, Quincy, Massachusetts, USA) with a constant 0.5 V potential across electrodes to allow direct recording of skin conductance (Reference Venables, Christie, Martin and VenablesVenables & Christie, 1980). Participants were made as comfortable as possible and asked to keep their hands completely still. They were then instructed that after a 3 min rest period they would hear a series of tones that would last about 5 min. The amount of time that elapsed from the end of electrode placement to the start of the rest period was approximately 2 min.
Stimuli
A set of ten orienting stimuli were presented with inter-stimulus intervals randomised between 25 s and 40 s. Orienting stimuli consisted of a series of six 75 dB tones of 1000 Hz frequency, 25 ms rise time and 1 s duration. These were followed by four more attentionally meaningful stimuli consisting (in order of presentation) of a reorienting stimulus (a 500 Hz tone of 75 dB intensity, 1 s duration), a consonant–vowel stimulus (‘da’, 0.35 s duration, 75 dB intensity), one 90 dB stimulus (1 s duration, 1000 Hz frequency) and one 90 dB white noise burst (1 s duration, 5 ms rise time).
Scoring
Skin conductance responses to each orienting stimulus were defined as increases in conductivity of more than 0.05 mS occurring within a latency window of 1–3 s post-stimulus. The number of non-specific skin conductance responses occurring during the 3 min rest period (using the same amplitude criterion as above) was also scored. Skin conductance levels (in mS) were recorded at the beginning and end of the rest period, and at end of the orienting procedure. Charts were scored with the rater masked to group membership. Amplitudes were subjected to square root transformation to reduce skew and kurtosis, as recommended by Venables & Christie (Reference Venables, Christie, Martin and Venables1980). Because levels for the right and left hands were strongly correlated with each other at the respective time points (correlations ranged from 0.77 to 0.83, P<0.001), the values for the two hands at each time point were averaged. If data were missing for one hand (which occurred on five occasions owing to equipment failure), then data for the available hand were used.
Statistical analyses
In cases in which serious violations of the assumptions underlying traditional statistical techniques were detected, additional modern methods were used. It is known that conventional methods for comparing means can have very poor power (e.g. Reference WilcoxWilcox, 2005). Comparing medians can reduce problems associated with methods for comparing means, but a concern about using medians is that they trim too much of the data, which again can result in relatively poor power. By trimming 20%, poor power due to outliers, skewness and variance can be reduced substantially, yet good power is still achieved under standard assumptions (Reference WilcoxWilcox, 2005). The employment of bootstrapping techniques (see Reference WilcoxWilcox, 2003) also seemed appropriate because of the small sizes of some of the groups in our study. Rather than assume normality to determine appropriate critical values, bootstrap methods estimate appropriate critical values using the available data (Reference Efron and TibshiraniEfron & Tibshirani, 1993; Reference Davison and HinkleyDavison & Hinkley, 1997).
RESULTS
The sample's demographic characteristics are summarised in Table 1.
Comorbidity
Chi-squared analysis was used to assess comorbidity between antisocial personality disorder and schizophrenia-spectrum personality disorder within the entire community sample. Results confirmed significant comorbidity between the two conditions (χ2=7.665, P=0.006). Among those diagnosed with schizophrenia-spectrum disorder (n=17), almost half (8, 47%) had a comorbid diagnosis of antisocial personality disorder compared with 17% (n=8) in those without schizophrenia-spectrum disorder (n=84) (Fig. 1). Of the total sample, 48 (47.5%) were diagnosed with no Axis II condition, 14 (13.9%) with antisocial personality disorder only, 9 (8.9%) with schizophrenia-spectrum disorder only and 8 (7.9%) with comorbid schizophrenia-spectrum/antisocial personality disorder.
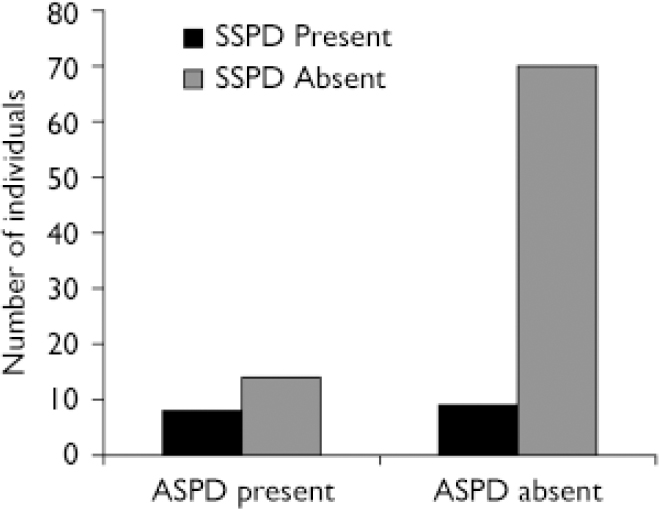
Fig. 1 Comorbidity between schizophrenia-spectrum personality disorder (SSPD) and antisocial personality disorder (ASPD).
Criminal offending
One-way analysis of variance (ANOVA) was used to assess differences in self-reported criminal offending among the four groups. Results indicated that the groups differed significantly: F(3)=15.687, P< 0.001. Post hoc (Bonferroni-corrected) tests indicated, as expected, that the ASPD group (n=13; mean=21.62, (s.d.=12.45) reported significantly more criminal offending than controls (n=46; mean=11.34, s.d.=5.61, P=0.004). Furthermore, the SSPD/ASPD group (n=8; mean=33.75, s.d.=17.37) reported significantly more criminal offending than the ASPD group (P=0.026), the SSPD group (n=8; mean=14.25, s.d.=9.00, P<0.001) and the control group (P<0.001). A boxplot of the self-report crime data indicated only minimal outliers and skewness. Consequently, additional modern bootstrapping methods involving trimmed means were not applied to augment this specific analysis.
Additional ANOVAs were used to assess differences in both the total number of charges and convictions among the four groups. Results indicated that the criminal records of the SSPD/ASPD group contained more charges and convictions than those of the other groups (Table 2); however, the groups did not differ significantly on the total number of either charges (F(3)=1.183, P=0.322), or convictions (F(3)=1.181, P=0.323). Boxplots of these data indicated significant outliers in some cases, thus the aforementioned modern methods were employed to augment conventional analyses. A percentile bootstrap method for 20% trimmed means (Reference WilcoxWilcox, 2005) indicated that the criminal records of both the ASPD group and the SSPD/ASPD group contained significantly more charges than the SSPD group and the controls (P=0.0005 and P<0.0001 respectively for the ASPD group; P=0.006 and P=0.003 respectively for the SSPD/ASPD group). Although the SSPD/ASPD group had 97% more charges than the ASPD group, this difference was not statistically significant.
Table 2 Criminal offending for the four diagnostic groups

| SSPD Mean (s.d.) | ASPD Mean (s.d.) | SSPD/ASPD Mean (s.d.) | Controls Mean (s.d.) | |
|---|---|---|---|---|
| Self-reported crime | 14.25 (9.00) | 21.62 (12.45)* | 33.75 (17.38)*† | 11.35 (5.61) |
| Charges | 0.44 (0.88) | 4.57 (4.00)* | 9.00 (8.82)* | 3.44 (11.54) |
| Convictions | 0.11 (0.33) | 1.64 (1.74)* | 3.25 (3.33)* | 1.25 (4.17) |
An additional percentile bootstrap method for 20% trimmed means indicated that the criminal records of both the ASPD group and the SSPD/ASPD group contained significantly more convictions than the SSPD group and the controls (P=0.0005 and P=0.001 respectively for the ASPD group; P=0.006 and P=0.007 respectively for the SSPD/ASPD group). Although the SSPD/ASPD group showed a 98% increase in convictions compared with the ASPD group, given the modest sample size this large difference was not statistically significant.
Psychophysiology
Skin conductance arousal
A conventional repeated-measures multivariate analysis of variance (MANOVA) was used to assess group differences in skin conductance level during testing (i.e. at the beginning and end of the initial 3 min rest period and at the end of the orienting phase). Results indicated that the groups differed significantly on the three skin conductance level readings; F(3)=3.224, P=0.027. Post hoc tests, however, indicated that comparisons were largely nonsignificant after Bonferroni correction, and that only the SSPD/ASPD group demonstrated a trend toward significantly lower skin conductance arousal when compared with the control group (P<0.069). Boxplots of these data indicated relatively few outliers but significant skewness in some cases. A repeated-measures bootstrap analysis confirmed that the SSPD/ASPD group demonstrated significantly lower skin conductance level than controls (P<0.001) and that all other group differences remained non-significant.
Skin conductance responsivity
Frequency. An ANOVA was used to assess the difference in total number of skin conductance responses during the entire orienting phase of the procedure. Additional ANOVAs were used to assess differences in the total number of responses during the first six orienting stimuli only, and then during the four meaningful stimuli only. Boxplots of these data indicated relatively few outliers but significant skewness in some cases. Consequently, modern methods were employed to augment conventional analyses.
Results indicated that the groups differed significantly in number of skin conductance orienting responses during the entire orienting phase: F(3)=4.104, P=0.009. Post hoc tests (Bonferroni-corrected) indicated that only the SSPD/ASPD group (n=8; mean=0.500, s.d.=0.598) demonstrated significantly fewer responses over the entire orienting phase than the control group (n=48; mean=2.583, s.d.=2.025, P= 0.024). A bootstrap test of linear contrasts using 20% trimmed means, however, indicated that both the ASPD and the SSPD/ASPD groups demonstrated significantly fewer responses over the entire orienting phase than controls (P=0.027 and P=0.002 respectively). Conventional ANOVA results also indicated that the groups did not differ significantly in number of responses during the first six orienting stimuli, although the same bootstrap linear contrast method revealed that the ASPD group demonstrated significantly fewer responses than did controls (P=0.045) during these stimuli. In addition, conventional results indicated that the groups differed significantly in number of responses during the four meaningful stimuli: F(3)=3.442, P=0.021. Post hoc tests (Bonferroni-corrected) indicated that only the SSPD/ASPD group (n=8; mean=0.375, s.d.= 0.582) demonstrated significantly fewer responses over the four meaningful stimuli than the control group (n=48; mean=1.990, s.d.=1.435, P=0.018). The bootstrap linear contrast method yielded identical results (P<0.0001).
Amplitude. A repeated-measures MANOVA was used to assess group differences in skin conductance amplitude across the entire orienting phase (group means are displayed in Fig. 2). Results indicated only a trend towards significance: F(3)=2.033, P=0.117). Additional repeated-measures MANOVAs indicated that group differences were nonsignificant for the first six orienting stimuli (F(3)=1.875, P=0.141) and the four meaningful stimuli (F(3)=1.915, P=0.135).
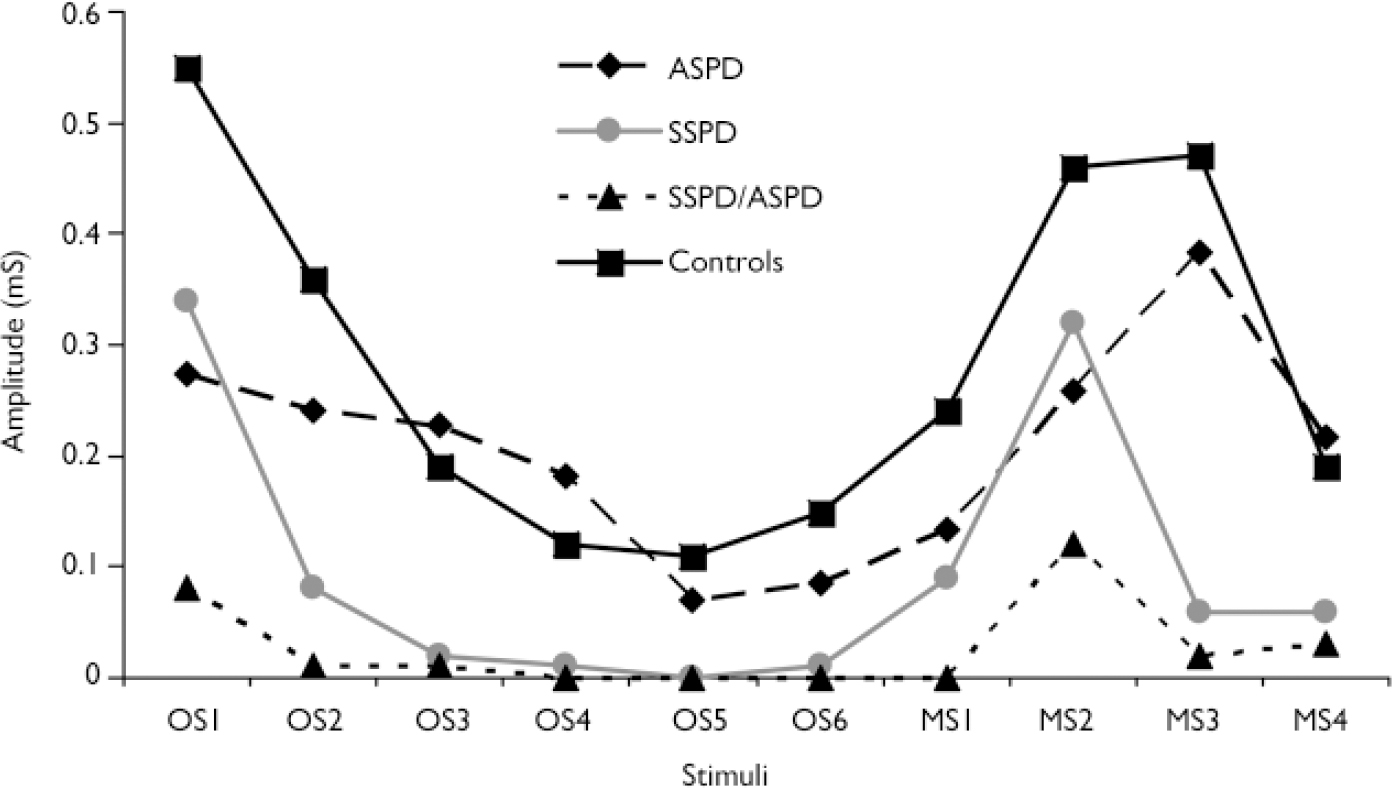
Fig. 2 Skin conductance amplitude: diagnostic group means by orienting paradigm stimuli (ASPD, antisocial personality disorder; MS, meaningful stimulus; OS, orienting stimulus; SSPD, schizophrenia-spectrum personality disorder).
A boxplot of the skin conductance amplitude data indicated significant skewness and numerous outliers (Fig. 3), which again made appropriate the application of modern statistical methods. A repeated-measures bootstrap method using 20% trimmed means (Reference WilcoxWilcox, 2005) was used to assess differences in skin conductance amplitude over the entire orienting phase. Results indicated that the SSPD/ASPD group demonstrated significantly lower amplitude than the control group (P=0.0004). This same repeated-measures bootstrap method was subsequently used to assess differences in skin conductance amplitude over the first six orienting stimuli and over the four meaningful stimuli. Results indicated that the the SSPD group demonstrated significantly lower amplitude over the first six orienting stimuli than controls (P<0.0001), and that the SSPD/ASPD group demonstrated significantly lower amplitude over these stimuli than the ASPD group (P=0.0004), the SSPD group (P<0.0001) and controls (P<0.0001). In addition, results indicated that the SSPD/ASPD group demonstrated significantly lower amplitude over the four meaningful stimuli than controls (P=0.002).

Fig. 3 Boxplot of skin conductance amplitude data, indicating skewness and outliers. Rectangular boxes represent distribution of data for each stimulus presentation (arranged side by side in ascending chronological order 1–10, from left to right), for each diagnostic group. Medians are represented by white bands within the boxes. Upper and lower quartiles (i.e. where the middle half of the data lie) are represented by the ends of the boxes. Adjacent values (i.e. smallest and largest values, not declared outliers) are indicated by the whiskers (![]() ). Outliers are represented by the single dashed lines (see Reference WilcoxWilcox, 2003). Distributions centred at 0.0 mS, with relatively minimal or no variability, appear as the symbol H. ASPD, antisocial personality disorder; SSPD, schizophrenia-spectrum personality disorder.
). Outliers are represented by the single dashed lines (see Reference WilcoxWilcox, 2003). Distributions centred at 0.0 mS, with relatively minimal or no variability, appear as the symbol H. ASPD, antisocial personality disorder; SSPD, schizophrenia-spectrum personality disorder.
Potential psychiatric confounds
Additional chi-squared analyses were used to assess group differences in comorbid Axis I disorders. Results indicated that groups did not differ significantly regarding the presence of comorbid mood, psychotic or anxiety disorders. Although there was significantly more substance use disorder (i.e. misuse and dependence) among the ASPD and SSPD/ASPD groups (χ=19.091, P>0.001), further chi-squared analysis revealed that these two groups did not differ significantly in rates of these disorders (χ2=1.257, P=0.262). Consequently, the significantly increased antisocial behaviour and skin conductance orienting to meaningful stimuli found in the comorbid group compared with the ASPD group could not be accounted for by a difference between these groups in substance use disorders.
DISCUSSION
This study set out to assess whether there was significant comorbidity between schizophrenia-spectrum personality disorders (a group of distinct yet related disorders) and antisocial personality disorder, and if so to determine whether the comorbid group differed in biological (autonomic) and socially meaningful (criminality) ways from individuals with each condition separately. Results indicated not only significant comorbidity but also that the comorbid group was characterised by reduced skin conductance orienting and arousal and more criminal offending. Specifically, the comorbid group demonstrated significantly lower skin conductance orienting response amplitudes to neutral orienting stimuli and reported significantly more criminal behaviour than all of the other groups. Results have potential implications for identifying a distinct subgroup of individuals with schizophrenia-spectrum and antisocial personality disorders, for improving understanding of the reasons for the comorbidity between antisocial behaviour and schizophrenia, and also for the differential treatment, care and management of these individuals in therapeutic and forensic settings.
Findings suggest that ignoring the comorbid link between schizophrenic-spectrum and antisocial personality disorders may obfuscate findings in investigations of either condition separately. Studies of antisocial populations have produced inconsistent results on electrodermal responding, findings that may be clarified when schizophrenia-spectrum disorders are considered as a moderator. For example, it has been observed that although autonomic underresponsivity does not characterise antisociality in general, a specific subgroup of people with schizoid antisocial personality disorder are characterised by autonomic non-response (Reference Raine and VenablesRaine & Venables, 1984). Subsequent research has indicated the same pattern of reduced skin conductance orienting in schoolboys with schizotypal–antisocial symptoms and in adults with schizoid psychopathy (Reference Raine, Bihrle and VenablesRaine et al, 1999). Conversely, disparate findings of both reduced and increased orienting in people with schizotypal disorder may reflect the need to consider antisociality as a moderating variable, where autonomic underresponding may specifically characterise schizotypal disorder with antisocial tendencies (Reference Raine, Bihrle and VenablesRaine et al, 1999). One implication of the current study is that future studies could significantly benefit by assessing both antisocial behaviour and SSPD within the same population in order to elucidate risk factors specific to each of these conditions.
The finding of reduced orienting particularly in the group comorbid for schizophrenia-spectrum and antisocial personality disorder can be viewed within a neuroanatomical context predicated on the frontal cortex. Specifically, skin conductance orienting response is thought to be a marker for structural and functional integrity of the prefrontal cortex, and impaired prefrontal structure and function have been associated with both antisocial behaviour and schizotypal personality. Structural magnetic resonance imaging (MRI) and neurological studies on humans have demonstrated that reductions in the integrity of the prefrontal cortex (i.e. lesions, or reduced area or volume) are associated with reduced skin conductance responsivity (Reference CritchleyCritchley, 2002). Functional MRI studies have shown that skin conductance responses during the Iowa gambling task are associated with activation of the ventromedial and orbitofrontal cortex (Reference Critchley, Elliott and MathiasCritchley et al, 2000). Visual orienting is associated with increased activation in the anterior cingulate, whereas reorienting is associated with increased activation in the middle frontal gyrus (Reference Thiel, Zilles and FinkThiel et al, 2004). Furthermore, stimuli that elicit a skin conductance response, compared with stimuli that do not, result in activation in the hippocampus, anterior cingulate and ventromedial prefrontal cortex (Reference Williams, Brammer and SkerrettWilliams et al, 2000). Although the circuitry underlying the skin conductance response is complex and involves multiple regions, including the right inferior parietal cortex and amygdala, the convergence of findings from structural and functional imaging studies identifies the frontal cortex as a key higher brain area primarily associated with this response.
This linkage of frontal structure and function with the skin conductance response, and the finding in our study that reduced skin conductance orienting response is particularly associated with comorbidity of schizophrenia-spectrum and antisocial personality disorder, suggest the hypothesis that impaired frontal structure and function would be observed in both conditions. There is increasing evidence from both neurocognitive and neuroimaging studies to support this hypothesis. There is growing neuropsychological evidence that juvenile delinquency, antisocial behaviour, criminality and criminal psychopathy are associated with poorer performance on tasks related to both orbitofrontal and ventromedial functioning (Reference Lapierre, Braun and HodginsLapierre et al, 1995; Reference Morgan and LilienfeldMorgan & Lilienfeld, 2000; Reference Brower and PriceBrower & Price, 2001; Reference Yechiam, Kanz and BecharaYechiam et al, 2007). Similarly, poor frontal functioning has been identified as one of the best-replicated neurocognitive correlates of schizotypal personality (Reference RaineRaine, 2006b ). Both structural and functional imaging studies have observed prefrontal impairments in antisocial populations (Reference Raine, Stoff, Breiling and MaserRaine, 1997; Reference Raine, Lencz and BihrleRaine et al, 2000) and also in schizotypal populations (Reference Raine, Lencz and YaralianRaine et al 2002a ), although there is stronger evidence for structural impairment in schizophrenia than in schizotypal disorder. Consequently, reduced skin conductance orienting response may particularly characterise the comorbid group because it is a peripheral marker for prefrontal impairment, which in turn represents a common risk factor for both personality disorders. It is proposed that reduced orienting may represent an attentional marker of prefrontal impairment predisposing to both antisocial and schizotypal personality disorders. Taking into account the comorbid relationship between antisocial and schizotypal personality disorders in future studies could help clarify the heterogeneity in findings for both these disorders.
Findings from our study underscore the importance of modern statistical methods such as trimmed means and bootstrapping (Reference WilcoxWilcox, 2003), and demonstrate how these techniques may be used to augment conventional analyses when the assumptions of these traditional statistics are violated. In addition, our study illustrates how important group differences may be elucidated using alternative methods when conventional methods fail, and how the nature of data distributions should be considered when choosing an appropriate statistical strategy. Although it is well recognised in the literature that type 1 errors are problematic, it is less well recognised but of importance in the early stages of an enquiry (in this case, understanding comorbidity) that type 2 errors can lead to equally misleading conclusions.
One discrepancy in findings is that although significant results were observed for self-report criminal offending, findings for official crime measures (charges and convictions) were non-significant when comparing the comorbid and ASPD only groups. This may reflect a true result, and may be explained by the fact that official records of charges and convictions are not comprehensive and do not reflect criminal offences that are undetected. Law enforcement clearance rates clearly indicate that the substantial majority of criminal activity goes unsolved (Reference SeigelSeigel, 2006). An alternative interpretation is that there are genuine differences between the comorbid and ASPD only groups, but that the study lacked power to detect these differences. That this explanation should not be ruled out is suggested by the fact that the comorbid group showed a 96.9% increase in criminal charges and a 98.2% increase in convictions compared with the ASPD only group, differences that exceed the (statistically significant) 56% increase in self-report crime. If correct, this in turn provides an example of how – in at least some circumstances – modern statistical techniques such as trimmed means (Reference WilcoxWilcox, 2005) cannot entirely compensate for the lack of power in traditional statistics, although the possibility of genuine null results cannot be discounted.
Limitations
A limitation of our study may be the relatively small number of stimuli used in the orienting test. Results from statistical analyses of only four attentionally meaningful stimuli should be interpreted with caution. Clearly, these results need to be replicated, though they do give provisional findings for future research. Subsequent studies should incorporate more stimuli, perhaps expanding upon the use of even more socially meaningful stimuli such as positively and negatively charged affective pictures.
One additional consideration to be made is that the results observed in this study may merely be attributable to the additive effects of both disorders. Although this is a distinct possibility, the data from our study may provide some evidence to the contrary. For example, rates of criminal offending in the SSPD group were not significantly different from those of the control group (in fact, the former had fewer charges and convictions than controls); intuitively the addition of SSPD criminal offending to that of the ASPD group should consequently reduce – not increase – levels of criminal offending. Such results may speak of a behaviourally (and possibly biologically) distinct subgroup whose dysfunction is more than the additive product of the two other groups.
Additional research in the area of comorbidity of schizophrenia-spectrum/antisocial personality disorder is clearly warranted. Being the first study of its kind, this study's findings require replication. More efforts are needed to validate the existence of the comorbidity, especially in community samples. Future studies should incorporate the use of corroborating information (collateral interviews, official crime records) to enhance the quality of self-report data.
Implications
Findings may have several implications at basic research, clinical intervention and forensic levels. At a research level, although researchers have made significant advances in the understanding of both schizophrenia-spectrum and antisocial personality disorders separately, conceptualising those comorbid for the two as a distinct group might clarify and strengthen findings for future investigations not only of this group, but also of ‘pure’ samples. The importance of this type of clarification and the exploration of an aetiologically divergent subgroup with both disorders has been underscored in the literature (Reference Fowles, Roy, Bouscein and FowlesFowles, 1993). At a clinical treatment level, outcomes of psychopharmacological treatment programmes may vary markedly between comorbid and ‘pure’ cases; techniques that demonstrate effectiveness in both disorders separately (see Reference HiroseHirose, 2001; Reference Lösel, Raine and SanmartínLösel, 2001; Reference Walker, Thomas and AllenWalker et al, 2003; Reference Bilder, Snyder, Nussbaum and RobinsBilder, 2006) may be less effective in individuals with comorbidity; ultimately, alternative treatment strategies may need to be developed for this comorbid group. At a forensic level, comorbidity may have practical implications in the evaluation of dangerousness and potential for re-offending. If a particular diagnostic entity has been shown to demonstrate increased rates of criminality, an offender with such a symptom presentation might require special sentencing requirements to both ensure public safety and facilitate more effective rehabilitation.
In conclusion, results of this initial study indicate that those with comorbid schizophrenia-spectrum and antisocial personality disorders differ in both behavioural and psychophysiological ways from those with either condition separately, beyond the additive effects of both conditions in combination. The finding of reduced electrodermal orienting in the comorbid group confirms and extends findings of three prior studies observing this same effect, and may reflect an attentional resource allocation deficit linked to the prefrontal cortex which is common to both clinical groups. Further research on this comorbid condition is warranted, particularly because it presents with significantly higher rates of increased criminal activity.
Acknowledgements
This study was supported by Independent Scientist Award K02 MH01114-01, and grant 5 R03 MH50940-02 from the National Institute of Mental Health and a grant from the Wacker Foundation to A.R. We thank Jennifer Bobier, Nicole Diamond, Kevin Ho, Blane Horvath, Sharon Ishikawa, Lori LaCasse, Todd Lencz, Shari Mills, Kristen Taylor and Pauline Yaralian for assistance in data collection and scoring.








eLetters
No eLetters have been published for this article.