Mental health disorders have been found to be three to five times more prevalent in children and adolescents with intellectual disability compared with their typically developing peers.Reference Allerton, Welch and Emerson1,Reference Einfeld, Ellis and Emerson2 Consistent with this general mental health inequity, adolescents with intellectual disability, Autism Spectrum Disorder (hereafter referred to as autism) or both conditions are at higher risk for depression than their peers of a similar age.Reference Allerton, Welch and Emerson1,Reference Maïano, Coutu, Tracey, Bouchard, Lepage and Morin3–Reference DeFilippis6 Specifically, children and young people with intellectual disability are 1.7 times more likely to experience depression compared with other children.Reference Emerson and Hatton5 Although young people with autism have demonstrated higher rates of depression than typically developing children and adolescents, reported rates vary considerably.Reference DeFilippis6
Treatment for mental health problems in people with intellectual disability has historically relied on pharmacological approaches.Reference Vereenooghe and Langdon7,Reference McCabe, McGillivray and Newton8 However, international guidelines and recommendations suggest that first-line treatments for depression in children and young people should include psychological therapies.9,10 There is some support for the use of cognitive–behavioural therapy (CBT) for depression in adults with mild-to-moderate intellectual disability,Reference Vereenooghe and Langdon7 but a lack of research for children and adolescents. Research evidence for the psychological treatment of depression in children and adolescents with autism is available but limited, with the evidence focused on the treatment of anxiety or disruptive behaviours.Reference Danial and Wood11,Reference Lang, Regester, Lauderdale, Ashbaugh and Haring12 The current study, therefore, had three review questions. First, what is the current evidence base for psychological interventions for depression in children and young people with an intellectual disability and/or autism? Second, what are the experiences of children and young people with intellectual disability and/or autism and their family members of psychological intervention for depression? And finally, what are the experiences of therapists delivering psychological intervention for depression to children and young people with intellectual disability and/or autism?
Method
The review protocol was prospectively registered with the International Prospective Register of Systematic Reviews (registration number CRD42019145495; https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42019145495). The review was conducted and is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.Reference Moher, Liberati, Tetzlaff and Altman13
Search strategy
PsycINFO, Medline, EMBASE via Elsevier, CINAHLPlus, Social Sciences Index and Sciences Index via Web of Science, and Scopus databases were searched by two authors (L.A.C and K.P.) on 1 May 2020 for articles published from inception to 30 April 2020. Searches were conducted with keywords identified for each domain (see Table 1 for an example search string) and limited to articles written in English. Hand searching of reference lists and citation searching of included studies were also conducted to identify potential additional articles.
Table 1 Search terms

Inclusion/exclusion criteria
Studies were included if they met the following criteria.
Study design
Pre–post single-group designs, case series, clinical case reports, single-case experimental designs and qualitative studies. Studies with a comparison group, control group or no control group were all included. Observational and case-control studies involving no treatment were excluded.
Participants
Children, adolescents or young people up to 21 years of age, with an intellectual disability (including borderline intellectual disability) and/or autism. Studies were included if the entire sample included the relevant population, or if outcomes were reported separately for the relevant population. Studies that involved young people up to the age of 25 years were included if 75% of the sample was under 21 years of age.
Participant diagnosis
Either (a) major depressive disorder as diagnosed by standardised criteria (e.g. the DSM, ICD, Diagnostic Manual-Intellectual Disability); (b) dysthymia or minor depression as diagnosed by standardised criteria; or (c) depressive status, as defined by meeting cut-offs on a standardised depression screening questionnaire (e.g., Children's Depression Inventory (CDI),Reference Kovacs14 Beck Depression Inventory (BDI-II),Reference Beck, Steer and Brown15 Glasgow Depression ScaleReference Cuthill, Espie and Cooper16).
Treatment or intervention
Any psychological or psychosocial intervention (e.g. life skills training, lifestyle intervention) with the aim of treating depression or depressive symptoms. Pharmacological or medical treatments, transcranial magnetic stimulation, complementary and alternative therapies and treatments were excluded.
Screening
First-stage title and abstract screening was undertaken by two authors (L.A.C. and K.P.) to identify any articles that were clearly not relevant to the review, with a randomly selected 20% screened by both authors to determine interrater reliability. Agreement was 99.1% (κ=0.940). Full-text review was independently undertaken by the same two authors, with any conflicts of inclusion/exclusion resolved through discussion, and consultation with additional authors (K.M.G. and G.A.M.) where necessary (n = 1).
Data extraction
Data were extracted from each article and coded for (a) study information (type of study, country of publication, year of publication), (b) participant information (age, gender, intellectual disability/autism diagnosis), (c) assessment of depression, (d) treatment information (type of treatment/intervention, length of treatment, length of follow-up) and (e) study outcomes.
Risk of bias
Risk of bias assessments were conducted by a panel of four authors (L.A.C., K.P., G.A.M. and K.M.G.). Assessments of controlled and uncontrolled trials were conducted using predeveloped proformas based on the Cochrane risk of bias tool and the Newcastle–Ottawa Scale.17,Reference Wells, Shea, O'Connell, Peterson, Welch and Losos18 These proformas have been used previously in large, international systematic reviews such as that by Blackmore et al.Reference Blackmore, Gray, Boyle, Fazel, Ranasinha and Fitzgerald19 Single case studies and case series were appraised with the criteria described by Horner et al.Reference Horner, Carr, Halle, McGee, Odom and Wolery20 Each study was assessed overall as being of low, moderate or high risk of bias.
Results
A total of 13 936 records were retrieved in the search. After removal of duplicates, 9343 records remained for abstract and title screening. A further 9231 records were excluded, leaving 114 for full-text review. No additional articles were identified through reference lists of included studies or forward citation searching. A total of 10 studies met inclusion criteria (Fig. 1).

Fig. 1 Study flow diagram.
Summary data for all included studies, including sample description, assessment of depression, description of treatment and treatment duration, outcome measures and study results related to the effect of treatment on depression or depressive symptoms, can be viewed in Tables 2 and 3.
Table 2 Clinical case reports
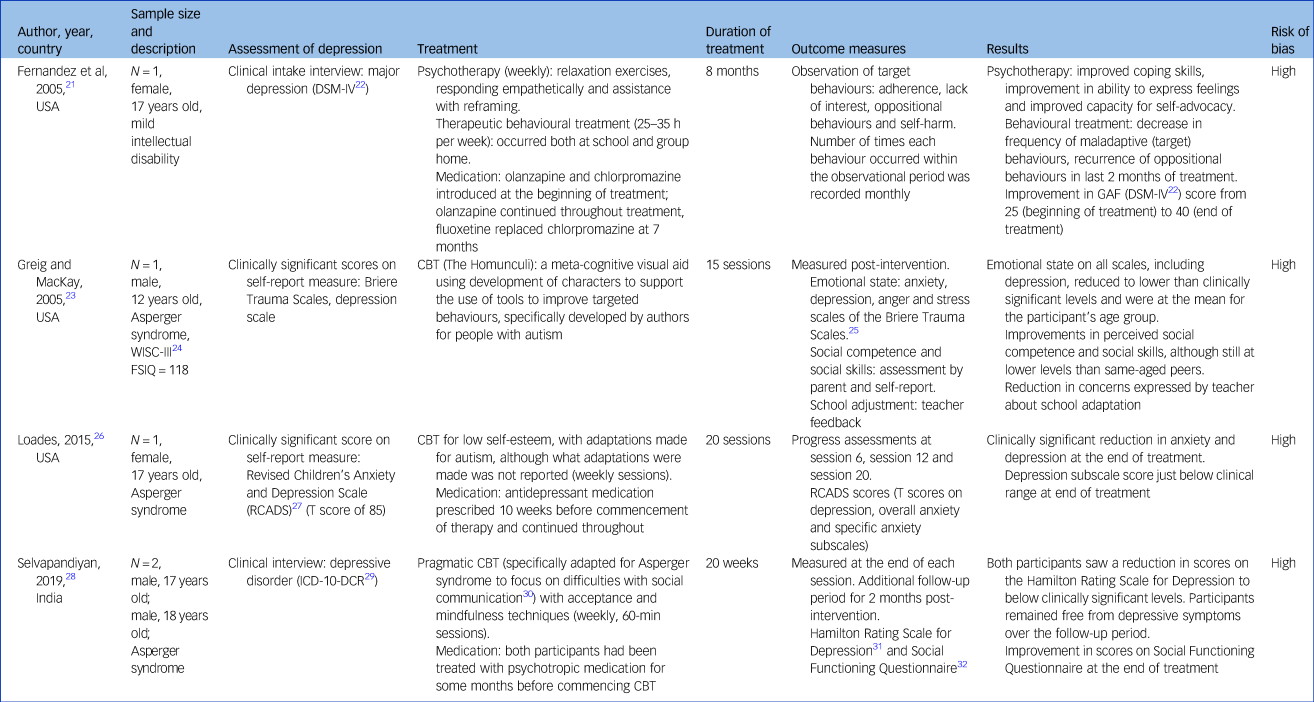
GAF, Global Assessment of Functioning; WISC, Wechsler Intelligence Scale for Children; FSIQ, Full Scale Intelligence Quotient; CBT, cognitive–behavioural therapy.
Table 3 Experimental and quasi-experimental designs
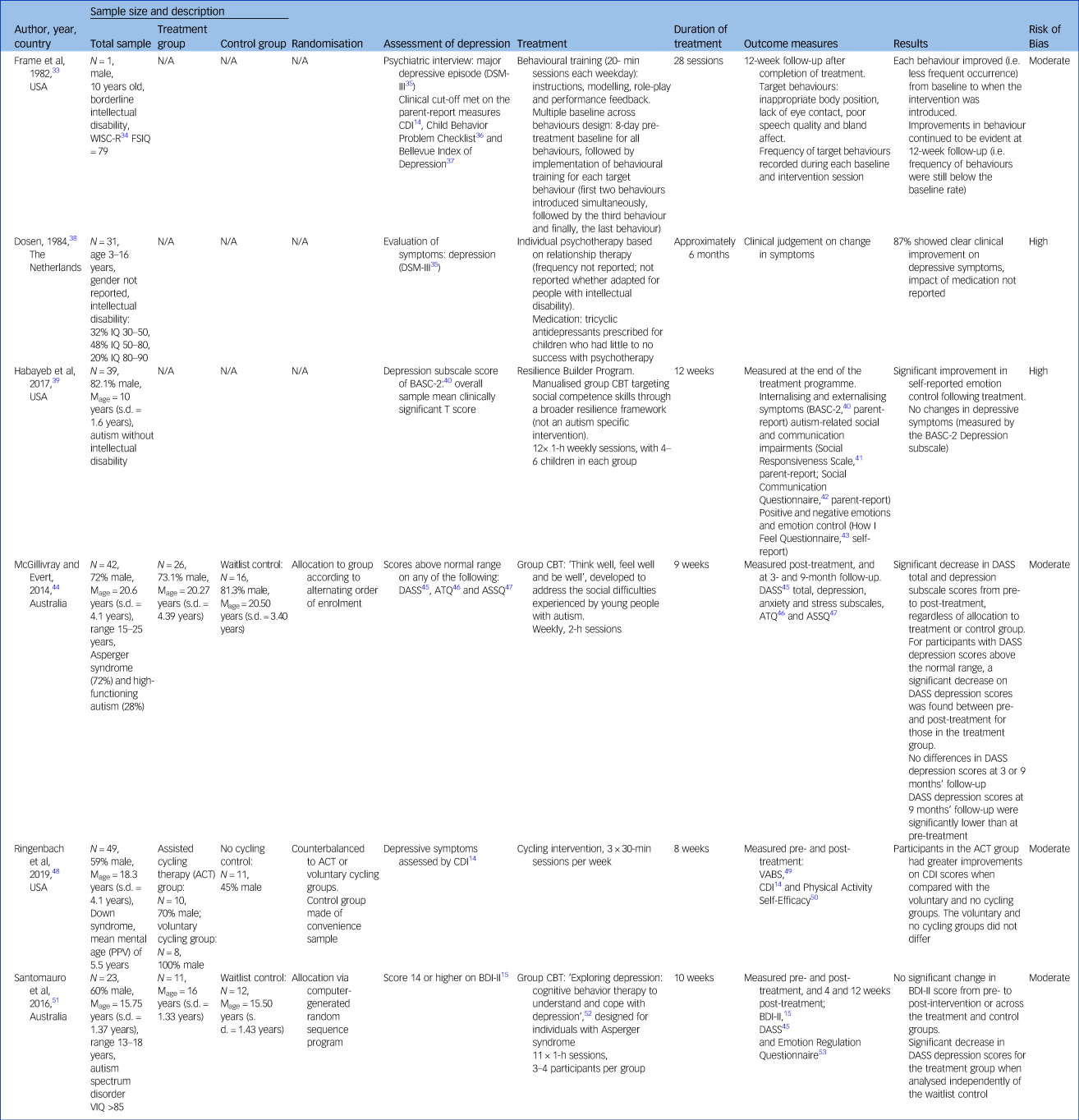
WISC, Wechsler Intelligence Scale for Children; FSIQ, Full Scale Intelligence Quotient; CDI, Children's Depression Inventory; Mage, mean age; BASC-2, Behavior Assessment System for Children, 2nd edition; CBT, cognitive–behavioural therapy; DASS, Depression, Anxiety and Stress Scales; ATQ, Automatic Thoughts Questionnaire; ASSQ, Anxious Self-Statements Questionnaire; PPV, Peabody Picture Vocabulary; VABS, Vineland Adaptive Behavior Scales; VIQ, verbal IQ; BDI-II, Beck Depression Inventory.
Research question 1: what is the evidence base for psychological interventions for depression for children and young people with intellectual disability and/or autism?
Of the ten included studies, four were clinical case reports,Reference Fernandez, Tom, Stadler, Cain and Knudsen21,Reference Greig and MacKay23,Reference Loades26,Reference Selvapandiyan28 and the remaining six were experimental or quasi-experimental designs, including one multiple baseline design study,Reference Frame, Matson, Sonis, Fialkov and Kazdin33 two uncontrolled group design trialsReference Dosen38,Reference Habayeb, Rich and Alvord39 and three controlled group design trials.Reference McGillivray and Evert44,Reference Ringenbach, Holzapfel, Arnold, Nam, Lopez and Chen48,Reference Santomauro, Sheffield and Sofronoff51
Clinical case reports
Across the four clinical case reports, five participants were described (n = 3 male, ages ranging from 12 to 18 years). One clinical case report used a combination of psychotherapy and behavioural training to target depressive symptoms in a 17-year-old female with a mild intellectual disability.Reference Fernandez, Tom, Stadler, Cain and Knudsen21 Three clinical case reports used adapted versions of CBT with a 12-year-old male,Reference Greig and MacKay23 a 17-year-old female,Reference Loades26 and two males aged 17 and 18 years old,Reference Selvapandiyan28 all with Asperger syndrome.
Three clinical case reports utilising CBT adapted their programme for young people with autism. Greig and MacKayReference Greig and MacKay23 highlight that their CBT programme, The Homunculi, involves the creation of characters to help the individual to visualise various processes and behaviours. LoadesReference Loades26 reported that the CBT programme was adapted for their participant with Asperger syndrome, but did not describe what these adaptations involved. SelvapandiyanReference Selvapandiyan28 implemented pragmatic CBT,Reference Gaus30 described by the author as being designed to target the social communication difficulties evident in Asperger syndrome. The duration of treatment varied across the clinical case reports, ranging from 15 individual sessions to 8 months. Behavioural training was more intensive, with sessions being undertaken almost daily, whereas CBT was undertaken weekly. Behavioural training in these instances involved behavioural modification of identified behaviours, including adherence, lack of interest, oppositional behaviours and self-harm.Reference Fernandez, Tom, Stadler, Cain and Knudsen21
Three clinical case reports relied on questionnaires to assess change in depressive symptoms over the course of treatment.Reference Greig and MacKay23,Reference Loades26,Reference Selvapandiyan28 One clinical case report used the depression scale of the Briere Trauma Scales (self-report)Reference Briere25 pre- and post-treatment.Reference Greig and MacKay23 Another clinical case report used the Revised Children's Anxiety and Depression Scales (self-report)Reference Chorpita, Moffitt and Gray27 at pre-treatment and throughout the course of treatment at sessions at 6, 12 and 20 weeks.Reference Loades26 The remaining clinical case report used the Hamilton Rating Scale for Depression (HRSD),Reference Hamilton31 rated by the clinician at the end of each treatment session. The study reported that progress was monitored over 2 months post-treatment; however, the HRSD scores at follow-up were not reported.Reference Selvapandiyan28
All clinical case reports reported an improvement in depressive symptoms following the intervention, whether that was a decrease in problem behavioursReference Fernandez, Tom, Stadler, Cain and Knudsen21 or a reduction in depressive scores on screening measures.Reference Greig and MacKay23,Reference Loades26,Reference Selvapandiyan28 Maintenance of improvements were reported for the one clinical case report that included longer term follow-up.Reference Selvapandiyan28 Two of the clinical case reports reported the use of medication throughout the study period; one introduced medication (olanzapine and chlorpromazine) at the beginning of the treatment period,Reference Fernandez, Tom, Stadler, Cain and Knudsen21 and the other reported that the client had been prescribed medication 10 weeks before the commencement of the CBT intervention, with a stable dose maintained throughout the intervention programme.Reference Loades26 Neither study considered the potential effects of the medication when reporting treatment outcomes.
Experimental and quasi-experimental designs
Multiple baseline design
One study employed an experimental multiple baseline across behaviours design with a 10-year-old male with borderline intellectual disability (Wechsler Intelligence Scale for Children-Revised IQ = 79).Reference Frame, Matson, Sonis, Fialkov and Kazdin33 The intervention took place on an in-patient unit, using behavioural treatment to target behaviours deemed to be reflective of depression in the participant. This included ‘inappropriate body position’, lack of eye contact, poor speech quality and bland affect. Behavioural intervention involved specific skill training incorporating instruction, modelling, role-playing and feedback of a more appropriate response in place of the inappropriate behaviour, over 20-min sessions each day. Baseline was established over 8 days for all behaviours, followed by introduction of the behavioural treatment for the first two behaviours simultaneously (six sessions), the third behaviour (five sessions) and the final behaviour (nine sessions). Frequency of target behaviours was recorded during each session, with reductions in each target behaviour observed following the introduction of the intervention, and maintenance of improvements at 12-week follow-up. Although the study involved administration of a number of depression screening tools during their initial diagnostic process, these were not used to assess post-treatment outcomes.
Uncontrolled trials
Two studies were uncontrolled trials. One study was a case series with a pre–post design, reporting on existing patients seen in an in-patient setting.Reference Dosen38 This study identified patients (N = 31) with an intellectual disability aged 3–16 years, diagnosed with depression according to the DSM-III.35 Gender was not reported. Treatment was described as the standard treatment used in the clinical setting. Individual psychotherapy was the primary treatment utilised, although frequency and duration of therapy were not specified. An unspecified number of participants were prescribed medication when it was clear the psychotherapy alone was not effective. The timing of the introduction of medication was not reported, and the impact was not considered in the reporting of outcomes. Outcomes were reported after approximately 6 months of treatment, before discharge from the facility. A total of 87% of patients (n = 27) were considered to have shown a clear clinical improvement by the time of discharge. However, a definition of clinical improvement was not reported, nor was the effect of medication.
The second pre–post design was an uncontrolled trial involving participants with autism and no co-occurring intellectual disability (n = 39, 82% male, mean age 10 years) to receive a structured intervention.Reference Habayeb, Rich and Alvord39 Participants in this study undertook a 12-week group CBT programme targeting social competence skills. Depressive symptoms were measured at the beginning and at the end of the treatment programme, using the depression subscale score of the Behavior Assessment System for Children,Reference Reynolds and Kamphaus40 a parent-report measure. No change was seen in depressive symptoms from the beginning to end of treatment, although improvements were seen in aggression, emotion control and autism symptoms.
Controlled trials
Three studies were controlled trials. One trial evaluated the effect of a lifestyle intervention, a physical exercise programme, specifically assisted cycling therapy (ACT), in a group of young people with Down syndrome (n = 49, 59% male, mean age 18.3 years, s.d. 4.1 years).Reference Ringenbach, Holzapfel, Arnold, Nam, Lopez and Chen48 ACT involved the use of a mechanical motor attached to a stationary exercise bicycle to increase the individual's cycling rate above their preferred voluntary cycling rate. Participants were counterbalanced to one of two active treatment groups, ACT or voluntary cycling, although how the counterbalancing was achieved was not described, nor was it clear whether participants were randomised to each group. A no-cycling control group was recruited through convenience sampling, by advertising in local communities. There were no group differences at baseline in terms of gender, chronological age, receptive language ability, hours of sport per week or body mass index. However, the ACT group scored significantly higher on a measure of cognitive planning compared to both the voluntary and no cycling groups. Depression symptoms were measured both pre- and post-treatment, using the CDI,Reference Kovacs14 with greater improvements on CDI scores seen in the ACT group when compared with both the voluntary and no cycling groups at the end of the 8-week therapy.
The second controlled trial reported on the effect of a group CBT programme for young people with Asperger syndrome or autism without intellectual disability (n = 42, 72% male, mean age 20.6 years, s.d. 4.1).Reference McGillivray and Evert44 Participants were allocated to either treatment or waitlist control, according to alternating order of study enrolment (i.e., pseudo-randomisations). The 9-week CBT programme was developed with particular regard to the social difficulties often experienced by young people with autism. Depression was measured by the depression subscale of the Depression, Anxiety and Stress Scales (DASS)Reference Lovibond and Lovibond45 at pre-treatment, post-treatment and again at 3 and 9 months’ follow-up. There was no significant difference between the groups post-treatment. However, there was a significant decrease in DASS depression scores for those participants with scores in the clinical range at pre-treatment. These improvements were maintained at both 3 and 9 months’ follow-up.
The final controlled study also evaluated a group CBT programme for young people with autism with no intellectual disability (n = 23, 60% male, mean age, 15.75 years, s.d. 1.37).Reference Santomauro, Sheffield and Sofronoff51 Participants were randomly allocated to either waitlist or control, via a computer-generated random sequence programme. The 10-week group CBT programme was designed specifically for young people with autism.Reference Attwood and Garnett52 Depression was measured with both the DASS depression subscale and the BDI-IIReference Beck, Steer and Brown15 at pre-treatment, post-treatment and at 4 and 12 weeks’ follow-up. The authors reported no significant change in BDI-II scores from pre- to post-treatment, although a significant decrease was seen in DASS depression scores for the treatment group.
Research questions 2 and 3: what are the experiences of young people and their families and treatments for depression, and what are the experiences of professionals in delivering treatment for depression?
No studies were identified that had a focus on evaluating the experiences of young people with intellectual disability and/or autism and their families in receiving psychological treatment for depression. Only two of the included studies reporting on treatments for depression also reported on participant experience.Reference Greig and MacKay23,Reference Santomauro, Sheffield and Sofronoff51 Santomauro et alReference Santomauro, Sheffield and Sofronoff51 gathered feedback from 15 young people with autism during their final group booster session, as a group discussion. Fourteen out of 15 young people reported enjoying the programme, with the 15th participant still recommending the programme for its usefulness. Participants considered the group setting the most beneficial aspect of the CBT programme. Greig and MacKayReference Greig and MacKay23 briefly noted that the participant in their single case study felt that the intervention had worked for them in real-life situations.
No studies evaluated the professional or clinician experience of delivering treatment for depression to children and young people with intellectual disability and/or autism.
Risk of bias
Risk of bias was assessed for all studies. No studies were considered to have a low risk of bias. All of the clinical case reports (n = 4) were assessed as high risk of bias.Reference Fernandez, Tom, Stadler, Cain and Knudsen21,Reference Greig and MacKay23,Reference Loades26,Reference Selvapandiyan28 Clinical case reports are inherently biased: they have a high risk of publication bias; they are retrospective reports and subject to information bias in that they involve subjective interpretation by the author who is often the treating clinician; outcome assessment measures are often administered by the clinician; and causal relationships and generalisation are not possible because of the nature of describing treatment outcome for one individual, often leading to overinterpretation of results and treatment effectiveness.Reference Nissen and Wynn54 In addition to these overarching issues, the included clinical case reports had particular problems with outcome measures, including selecting inappropriate measures, not reporting how scores were calculated and not reporting on all outcomes as stated.
Of the quasi-experimental and experimental studies, four studies were rated to have a moderate risk of bias, including the multiple baseline design,Reference Frame, Matson, Sonis, Fialkov and Kazdin33,Reference McGillivray and Evert44,Reference Ringenbach, Holzapfel, Arnold, Nam, Lopez and Chen48,Reference Santomauro, Sheffield and Sofronoff51 and the remaining two rated as high risk of bias (both uncontrolled trials).Reference Dosen38,Reference Habayeb, Rich and Alvord39 Reasons for ratings of moderate and high risk of bias included no control group, no or poor randomisation when there was a control group, outcome measures administered by the clinician delivering treatment, use of outcome measures with unestablished psychometric properties in intellectual disability and/or autism, and not considering effects of confounding variables (e.g. medication).
Discussion
This systematic search identified 10 studies that evaluated psychological treatments for depression in children and young people with intellectual disability and/or autism. However, four of these were clinical case reports with a high risk of bias and thus are unable to directly inform a research evidence base to guide treatment.Reference Fernandez, Tom, Stadler, Cain and Knudsen21,Reference Greig and MacKay23,Reference Loades26,Reference Selvapandiyan28 The remaining six studies included four studies with either a single case experimental design,Reference Einfeld, Ellis and Emerson2 an uncontrolled group designReference Dosen38,Reference Habayeb, Rich and Alvord39 or a controlled group design.Reference McGillivray and Evert44,Reference Ringenbach, Holzapfel, Arnold, Nam, Lopez and Chen48,Reference Santomauro, Sheffield and Sofronoff51 The six experimental/quasi-experimental studies each focused on different treatments, different population groups, used different outcome measures for depression and were all rated with a moderate or high risk of bias. Therefore, no conclusions can be drawn with any confidence about the suitability or effectiveness of any particular psychological or psychosocial intervention for treating depression in children and young people with intellectual disability and/or autism. There was also essentially a complete lack of information about the experiences of young people or their families who received psychological intervention for depression, or the therapists who delivered the intervention.
Study design
High-quality, randomised controlled trials are essential to improve the evidence-base for effectiveness of these interventions. Only one of the three controlled trials employed adequate randomisation strategies,Reference Santomauro, Sheffield and Sofronoff51 with the others allocating participants based on order of enrolment,Reference McGillivray and Evert44 or through counterbalancing, which was not thoroughly described.Reference Ringenbach, Holzapfel, Arnold, Nam, Lopez and Chen48 Future studies should focus on developing well-designed, randomised controlled trials to address this important gap in the literature.
In addition to the need for well-designed trials, future research should evaluate existing evidence-based psychological and psychosocial treatments for depression adapted specifically to meet the needs of children and young people with intellectual disability and/or autism. Although a range of psychological and psychosocial interventions were identified in this review, only two of the experimental studies reported that the intervention used had been adapted for young people with autism.Reference McGillivray and Evert44,Reference Santomauro, Sheffield and Sofronoff51 Importantly, none of the interventions described had been adapted for young people with intellectual disability. Development of new interventions tailored specifically for this population is also important. New interventions should be developed and evaluated through pilot studies, and further trialled in randomised controlled trials. The role of a parent/caregiver as support or facilitator within psychological interventions should also be considered. This approach has been successfully demonstrated in interventions with adults with intellectual disability (for example, Jahoda et alReference Jahoda, Hastings, Hatton, Cooper, Dagnan and Zhang55,Reference Jahoda, Melville, Cooper, Hastings, Briggs and Dagnan56 ). Particularly important in any adaptation or development of interventions is collaboration with the key stakeholders: young people with intellectual disability and/or autism, their families and the therapists delivering the interventions.
Exclusion of intellectual disability
Inclusion of children and young people with intellectual disability was extremely limited. Only four studies included participants with an intellectual disability,Reference Fernandez, Tom, Stadler, Cain and Knudsen21,Reference Frame, Matson, Sonis, Fialkov and Kazdin33,Reference Dosen38,Reference Ringenbach, Holzapfel, Arnold, Nam, Lopez and Chen48 and six studies included participants with a diagnosis of Asperger syndrome or autism without co-occurring intellectual disability.Reference Greig and MacKay23,Reference Loades26,Reference Selvapandiyan28,Reference Habayeb, Rich and Alvord39,Reference McGillivray and Evert44,Reference Santomauro, Sheffield and Sofronoff51 No studies involved participants with both intellectual disability and autism, consistent with a recent meta-analysis demonstrating selection bias against participants with intellectual disability in autism research.Reference Russell, Mandy, Elliott, White, Pittwood and Ford57 The exclusion of young people with intellectual disability was particularly evident in the controlled trials, with only the physical exercise intervention, involving no cognitive component, including participants with intellectual disability.Reference Ringenbach, Holzapfel, Arnold, Nam, Lopez and Chen48 This is a significant gap in the literature in that we have limited evidence of effective interventions for depression in children with intellectual disability, despite knowledge that rates of mental health problems, and in particular, depression, are prevalent in this population.
Outcome measures
Outcome measures of depression and depression symptoms were inconsistent, and in some cases, not valid measures of depression. Although a number of studies used validated measures and screening tools for depression, including the Revised Children's Anxiety and Depression Scale (RCADS) depression subscale,Reference Loades26 the HRSD,Reference Selvapandiyan28 the DASS Depression scale,Reference McGillivray and Evert44,Reference Santomauro, Sheffield and Sofronoff51 the CDI-IIReference Ringenbach, Holzapfel, Arnold, Nam, Lopez and Chen48 and the BDI-II,Reference Santomauro, Sheffield and Sofronoff51 others relied on subjective measurement such as clinical judgement, or changes in behaviour not necessarily indicative of depression. None of the depression measures used were developed or adapted for people with intellectual disability and/or autism. Further, selection of outcome measures was not suitable in all studies. For example, the DASS is a tool designed for use with a typically developing adult population, yet was used with children and young people as young as 13 years in these studies. Use of suitable depression outcome measures is critical for future studies to ensure effectiveness in treating depression presenting in children and young people with intellectual disability and/or autism. Some caregiver-report measures of depressive symptoms in children and young people with intellectual disability already exist, such as the Developmental Behavior Checklist 2Reference Gray, Tonge, Einfeld, Gruber and Klein58 and the Anxiety, Depression and Mood Scale,Reference Esbensen, Rojahn, Aman and Ruedrich59 and could be used to assess change in depressive symptoms. Some research has used adapted versions of the CDIReference Kovacs14 for young people with intellectual disability.Reference Klein, Houtkamp, Salemink, Baartmans, Rinck and van der Molen60,Reference Weeland, Nijhof, Otten, Vermaes and Buitelaar61 In addition, adapting existing self-report measures of depression validated for use with adults with intellectual disability, such as the Glasgow Depression Scale,Reference Cuthill, Espie and Cooper16 for use with children and young people, could be considered in future research.
Strengths and limitations
The current review was conducted with strong methodological rigour, in line with PRISMA guidelines and following a pre-registered protocol. Strengths of this review include the broad definition of psychological and psychosocial therapies used, ensuring all relevant treatments and interventions were identified, the inclusion of all publication types, including theses, and no restrictions on date of publication. Non-English publications were excluded; however, two studies were identified from countries without English as a first language. A meta-analysis was not undertaken because of the small number of studies identified, their poor quality and moderate-to-high risk of bias.
Summary and future directions
This systematic review highlights a number of significant gaps in the literature for treatment of depression for children and young people with intellectual disability and/or autism. The lack of well-designed, randomised controlled trials was clear, as was the exclusion of young people with intellectual disability. The complete lack of research on psychological interventions for young people with intellectual disability was striking and concerning. Adaptation and development of specifically tailored psychological and psychosocial interventions for depression in children and young people with intellectual disability and/or autism, as well as measures of depression and depressive symptoms, is an essential next step in the research. Future research should also ensure accurate records of medication are taken and considered when interpreting the effectiveness of a psychological intervention.
Further, evaluating experiences of both receiving treatment for depression (children and parents) and delivering treatment (therapists and professionals) is paramount in ensuring that interventions, both existing, adapted and newly developed, meet the needs of the patient. Future research should ensure that families and professionals are consulted on the design of interventions and evaluations of their experiences are embedded within any study design.
It is important to note that these findings are not unique to the treatment of depression for children and young people with intellectual disability and/or autism. There is an absence of intervention research of any psychological treatments for any mental health disorder in this population.Reference Vereenooghe and Langdon7 Further, children and young people with severe intellectual disability are a particularly vulnerable group, and often neglected in research of mental health problems and intervention.Reference Vereenooghe, Flynn, Hastings, Adams, Chauhan and Cooper62 As highlighted in a recent systematic review, future research into psychological treatments for depression for children and young people with intellectual disability and/or autism should also be supported by the development of appropriate outcome measures of any mental health symptoms for this population.Reference Flynn, Vereenooghe, Hastings, Adams, Cooper and Gore63
Funding
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors. L.A.C. is supported by an Australian Government Research Training Program (RTP) Scholarship. K.P. is the recipient of a University of New South Wales Australia Scientia PhD Scholarship.
Author contributions
K.M.G., K.P. and R.P.H. formulated the research questions. K.M.G., K.P., R.P.H. and G.A.M. designed the study. K.P. and L.A.C. carried out the database search. K.P., L.A.C., K.M.G., G.A.M. and R.P.H. collected and analysed the data. L.A.C., K.M.G., R.P.H., G.A.M. and K.P. wrote the manuscript or contributed to substantive reviews and revisions. All authors approved the final version of the manuscript and agree to be accountable for all aspects of the work.
Declaration of interest
None.








eLetters
No eLetters have been published for this article.