The elderly population is increasing both in absolute numbers as well as in the percentage of the total population worldwide,1 with no exception for those with bipolar disorder, major depressive disorder (MDD), schizoaffective disorder or schizophrenia.Reference Whiteford, Degenhardt, Rehm, Baxter, Ferrari and Erskine2 Although there is premature mortality among people with MDD, bipolar disorder and schizophrenia, some individuals with these conditions reach an advanced age and may experience considerable physical health burdens and multimorbidity; therefore, they may be more likely to need admission to a nursing home environment.Reference Stubbs3, Reference Correll, Solmi, Veronese, Bortolato, Rosson and Santonastaso4
Essential epidemiology of MDD in the elder population
MDD is one of the most common mental disorders worldwide and is prevalent throughout the lifespan, with prevalence estimates of 1–5% in those 65 years of age and older.Reference Lackamp, Schlachet and Sajatovic5 Regrettably, little is known about the actual rates and clinical features associated with MDD among nursing home residents, essentially because of almost invariable systematic exclusion of elderly patients from selection into studies and subsequent publication bias. Also, nursing home residents with MDD may be either patients with disorder onset early in life (then lasting or recurring at an old age) or patients whose onset first occurs in late life, representing differential clinical and neurobiological phenotypes of depression.Reference Bukh, Bock, Vinberg, Gether and Kessing6–Reference Ulrich, Nogueira, Teixeira and Ely Filho8
MDD deserves further accurate clinical epidemiological assessment focusing on the cases in individuals not related to or overlapping with dementias, ideally providing clear-cut prevalence estimates of MDD among residents in nursing homes, which are most likely populated with elderly people. Patient-tailored treatment and prevention of depression in the elderly population should promote cognitive health, enhancing the chances of independent living and overall quality of life.
Goals of the study
To the best of our knowledge, the only systematic review on the prevalence of psychiatric disorders among nursing home residents dates back to the year 2010, did not use any quantitative pooling and documented long-term point-prevalence rates of an MDD diagnosis up to 10% for nursing home residents and 29% for depressive symptoms overall.Reference Seitz, Purandare and Conn9 However, it must be noted that the study merged a variety of different clinical phenotypes of depression, including bipolar disorder and those ‘confounded’ by comorbid dementia(s), lifetime substance abuse and/or anxiety disorders. The study also limited the search strategy to only the EMBASE data-setReference Seitz, Purandare and Conn9 and did not adopt a reliable (semi-) structured interview based on any major standard diagnostic coding. Therefore, considerable uncertainty still surrounds the actual prevalence rates and clinical correlates associated with MDD, bipolar disorder and schizophrenia among nursing home residents.
We aimed to conduct a systematic review and meta-analysis of the prevalence and clinical correlates of MDD, bipolar disorder and schizophrenia among nursing home residents without dementia, with diagnoses assessed using structured interviews based on either the DSM or ICD systems, and to strive to control or avoid as many confounding factors as possible (with a special emphasis on dementia-related processes).
Method
Search strategy and study selection
The present systematic review adhered to the PRISMAReference Moher, Liberati, Tetzlaff, Altman and Group10 and the MOOSE guidelines.Reference Stroup, Berlin, Morton, Olkin, Williamson and Rennie11 It is registered in the international prospective register of systematic reviews (PROSPERO) (https://www.crd.york.ac.uk/PROSPERO/), registration number is CRD42018088312. We divided into two teams (M.F., A.F., S.N. and A.A.; M.S. and F.M.) and independently searched PubMed, PsycINFO and EMBASE databases for records indexed from the year 1980 onwards (last updated, June 2017). The string was searched in PubMed and was adapted across varying data-sets: ((nursing home*[Title/Abstract] OR long-term care[Title/Abstract] OR homes for the aged[Title/Abstract])) AND ((((((((“Psychotic Disorders”[Mesh] OR “Bipolar Disorder”[Mesh]) OR “Depressive Disorder, Major”[Mesh]) OR (“Mood Disorders”[Mesh] OR “Seasonal Affective Disorder”[Mesh] OR “Affective Disorders, Psychotic”[Mesh])) OR (“Depression”[Mesh] OR “Depressive Disorder”[Mesh])) OR “Schizophrenia”[Mesh]) OR “Schizophrenia Spectrum and Other Psychotic Disorders”[Mesh])) OR (psychosis)). Additional details for the search strategy across varying data-sets have been provided in supplementary Data 1 available at https://doi.org/10.1192/bjp.2019.5. Finally, the results were augmented by a manual search and cross-references as detailed in Fig. 1.
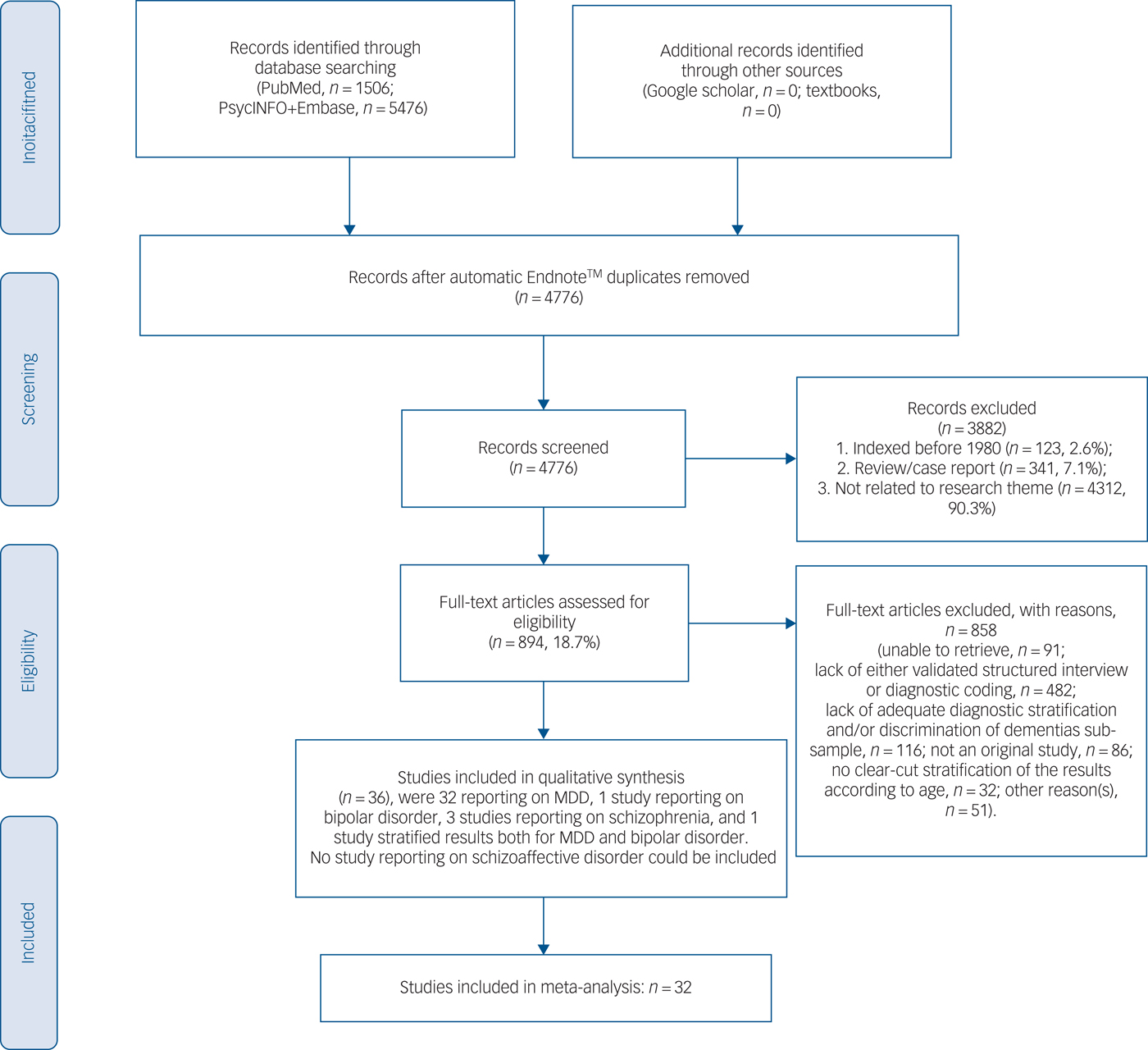
Fig. 1 PRISMA 2009 flow diagram.
Studies were deemed eligible if they were original peer-reviewed articles (any language), but not case report/series (i.e. with a sample size <10), that reported the prevalence of either MDD, bipolar disorder or schizophrenia/schizoaffective disorder among nursing home residents, or contained data allowing us to compute the prevalence. Patients whose bipolar disorder started at age 60 years or older were consider to have late-onset bipolar disorder,Reference Sajatovic, Strejilevich, Gildengers, Dols, Al Jurdi and Forester12 and this age threshold was likewise applied to MDD and schizophrenia as well. Either naturalistic studies or interventional studies with baseline prevalence data were included. The diagnosis of MDD, bipolar disorder or schizophrenia had to be made according to any version of the DSM or ICD.
Data extraction
We divided into two teams (M.F., A.F., S.N. and A.A; M.S. and F.M.) and independently extracted data using a predetermined extraction form, and including the following: MDD, bipolar disorder or schizophrenia prevalence (or variables needed to compute it), author, year of publication, year of data collection, country/continent of data collection, study design, demographic characteristics, underlying main condition, employed clinical rating scales and the diagnostic criteria that were used in conjunction with a validated structured interview, and essential clinical and pharmacological moderators, including but not limited to, prescription of first (FGAs) or second-generation (SGAs) atypical antipsychotics as well as the percentage of major medical comorbidities. Any eventual within- and between-team disagreements were solved by the corresponding team principal investigator (M.F. and M.S.) and between-team resolution was performed by a senior author (A.F.C.) as necessary.
Quality assessment
We assessed the quality of the randomised controlled trials (RCTs) using the Cochrane Risk of Bias Assessment Tool and for the other design studies we used the Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies (National Heart, Lung and Blood Institute (NIH), https://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools/cohort). The quality of the interventional studies was assessed using the Cochrane Risk of Bias Assessment Tool.Reference Higgins, Altman, Gøtzsche, Jüni, Moher and Oxman13 For both rating tools, higher scores indicated poorer quality of the study. Acceptable, good scores were computed based on percentile distribution.
Meta-analysis
Because of the anticipated heterogeneity, we used a random-effects meta-analysis and computed the pooled prevalence and 95% CIs with Comprehensive Meta-Analysis (CMA, version 2).Reference Borenstein, Hedges, Higgins and Rothstein14 Heterogeneity was assessed with the Cochrane Q and I 2 statistics for each analysis.Reference Higgins, Thompson, Deeks and Altman15 We conducted mixed-effect model meta-regression analyses with CMA, for outcomes with high heterogeneity (I 2>50% and/or P≤0.05) and reported by ≥4 studies, to investigate potential moderators of the observed prevalence of MDD, bipolar disorder and schizophrenia in nursing homes. We conducted sensitivity analyses according to country, continent, criteria used to define a given mental condition, period of data collection (in decades), specific psychiatric diagnosis (MDD, bipolar disorder and schizophrenia), and the quality of the study (post hoc assessment of good, fair, or poor quality) based on either the NIH or the Cochrane tools mentioned earlier, and using quartiles, we then merged the studies into two main categories (poor–moderate and fair–good quality) to allow sensitivity prevalence analysis across the two main categories (as detailed in in the results section).
Depending on the available data, we aimed to investigate the following moderators: sample size, year of data collection, mean age, percentage of men, ethnicity, country, diagnostic criteria (DSM/ICD), major medical or psychiatric comorbidities whenever available and quality of the study according to the NIH rating.
Publication bias was assessed via visual inspection of funnel plots and with the Begg-Mazumdar Kendall's tauReference Begg and Mazumdar16 and Egger bias tests.Reference Egger, Davey Smith, Schneider and Minder17 In cases where publication bias was identified, we computed the trim and fill adjusted analysisReference Duval and Tweedie18 to remove the most extreme small studies from the positive side of the funnel plot, and recomputed the effect size at each iteration until the funnel plot was symmetric around the (new/adjusted) effect size.
Results
Out of the initial title and abstract assessment of 4776 hits after duplicate removal, we excluded 3882 papers, thus, 894 full-texts were further assessed (see Fig. 1). A total of 36 studiesReference Falck, Pot, Braam, Hanewald and Ribbe19–Reference Streim, Oslin, Katz, Smith, DiFilippo and Cooper53 could be included in the qualitative synthesis. Table 1 outlines the main details of the studies, including the clinical features documented among nursing home residents with MDD, bipolar disorder and schizophrenia; in total there were 13 754 participants included with a weighted mean age of 80.65 years. Most of the studies were conducted in North America (n = 21, Europe n = 9, Oceania n = 3, Asia, n = 2, other n = 1) using DSM criteria (DSM-IV n = 17, DSM-III n = 13, ICD-9/10 n = 6). In most, women were overrepresented among the nursing homes residents without a current diagnosis of dementia (any type). Notably, major non-psychiatric medical comorbidities (for example diabetes, other cardio- or cerebrovascular conditions) were rarely documented; similarly, prominent cognitive impairment (but not dementia) was relatively uncommon.
Table 1 Qualitative synthesis of records (n = 36 studies, n = 13 754 participants)a

MDD, major depressive disorder; NIH, National Heart, Lung and Blood Institute; SCZ, schizophrenia; BD, bipolar disorder; NMES, National Medical Expenditure Survey; IPC, Institutional Population Component; RCT, randomised clinical trial; GAD, general anxiety disorder.
a. Please note that the actual number of studies included in the meta-analysis exceeded n = 36 since a couple of original records included multiple multidiagnostic arms. A total of 10 out of 36 studies were indexed after the year 2010 (27% of the sample); studies indexed after the year 2010 may have nonetheless accounted for data collected earlier in the research process.
b. Year of data collection may differ from the year of the publication of the study.
c. Mean age for participants with MDD, bipolar disorder and schizophrenia only, other than for Goodwin & SmyerReference Goodwin and Smyer44
d. Percentage of men in study only for those with MDD, bipolar disorder and schizophrenia other than for Gerety et al,Reference Gerety, Williams, Mulrow, Cornell, Kadri and Rosenberg49 Koenig & KuchibhatlaReference Koenig and Kuchibhatla46 Laprise & Vezina,Reference Laprise and Vezina47 Goodwin & Smyer,Reference Goodwin and Smyer44 Anderson et al Reference Anderson, Buckwalter, Buchanan, Maas and Imhof40 and Damian et al.Reference Damian, Valderrama-Gama, Rodriguez-Artalejo and Martin-Moreno39
e. Ethnicity percentages are only given when specified in the study. Data are either for all sample or those with MDD, bipolar disorder and schizophrenia only.
There were 31 cross-sectional studies, 3 prospective open studies and 2 RCTs. Among the 36 studies, 3Reference Rabins, Black, Roca, German, McGuire and Robbins42, Reference Erlandsen43, Reference Streim, Oslin, Katz, Smith, DiFilippo and Cooper53 were assessed using the Cochrane quality evaluation tool as they were interventional studies (2 of which were RCTsReference Rabins, Black, Roca, German, McGuire and Robbins42, Reference Streim, Oslin, Katz, Smith, DiFilippo and Cooper53 and 1 was a non-controlled prospective trialReference Erlandsen43). The quality of the 33 out 36 studies assessed using the NIH tool have been further appraised in Table 1 by stratification into quartiles, with scores ranging 2–5 (first and second quartiles merged) regarded as moderate–poor quality (n = 24/33 or 75% of the records) in contrast to higher scores (up to 7) regarded as fair–good quality studies (third and fourth quartiles merged) (n = 9/33 or 24% of the records). Of the studies appraised using the Cochrane toolReference Higgins, Altman, Gøtzsche, Jüni, Moher and Oxman13 two records were scored as 7 (i.e. considered of fair quality) versus one record scored as 4 (considered of poor quality).
In total, 32 studies reported on MDDReference Falck, Pot, Braam, Hanewald and Ribbe19, Reference Parmelee, Katz and Lawton21–Reference Anderson, Buckwalter, Buchanan, Maas and Imhof40, Reference Rabins, Black, Roca, German, McGuire and Robbins42, Reference Goodwin and Smyer44–Reference Streim, Oslin, Katz, Smith, DiFilippo and Cooper53 and 3 studies reported on schizophreniaReference Class, Unverzagt, Gao, Hall, Baiyewa and Hendrie20, Reference Erlandsen43, Reference Bartels, Mueser and Miles54 (1 schizophrenia study also documented a subset of people with bipolar disorderReference Bartels, Mueser and Miles54 and 1 study provided stratified results both on MDD and bipolar disorder samplesReference Choi, Ransom and Wyllie37). We could not locate any study reporting on schizoaffective disorder. The 32 studies reporting on MDD were included in the meta-analysis.Reference Falck, Pot, Braam, Hanewald and Ribbe19, Reference Parmelee, Katz and Lawton21–Reference Anderson, Buckwalter, Buchanan, Maas and Imhof40, Reference Rabins, Black, Roca, German, McGuire and Robbins42, Reference Goodwin and Smyer44–Reference Streim, Oslin, Katz, Smith, DiFilippo and Cooper53
Meta-analysis of MDD prevalence, publication bias, heterogeneity and categorical subgroup comparisons
The overall pooled MDD prevalence across 32 samples and 2110 people with MDD out of 13 394 nursing home residents pooled for quantitative analysis was 18.9% (95% CI 14.8–23.8), see Fig. 2 for details. Heterogeneity was high (I 2 = 97%, P≤0.001). Publication bias seemed unlikely (see Fig. 3 for visual inspection of the funnel plot) (Egger test intercept 0.726, (P not significant); Begg and Mazumdar's test, continuity-adjusted tau 0.00202, P not significant).
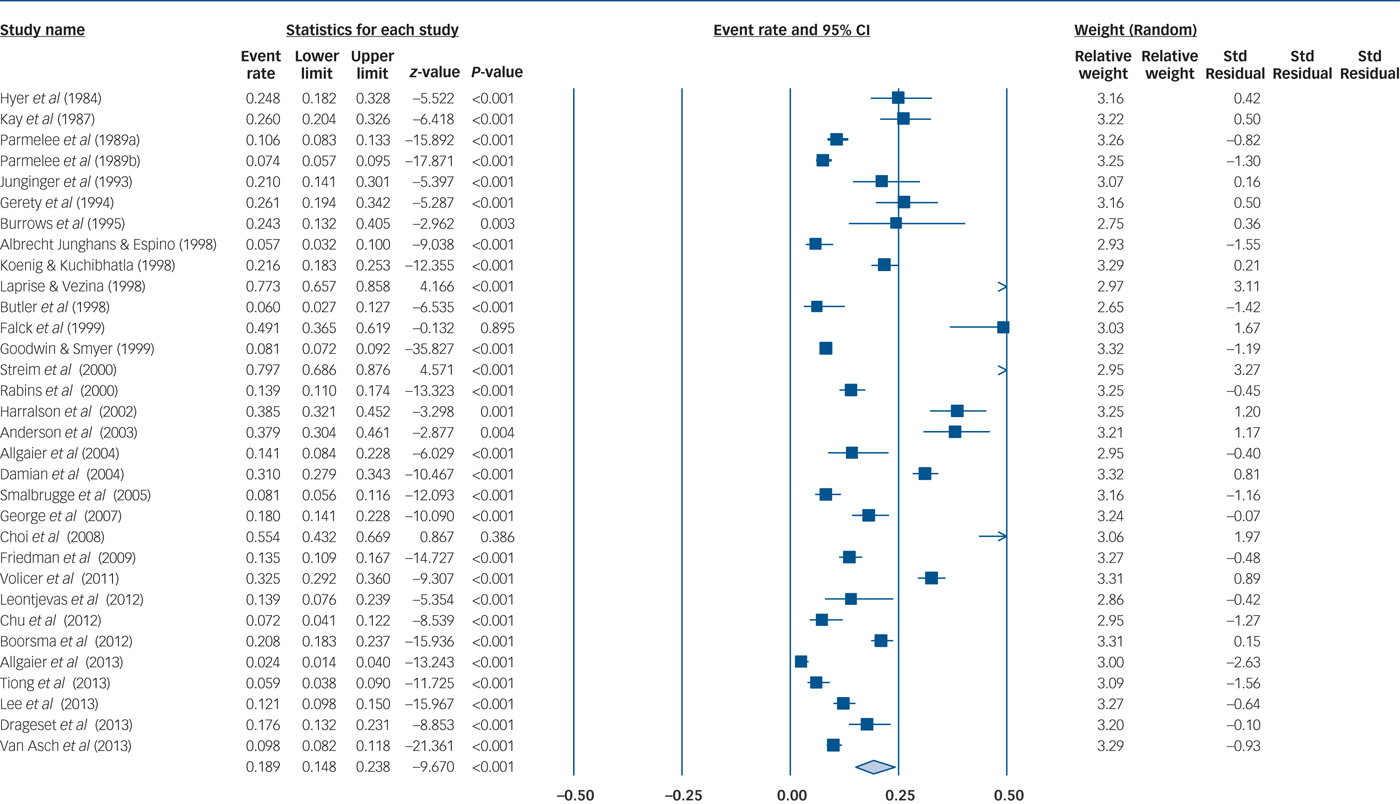
Fig. 2 Major depressive disorder (MDD) prevalence among nursing homes residents.
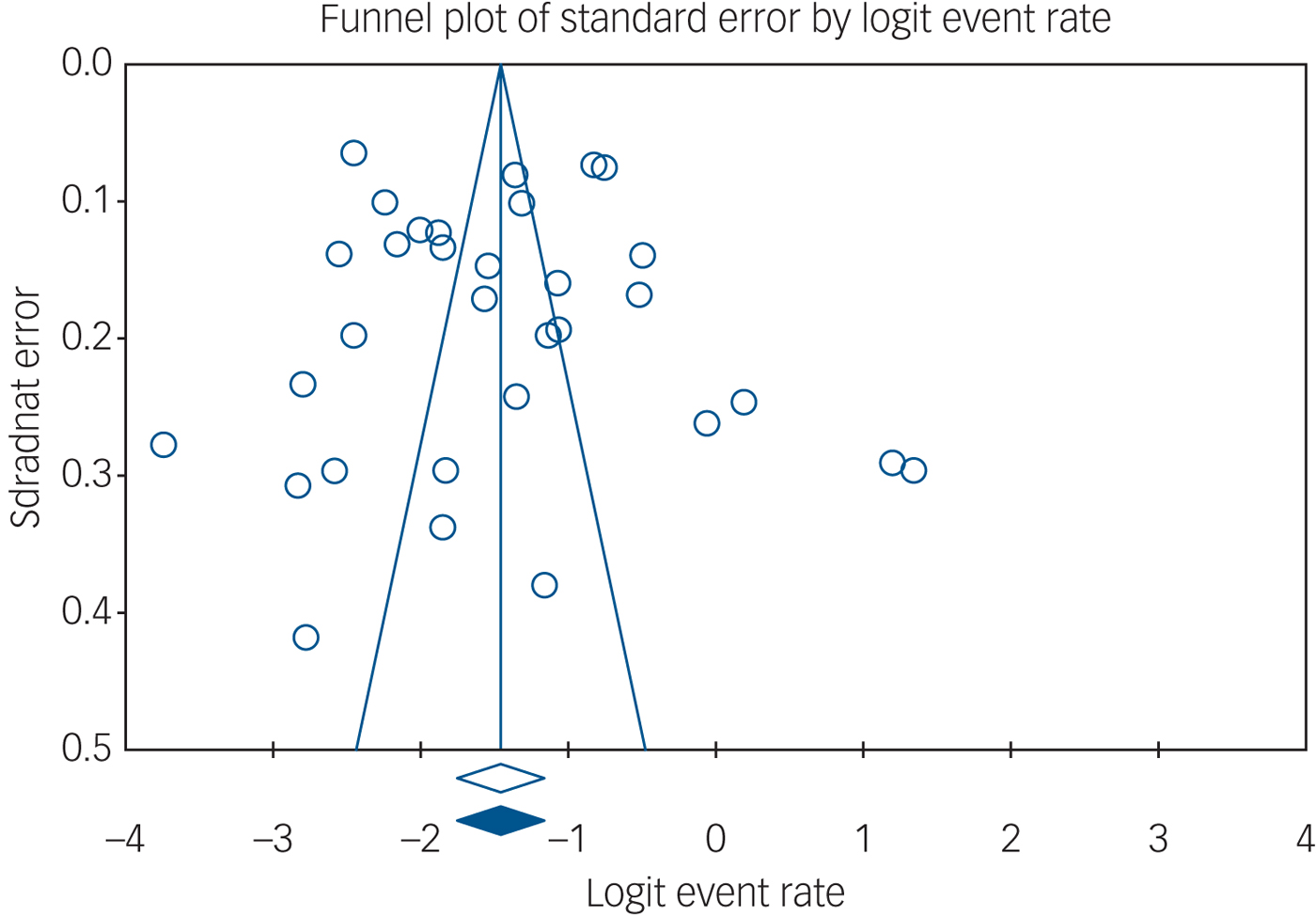
Fig. 3 Funnel plot.
Subgroup analysis of MDD in nursing home residents
As detailed in Table 2, the prevalence rates of MDD among nursing home residents significantly varied across geographical regions, being highest (point-prevalence rates 25.4%, 95% CI 18–34.5, P≤0.001) in North America and lowest in Oceania (5.7%, 95% CI 3.2–10, P≤0.001), although publication bias for North American studies could not be excluded (P = 0.015). The total overall between-region difference (P≤0.001) means that the estimated prevalence rates statistically significantly differed across varying subgroups according to geographical region.
Table 2 Random-effect meta-analysis with sensitivity analyses of the prevalence of major depressive disorder (MDD) in nursing homes

NS, not significant,
a. Publication bias could not be evaluated in the case of three studies or fewer.
Similarly, the prevalence estimates of MDD varied according to the design of the study, being the highest for prospective, non-controlled studies (44.1%, 95% CI 33.3–94.7, P not significant) and lowest for cross-sectional studies (17.2%, 95% CI 13.2–22, P≤0.001). There was a total overall between-design difference (P≤0.001).
In addition, the prevalence of MDD was higher among White nursing home residents (35.2%, 95% CI 16.7–59.7, P not significant) versus Black/African American counterparts (17.5%, 95% CI 11.2–26.4, P≤0.001) and was lowest among Hispanic or Latino Americans (5.7%, 95% CI 3.2–10, P≤0.001). There was a total overall between-ethnicity difference (P≤0.001).
A DSM-III diagnosis of MDD was documented among 12.4% of the residents (95% CI 8.2–18.2, P≤0.001), and a DSM-IV diagnosis of MDD was documented among 21.3% of the residents (95% CI 15.2–29.2, P≤0.001). A diagnosis of MDD made according to the ICD-9 or the ICD-10 criteria was documented among 30.9% of the residents (95% CI 13.3–56.6, P not significant). There was a total overall difference based on diagnostic criteria (P≤0.001).
Concerning major psychiatric or other medical comorbidities, diabetes was recorded among 18.3% of the residents (95% CI 5.8–44.9, P = 0.023), anxiety comorbidity was seen among 43.1% of the residents (95% CI 10.8–82.7, P not significant), and cognitive impairment (yet not leading to dementia) was recorded among 18.5% of the residents (95% CI 6–44.5, P = 0.021). There was a total overall difference in psychiatric or other medical comorbidities (P≤0.001).
Finally, those observational studies appraised as moderate-to-poor quality according to the NIH tool mentioned earlier and the ad hoc created percentile recoding documented point-prevalence rates of MDD up to 17.1% (95% CI 12.1–23.4, P≤0.001). In contrast, those non-interventional studies appraised as fair-to-good quality documented point-prevalence rates of MDD of 18.3% (95% CI 12.5–26, P≤0.001). There was a total overall difference between studies with varying quality (P≤0.001).
Mixed-effect meta-regression analysis of potential continuous variable moderators in patients with MDD
Supplementary Figs 1–4 provide a graphic synthesis of sex, mean age and publication year predictors. Mixed-effect meta-regression analysis demonstrated that the publication year predicted higher rates of MDD among nursing home residents (β = 0.007, 95% CI 0.001–0.013, P = 0.019, k (number of studies) = 32) and that age inversely predicted MDD prevalence (β = −0.031, 95% CI 0.008–0.046, P≤0.001, k = 22). Additionally, the higher the proportion of men among nursing home residents was, the higher the overall rate of MDD was (β = 0.017, 95% CI 0.010–0.024, P≤0.001, k = 25). As largely expected, the higher the antidepressant drug use was, the higher the overall rate of MDD diagnosis was (β = 0.006, 95% CI 0.002–0.015, P = 0.014, k = 8).
Variables unable to be included in the analyses
We were unable to extract sufficient data to allow reliable pooling of the following clinical moderators: mean age at onset of MDD, current use of lithium, anticonvulsant mood stabilisers, benzodiazepines, FGA or SGA drugs, current psychotropic polypharmacy (namely, two or more psychiatric drugs at once), obsessive–compulsive disorder, post-traumatic distress disorder, impulse-control disorder, suicidal behaviour, substance use (including misuse of over-the-counter pain-killer medications), tobacco use, and cardio-/cerebrovascular diseases (including obesity). In addition, we could not even run an exploratory meta-analysis of schizophrenia prevalence among nursing home residents because of the paucity of corresponding original studies (n = 3) and the fact that these studies did not follow a naturalist approach. Similarly, nursing home residents with bipolar disorder could be appraised only for qualitative synthesis since the corresponding original studies were too few in number (n = 2).
Major biases found across the included studies reporting on MDD
The following issues were documented in at least three studies: a relatively small sample size, a lack of clear-cut definition of the time frame when the MDD symptoms were assessed, and/or a lack of an accurate description of the severity of the underlying psychiatric or other medical condition(s). See supplementary Table 1 for the PRISMA 2009 checklist for the study.
Discussion
This systematic review included 36 studies encompassing 13 754 individuals. Of these, it was possible to pool data from 13 394 individuals identifying 2110 people with MDD (documented by 32 original studies). In addition, we identified 192 individuals with schizophrenia described in three studies, but it was not possible to reliably pool data from these for quantitative synthesis because of the non-naturalistic designs (the qualitative synthesis is nonetheless summarised in Table 1). The mean prevalence of MDD across varying geographical regions was 18.9%. Mixed-model meta-regression analysis of the MDD subset revealed that the more recent the publication year the higher the reported prevalence of MDD among the nursing home residents; the older the mean age of the residents the lower the reported prevalence of MDD among the nursing home residents; the higher the proportion of men among the nursing home residents the higher the rates of MDD overall; and, as expected, the higher the antidepressant drug use the higher the rates of MDD overall.
Finally, despite substantial heterogeneity, MDD prevalence was significantly affected by geographical region, study design and ethnicity moderators. Nonetheless, concerning the study design, the only statistically significant rates of MDD were the ones related to cross-sectional reports because of the paucity of prospective studies.
Overall, this study provides a more accurate insights into the prevalence and clinical features associated with nursing home residents without dementia diagnosed with MDD than was previous available as Seitz et al 9 provide only a qualitative synthesis of the evidence and did not discriminate comorbid MDD with or without dementia, despite the intricate relationship that exists between depression and cognitive deficits, especially in elderly people.Reference Austin, Mitchell and Goodwin55 In addition, we retained only those studies relying on the structured interview(s) validated according to mainstream diagnostic codes rather than merging overt MDD with depressive symptoms. Aiming at enhancing the quality of reporting, we purposely excluded those studies in which the diagnosis of MDD was not assessed by a structured interview. Nonetheless, we acknowledge that the use of structured interviews among nursing home residents may not be as popular as it is among the non-elderly adult population. Therefore, future primary studies should promote the use of standardised clinical ratings among elderly people with MDD, bipolar disorder and schizophrenia.
Strengths and limitations
There are several limitations of the present study that should be acknowledged, allowing a critical interpretation of the results. The limitations include the high heterogeneity of the studies and populations, the relatively narrow range of the queried databases, as well as the assessment and diagnostic strategies for MDD, bipolar disorder and schizophrenia. This is with special reference to the lack of original studies about people with bipolar disorder and schizophrenia, and the total lack of studies providing clear-cut stratification of schizophrenia spectrum disorders.
Moreover, the studies assessing patients with schizophrenia did not follow a naturalistic approach, in contrast to the ones documenting MDD (or bipolar disorder). This issue coupled with the paucity of corresponding primary studies following a naturalistic approach precluded meta-analytic assessment. In addition, because of the scarcity of corresponding data, we could not further stratify for earlier versus later onset of MDD. Similarly, additional information is critically needed with respect to further potential confounding factors (namely, specific non-psychiatric medical comorbidities or accurate records of pharmacological resource utilisation).In this regard, it must be remarked that many elderly patients diagnosed with MDD are exposed to benzodiazepines, antipsychotics and other tranquilisers, whereas antidepressant drugs could be underused.Reference DeJesus56, Reference Sanyal, Asbridge, Kisely, Sketris and Andreou57
People with highly disabling severe mental illness (namely, schizophrenia as well as bipolar disorder), the onset of which usually occurs earlier in life than MDD onset and that require exposure to higher/prolonged doses of drugs with significant cardiometabolic side-effects, may have reduced life expectancy compared with their counterparts diagnosed with MDD.Reference Dembling, Chen and Vachon58, Reference Chang, Hayes, Perera, Broadbent, Fernandes and Lee59 Although one may assume that most people with severe mental illness would be admitted either to long-term psychiatric institutions or even to correctional institutes (as bipolar disorder may lead to antisocial behaviour associated with higher use of an illicit substance)Reference Swann60 rather than general medicine or multidisciplinary nursing home facilities, the actual current practice suggests that there was a reduction in long-term institutional care places, with more patients, especially those that are functional, receiving treatment in the community rather than in care homes, which possibly contain more patients who are severely disabled. This perspective may explain the higher rates of MDD (and possibly severe mental illness as well) over time (in line with the publication year trend).
Clinical implications
Taken together, the results from the present systematic review and meta-analysis lay the groundwork for replication studies to specifically address the above-raised issues considering that the actual prevalence of MDD among nursing home residents without dementia is high, which may also be the case for bipolar disorder and schizophrenia, and where systematic assessment is particularly urged. There are several areas of research and a need for stratification of nursing home residents with MDD, bipolar disorder and schizophrenia that need to be addressed by future clinical research. For example, little is known about the rates of suicidal behaviour in such populations, although the finding of lower rates of MDD among the older residents could be explained by increased mortality among the individuals who have died by suicide and/or had lower life expectancy because of severe medical morbidity. Similarly, nursing home residents who experience prolonged bed rest are at increased risk both for depression and for cardiometabolic issues, urging for patient-tailored physical therapy interventions as well. In addition, future clinical research on nursing home residents without dementia needs to systematically assess the cognitive and the treatment adherence profile of those individuals admitted to long-term facilities for older people.
The management of elder people with MDD, bipolar disorder and schizophrenia accounts for significant socioeconomic burden and resources utilisation. The life expectancy of people with MDD, bipolar disorder and schizophrenia is also increasing over time, although several factors such as the exposure to the SGAs may inflate the risk for cerebravascular diseases, thus leading to shorter life expectancy overall compared to age-matched healthy controls. Thus, the present topic of research represents a crucial priority for practising clinicians, nursing personnel and those involved in insurance plan-making, as well as policy-makers.
Funding
B.S. is supported by Health Education England and the National Institute for Health Research HEE/ NIHR ICA Programme Clinical Lectureship (ICA-CL-2017-03-001). B.S. is part supported by the Maudsley Charity and the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care South London at King's College Hospital NHS Foundation Trust. The views expressed in this article are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
Supplementary material
Supplementary material is available online at https://doi.org/10.1192/bjp.2019.5.







eLetters
No eLetters have been published for this article.