Autism is a developmental disorder characterised by deficits in social interaction and communication, and restricted, repetitive interests and behaviours beginning in infancy and toddler years. 1,Reference Ozonoff, Goodlin-Jones and Solomon2 The prevalence of autism has been estimated at 13/10 000 and is believed to be rising. Reference Fombonne3 The aetiology is unknown. Although the estimated 60–92% concordance rate in monozygotic twins as compared with 0–10% in dizygotic twins underscores the importance of genetic influences, the incomplete concordance in monozygotic twins also indicates a role of environmental factors. Reference Klauck4,Reference Bailey, Le Couteur, Gottesman, Bolton, Simonoff and Yuzda5 It is now believed that the mechanism underlying autism aetiology is most likely polygenic and potentially epistatic and that environmental factors may interact with genetic factors to increase risk. Reference Newschaffer, Fallin and Lee6,Reference Santangelo and Tsatsanis7
Although the distinctive neuropathology remains elusive, studies have shown macroscopic, microscopic and functional brain abnormalities. Reference Newschaffer, Fallin and Lee6,Reference DiCicco-Bloom, Lord, Zwaigenbaum, Courchesne, Dager and Schmitz8 These brain abnormalities suggest that the aetiologically relevant period may be in utero because the pathogenesis may begin during the prenatal period. Reference Newschaffer, Fallin and Lee6
Pregnancy-related exposures have been the focus of a significant amount of epidemiological research on possible risk factors for autism. Although many studies support the hypothesis that obstetrical complications may increase the risk of autism, Reference Kolevson, Gross and Reichenberg9 the specific complications, magnitude of effect and overall conclusions of these studies are inconsistent. These inconsistencies may be because of methodological variations including diagnostic criteria, comparison groups, sample size and exposure assessment methods.
The purpose of this study is to provide a systematic review and meta-analysis of the epidemiological literature on the relationship between prenatal complications/exposures and autism. A review article by Kolevson and colleagues discussed seven studies on this topic. Reference Kolevson, Gross and Reichenberg9 Our study expands upon this review by providing the first formal meta-analysis as well as a quantitative review of all 64 studies of prenatal risk factors for autism published up to March 2007. We review the evidence for all prenatal factors examined in the literature, and provide a summary effect estimate for all factors examined in two or more studies. The scope of literature reviewed allows for meta-regression analyses to examine whether study design characteristics explain the heterogeneity in results across studies.
Method
Data sources and review methods
PubMed, Embase, and PsycINFO databases were searched using the keywords ‘autism’ in combination with ‘prenatal’ or ‘perinatal’ or ‘pregnancy’ or ‘neonatal’, limited to peer-reviewed studies published in any language through to March 2007. The search identified 698 studies in PubMed, 176 in Embase and 416 in PsycInfo. The literature search sought to identify all epidemiological studies that have examined the association of pregnancy and delivery factors and neonatal complications to the risk of autism. Based on a review of all abstracts, 83 papers were identified as potentially relevant and reviewed further. Those studies that were not reviewed included case series, animal studies, autism prevalence studies, medical hypotheses, studies of other psychiatric diseases (e.g. schizophrenia) and studies of unrelated exposures (e.g. demographics, familial psychiatric diseases, genetics, infant behaviours). Forty-one additional potential papers were identified after screening the reference lists of original and review articles. Among the 124 studies that were reviewed, we excluded those that did not include a comparison group (n = 13) or any formal statistical analyses (n = 3), did not examine exposures during pregnancy or the first month of life (n = 10), grouped their autism cases with other childhood psychotic disorders (n = 15) and were review or commentary articles (n = 18). The control group had to be non-autistic but could be otherwise affected. In total, 65 studies were eligible for inclusion Reference Bailey, Le Couteur, Gottesman, Bolton, Simonoff and Yuzda5,Reference Akçakin and Polat10–Reference Zwaigenbaum, Szatmari, Jones, Bryson, MacLean and Mahoney73 in the quantitative review. Two studies Reference Burd, Severud, Kerbeshian and Klug15,Reference Klug, Burd, Kerbeshian, Benz and Martsolf30 reporting on the same data-set were considered together, resulting in 64 studies for review.
Although the literature search covered the scope of prenatal, perinatal and neonatal factors, the current report reviews the pregnancy-related factors only, and a future publication will address factors related to labour and delivery as well as neonatal complications in relation to autism. However, it is important to recognise that prenatal, perinatal and neonatal complications are interrelated, and are therefore difficult to disentangle and reliably categorise. Many perinatal and neonatal complications are often the result of both observed and unobserved prenatal insults and compromises to fetal development. This report focuses on those potential risk factors that were commonly identified as being specifically related to the prenatal period in the extant literature.
The first author abstracted each article on two separate occasions spaced 1 year apart. For each study the following information was recorded:
-
(a) study design (cohort, case–control);
-
(b) sample size and description (e.g. clinic based, population based);
-
(c) comparison group description (e.g. matching criteria, sibling controls, healthy v. otherwise affected controls, diagnoses of otherwise affected controls);
-
(d) autism diagnostic criteria and mode of reporting (e.g. DSM–III v. DSM–IV, parental report v. medical record review v. study physician assessment, diagnostic measures used);
-
(e) risk factors examined and mode of reporting (e.g. parental interview, medical record review);
-
(f) covariates included in multivariate models;
-
(g) study results, including indicators of statistical significance, prevalence of exposures among cases and controls, rates or risks of autism across exposure levels, relative risks (RRs) and 95% confidence intervals (CIs).
Studies were classified as prospective v. retrospective if exposures were assessed and recorded before or after the onset of autism, regardless of when they were analysed for the purposes of the given study. For the quantitative review, we counted the number of studies that examined each prenatal factor in relation to the risk of autism and the number of null findings, significant and marginally significant positive findings, significant and marginally significant negative findings.
Statistical analysis
Meta-analysis
Of the 64 studies reviewed, 40 were appropriate for inclusion in the meta-analysis. Reference Akçakin and Polat10–Reference Williams, Oliver, Allard and Sears49 Twenty-four studies were excluded from the meta-analysis because they did not report relative risks and confidence intervals or did not provide information needed to calculate them. A separate meta-analysis was conducted for each exposure variable that was examined in two or more studies. For each exposure, a summary effect estimate was calculated using a random-effects model. Reference DerSimonian and Laird74 Because power to detect heterogeneity is low in meta-analyses such as these, Reference Takkouche, Cadarso-Suarez and Spiegelman75 we took a conservative approach and used random-effects models to form confidence intervals, because random-effects models account for any observed heterogeneity regardless of whether the heterogeneity is statistically significant. When available, the estimate used for each study was the multivariate estimate controlling for the maximum number of covariates.
If an effect estimate was reported without the corresponding 95% CI, the confidence bounds were derived from the P-value provided. If no P-value was provided, then a P-value of 0.05 or 0.50 was assumed for factors that did and did not reach statistical significance respectively.
Several studies included autism-spectrum disorders in their case definition. Five studies reported results for both the broader phenotype and for narrowly-defined autism, Reference Eaton, Mortensen, Thomsen and Frydenberg22,Reference Gillberg25–Reference Guillem, Cans, Guinchat, Ratel and Jouk27,Reference Juul-Dam, Townsend and Courchesne29 in which case the study-specific exposure effect estimates using the narrowest diagnostic criteria were recorded.
The relationships between autism and maternal/paternal age at birth as well as birth order were assessed categorically and meta-analytic tests of trend (details available from the authors on request) Reference Greenland and Longnecker76 were conducted using ordinal categorical variables with the score of each category equal to the mid-point of the exposure range, using SAS version 9 on UNIX (SAS Institute, Cary, NC). These trend tests were restricted to studies that provided information on the number of cases and participants at each exposure level.
As a result of the rarity of many of the exposures and small sample sizes, there were tables in some (<5%) of the meta-analyses with zero cell counts. In these instances, 0.5 was added to each cell of the 2 × 2 table. Reference Sterne, Bradburn, Egger, Egger, Smith and Altman77
Several studies used multiple control groups (e.g. individuals with intellectual disability (also known as mental retardation) and healthy controls). In these studies, the comparison groups were pooled and compared with the cases as a single group.
Some studies classified the exposures of interest into distinct subcategories (e.g. bleeding by trimester). In addition to providing a summary estimate for the primary exposure of interest (e.g. pregnancy bleeding), we also calculated summary estimates for each subcategory. If only the crude estimates were provided then the exposures were pooled by simply adding the cases–controls who experienced each subcategory type. If multivariate adjusted estimates were provided then the adjusted estimates for each exposure subcategory were combined using the method proposed by Greenland & Longnecker Reference Greenland and Longnecker76 to adjust the variance of the summary estimate by accounting for the covariance due to the inclusion of overlapping comparison groups across exposure subcategories.
Meta-regression
For each risk factor assessed in multiple studies we examined the heterogeneity in the relative risks estimated across studies using the Q statistic. Reference DerSimonian and Laird74,Reference Higgins, Thompson, Deeks and Altman78 As a result of the limited power of this test Reference Takkouche, Cadarso-Suarez and Spiegelman75 a liberal P of <0.10 was used to identify meta-analyses that required further examination to assess potential sources of heterogeneity. If we found evidence of suggested heterogeneity, a meta-regression Reference Sharp79,Reference Stram80 was conducted to identify measured methodological factors that could explain the between-study variability (i.e. between-study effect modification).
The analyses of effect modification were conducted using the ‘metareg’ command in Stata 8 on Windows. Reference Sharp79 The study characteristics that were examined included: diagnostic criteria (inclusion of spectrum disorders: yes v. no); exposure information quality (0, retrospective exposure assessment; 1, mix of retrospective and prospective exposure assessment; 2, prospective exposure assessment); control for confounding (0, univariate analysis; 1, control for select demographic factors, birth order, or IQ; 2, full multivariate analysis or matching with sibling controls); normal v. abnormal controls; and case selection (clinic based v. population based). If effect modification was suggested for a given study characteristic (P<0.10), then a stratified analysis was performed.
Publication bias was assessed for each factor by conducting tests for funnel plot asymmetry Reference Khoshdel, Attia and Carney81 using the ‘metabias’ command in Stata 8. Two statistical approaches were used to examine the association between study size and the effect of the exposure: the Begg test Reference Begg and Mazumdar82 and the Egger test. Reference Egger, Smith, Schneider and Minder83
Results
Table 1 and Table 2 list the prenatal factors that were not included in the meta-analysis due to unavailability of two or more effect estimates and 95% CIs, as well as an indication of whether they were associated with autism in the studies in which they were examined. Online Table DS1 lists the prenatal factors included in the meta-analyses, as well as the number of null findings, significant and marginally significant positive findings, and significant and marginally significant negative findings (protective association). For each factor that was examined in the meta-analysis, online Table DS1 reports the summary effect estimate and 95% CI from the random-effects model, and the P-value for the test of heterogeneity.
Table 1 Pregnancy-related risk factors examined in only one study and not eligible for meta-analysis

| Association with autism | Risk factor |
|---|---|
| None | Chronic maternal disease, maternal cytomegalovirus, autoimmune disease, severe cholecystitis, endocrine diseases, venous thrombosis, infertility requiring medical intervention, previous live births now dead, frequency of intercourse during pregnancy, irregular menstrual periods, maternal immunisation, maternal transfusions, previous X-rays, chorionic villi sampling, amniocentesis, pre-pregnancy body mass index, drug use during pregnancy, fetal oxygenation, maternal age at first birth 30+, father with foreign citizenship |
| Positive | Maternal asthma, allergies, maternal toxaemia or bleeding, prenatal stressors, month prenatal care began, urbanisation of birth place |
| Negative | Maternal alcohol use during pregnancy |
Table 2 Pregnancy-related risk factors examined in multiple studiesa but not eligible for meta-analysis
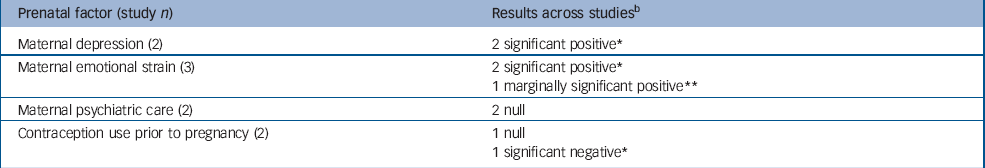
| Prenatal factor (study n) | Results across studiesb |
|---|---|
| Maternal depression (2) | 2 significant positive* |
| Maternal emotional strain (3) | 2 significant positive* |
| 1 marginally significant positive** | |
| Maternal psychiatric care (2) | 2 null |
| Contraception use prior to pregnancy (2) | 1 null |
| 1 significant negative* |
The meta-analysis found few statistically significant risk factors. Maternal gestational diabetes was associated with a two-fold increased risk of autism. In addition, a significant 81% elevated risk was observed in relation to maternal bleeding during pregnancy. Maternal medication use was also associated with a 46% increased risk. Although 15 studies examined the relationship between prenatal medication use and risk of autism, the majority studied the general use of any medications during pregnancy, whereas only a few examined the association with specific classes of medications. A meta-analysis of the two studies that looked specifically at psychiatric medication use during pregnancy suggested a significant positive association with the risk of autism (RR = 1.68).
Maternal age at birth over 30 was associated with an increased risk with effect estimates ranging from a 27% increased risk (30–34 v. 25–29) to a 106% increase in risk (40+ v. <30). Thirteen studies were included in the meta-analyses of maternal age at birth. The trend test included nine studies and indicated a significant increase in risk of autism with increasing maternal age at birth (trend P = 0.02). A 5-year increase in maternal age was associated with a 7% increase in risk.
Increased paternal age at birth was also found to be a significant risk factor (trend P = 0.004), with a 5-year increase in paternal age associated with a 3.6% increase in risk. Individual exposure category effect estimates ranged from 1.24 (30–39 v. <30) to 1.44 (40+ v. 25–29). In addition, the three studies that examined the effect of young paternal age at birth indicated a 26% decrease in risk for paternal age <25 v. 25–29. Only four studies were included in the meta-analyses of paternal age.
Of the nine studies that indicated a significant relationship between birth order/parity and risk of autism, six indicated a mixed trend. Specifically, autism was associated with being first or later born (≥third), often depending on the size of the sibship. The meta-analysis found a statistically significant 61% increase in risk for first-born children compared with children born third or later. This meta-analysis included four studies. No significant associations were observed in the comparisons of other birth order categories and the trend test did not indicate a linear relationship between birth order and autism risk.
Maternal birth abroad was marginally associated with risk of autism. In the five studies included in the meta-analysis, maternal birth abroad was associated with a 28% increased risk (P = 0.06). However, the definition of ‘abroad’ varied as the studies were conducted in different countries and areas of the world. In the studies conducted in Nordic countries, a statistically significant 58% increased risk of autism was observed among the offspring of mothers born abroad.
Heterogeneity in effect estimates across studies was observed for the following factors (P<0.10): infections during pregnancy, nausea/vomiting, bleeding, weight gain, maternal age at birth, paternal age at birth (40+ v. <30), birth order, smoking during pregnancy, mother born abroad and pre-eclampsia. Table 3 shows the results of the regression analyses that examined the potential between-study sources of heterogeneity.
Table 3 Analysis of effect modification by study characteristics: prenatal risk factors with heterogeneity (P<0.10)

| Prenatal risk factors | Significant sources of between-study heterogeneity: study characteristics (P<0.10)a | Summary effect estimate (95% CI) |
|---|---|---|
| Infections during pregnancy | Multivariate v. univariate analysis (P = 0.09) | 1.18 (0.76–1.83) |
| 4 studies: controlled for multiple covariates | 1.82 (1.01–3.30) | |
| 7 studies: no control for covariates | 0.89 (0.56–1.42) | |
| Nausea/vomiting | Exposure data collection (P = 0.004) | 1.16 (0.65–2.09) |
| 3 studies: prospective | 1.48 (1.03–2.14) | |
| 3 studies: retrospective | 0.55 (0.31–0.98) | |
| Maternal age: linear trend | None | 1.07 (1.01–1.13) |
| Birth order: linear trend | None | 0.95 (0.89–1.02) |
| Smoking during pregnancy | Population-based (P = 0.06) | 1.00 (0.75–1.36) |
| 3 studies: population based | 1.15 (0.90–1.47) | |
| 2 studies: clinic based | 0.63 (0.37–1.08) | |
| Mother born in another country | None | 1.28 (0.99–1.65) |
| Bleeding | None | 1.81 (1.14–2.86) |
| Toxaemia/pre-eclampsia, hypertension, swelling | None | 1.01 (0.80–1.27) |
The analysis of infections during pregnancy indicated significant effect modification based on control for covariates. Exposure to intrauterine infections was associated with a significant increase in risk for autism in the analysis limited to the four studies that controlled for multiple covariates or used sibling controls. However, there was no relationship between infections during pregnancy and autism in the studies that did not control for covariates or use sibling controls. For nausea/vomiting, there was significant effect modification based on whether the exposure was assessed prospectively or retrospectively. The positive relationship between nausea/vomiting and autism was only significant among prospective studies (RR = 1.48, 95% CI 1.03–2.14). In fact, the meta-analysis restricted to the three retrospective studies that examined nausea/vomiting in relation to autism suggested a protective association (RR = 0.55, 95% CI 0.31–0.98).
The test for linear trend in birth order indicated significant heterogeneity across studies that could not be explained by variation in any of the study characteristics examined. The analyses of several maternal age at birth comparisons as well as the linear trend test also indicated heterogeneity in the effect estimates across studies. Variation in the methodological characteristics could not explain the heterogeneity in the trend estimates. However, heterogeneity in the effect estimates for the maternal age categorical comparisons may have been as a result of the control for covariates. In general, the elevation in risk observed in relation to older maternal age at birth was slightly attenuated in the studies that controlled for multiple covariates.
Heterogeneity in the effect estimates for maternal smoking during pregnancy may have been as a result of the study base (population based or clinic based). No significant relationship with autism was observed overall or within strata, although only five studies were included in this meta-analysis.
Lastly, for the analyses of toxaemia/pre-eclampsia (17 studies), maternal birth abroad (5 studies) and bleeding (13 studies), the heterogeneity of effect estimates across studies could not be explained by any of the study characteristics investigated.
Publication bias was assessed for all factors examined in three or more studies. Significant publication bias was only suggested for smoking during pregnancy (Begg's test P = 0.03, Egger's test P = 0.04). The test for publication bias for prenatal smoking in fact indicated a potential bias in the direction of publishing inverse associations, as suggested by the fact that the three (out of five) smaller studies in the meta-analysis all reported relative risks that were below the null. Both of the tests for publication bias lacked power because of the small number of studies included in each meta-analysis. Reference Sterne, Gavaghan and Egger84 However, as a result of the many tests of publication bias performed it is likely that we would observe one or more significant results due to chance alone.
Several studies examined the relationship between compromised prenatal health in general and risk of autism, although none provided the necessary data for inclusion in the meta-analysis. Specifically, six studies utilised prenatal optimality scales to assess the number of prenatal complications experienced in cases and controls (Gillberg Optimality Scale, Reference Gillberg and Gillberg55,Reference Lord, Mulloy, Wendelboe and Schopler61 modified Gillberg Optimality Scale, Reference Piven, Simon, Chase, Wzorek, Landa and Gayle41,Reference Bryson, Smith and Eastwood53 Lewis-Murray Scale, Reference Stein, Weizman, Ring and Barak44 Rochester Research Obstetrical Scale Reference Links, Stockwell, Abichandani and Simeon60 ). Four of these studies reported a significant association between reduced prenatal optimality and risk of autism. Reference Bryson, Smith and Eastwood53,Reference Gillberg and Gillberg55,Reference Links, Stockwell, Abichandani and Simeon60,Reference Lord, Mulloy, Wendelboe and Schopler61
Discussion
This study is the first meta-analysis of the relationship between prenatal factors and risk of autism. Over 50 prenatal factors have been studied in relation to autism in 64 epidemiological studies, of which 40 were eligible for meta-analysis. However, few factors have been examined in multiple well-conducted studies. Therefore, attempted replication in methodologically strong studies remains necessary. Although the majority of factors examined in multiple studies have given inconsistent results, the preponderance of findings overall have not been statistically significant. The factors with the strongest evidence for an association with autism risk included advanced maternal and paternal age at birth, maternal gestational bleeding, gestational diabetes, being first born v. third or later, maternal prenatal medication use and maternal birth abroad. The factors with the strongest evidence against a role in autism risk included previous fetal loss and maternal pre-eclampsia, proteinuria, hypertension and swelling.
Although there is insufficient evidence to implicate any one prenatal factor in autism aetiology, the studies using prenatal optimality scales provide some evidence to suggest that exposure to pregnancy complications in general may increase the risk of autism. It is also important to note that the aetiological importance of the prenatal period may not be fully captured by examining only those complications and characteristics that are manifested and observed during the period of gestation. Many perinatal and neonatal complications also reflect what was occurring during pregnancy, and it may be that only those compromises to the prenatal environment that are manifested in labour and delivery as well as neonatal health complications are aetiologically relevant. The potential effects of a non-optimal prenatal environment as manifested in perinatal and neonatal complications will be addressed in our subsequent manuscript on this topic.
Parental age
The current meta-analysis shows that increased maternal and paternal age at birth are both associated with an elevated risk of autism. The biological mechanisms underlying these relationships are not known. Maternal age may be associated with autism because of the increased risk of chromosomal abnormalities in ova of increased age or as a result of unstable trinucleotide repeats. Reference Kolevson, Gross and Reichenberg9 Although advanced maternal age has been shown to be associated with an increased risk of obstetrical complications, Reference Rosenthal and Paterson-Brown85,Reference Ezra, McParland and Farine86 it is unknown which, if any, of these complications may affect the risk of autism. Reichenberg et al Reference Reichenberg, Bresnahan, Rabinowitz, Lubin and Davidson42 suggested that the relationship between paternal age and autism may be because of imprinted genes, de novo spontaneous mutations that accumulate with advancing age in spermatagonia or confounding by sociocultural environmental factors. Maternal and paternal age at birth are likely correlated Reference Kazmaura and Lie87,Reference Tang, Wu, Liu, Lin and Hsu88 and many of the studies included did not adjust paternal age for maternal age and vice versa. It is possible that advanced age of both parents plays a role in the susceptibility to autism or perhaps only maternal age or paternal age is aetiologically relevant. There is evidence to suggest that paternal age may be more important. Of the four studies that controlled for the age of the co-parent, three found only a significant association for paternal age at birth, Reference Larsson, Eaton, Madsen, Vestergaard, Olesen and Agerbo33,Reference Lauritsen, Pedersen and Mortensen34,Reference Reichenberg, Bresnahan, Rabinowitz, Lubin and Davidson42 and one found only a significant association for maternal age. Reference Maimburg and Vaeth38 When the analysis of maternal age was restricted to the four studies that controlled for paternal age the relative risk for a 5-year increase in maternal age was 1.06 (P = 0.08). All studies of paternal age included in the meta-analysis were adjusted for maternal age.
Birth order
Perhaps the factor that was most commonly associated with the risk of autism in the literature was birth order. Nine studies reported a significant relationship between birth order/parity and autism. However, the nature of the relationship was inconsistent across studies and was generally not found to be linear. The difficulty in elucidating the relationship between birth order/parity and autism may be as a result of potential effect modification by sibship size, as individuals with autism are more likely to be first-born in sibship sizes of two and later-born in families with larger sibship sizes. Reference Lord, Mulloy, Wendelboe and Schopler61,Reference Tsai and Stewart69 The latter trend has been attributed to parents deciding not to have additional children after one has developed autism. Reference Jones and Szatmari89
Maternal birth abroad
Maternal immigration has also been highlighted as a potential risk factor for autism. Reference Kolevson, Gross and Reichenberg9 In the meta-analysis, the elevated risk of autism among the offspring of women born abroad was just shy of statistical significance. In the three studies conducted in Nordic countries there was a significant 58% increased risk among the offspring of mothers born abroad, although the definition and categorisation of ‘abroad’ differed across the studies. The strength of the association in the Nordic studies may be because of an unknown mechanism particular to this area, or, perhaps more likely, may have been as a result of the methodological strengths of these three studies.
Several hypotheses have been postulated, including the idea that fathers with social disability potentially as a result of a genetic mechanism associated with autism may be less able to find a spouse from their own country and may therefore find a wife from a foreign country with whom to have children. Reference Gillberg, Schaumann and Gillberg90 More likely, Gillberg et al Reference Gillberg, Schaumann and Gillberg90 suggested that women born in another country may not be immunised against the common infectious agents in the country in which she gives birth and may therefore be more susceptible to relatively innocuous infections that may increase the risk for autism. Other possible explanations include a potential role of maternal stress because of the demands of residing in a new country, particularly with limited social support, or stress resulting from the experience of emigrating, perhaps as a result of economic or social factors. These hypotheses do not explain the relationship with maternal place of birth seen in a cohort study of children born in California between 1989 and 1994, Reference Croen, Grether and Selvin16 which showed a 40% decreased risk of autism among the children of women born in Mexico as compared with California. The association between maternal immigration and autism risk requires further examination in other areas of the world to examine whether the relationship can truly be generalised.
Gestational bleeding
Fetal hypoxia may underlie a potential relationship between gestational bleeding and autism. Maternal bleeding is one of several complications believed to be associated with fetal hypoxia. Reference Kolevson, Gross and Reichenberg9 Fetal distress, maternal hypertension, prolonged labour, cord complications, low Apgar score and Caesarean delivery are other pregnancy-related factors that are believed to be related to hypoxia and have been associated with autism risk in some, but not all, studies. Although some brain abnormalities observed in individuals with autism may reflect a potential role of oxygen deprivation during development, this possibility requires further examination. Hypoxia has also been shown to increase dopaminergic activity, and there is evidence for dopamine overactivation in autism. Reference Previc91
Bleeding in the second half of pregnancy in particular may reflect severe complications including placenta praevia or abruptio placenta. Reference Juul-Dam, Townsend and Courchesne29 Although the analyses stratified by trimester did not produce significant associations, only two studies were available to calculate the trimester-specific estimates.
Gestational diabetes
A biological mechanism underlying the potential elevated risk of autism associated with gestational diabetes is unknown. Gestational diabetes has been associated with various adverse pregnancy outcomes, Reference Ben-Haroush, Yogev and Hod92–Reference Eidelman and Samueloff94 and the hormonal and metabolic abnormalities and oxidative stress because of gestational diabetes may have lasting consequences for offspring health and development. Reference Ben-Haroush, Yogev and Hod92,Reference Biri, Onan, Devrim, Babacan, Kavutcu and Durak95 It is possible that the reported increasing maternal and paternal age at birth and rate of gestational diabetes may be contributing factors to the rising prevalence of autism. Reference Martin, Hamilton, Ventura, Menacker and Park96
Medication use
The mechanism underlying the suggested association with maternal medication use is also unclear because of the variety of medications consumed during pregnancy and assessed in these studies. Although many medications may cross the placenta and affect fetal development, the current analysis cannot indicate which medications may be detrimental. However, the meta-analysis of two studies that looked at psychiatric medication use suggested a significant 68% increased risk of autism, and one small Croatian study Reference Kocijan-Hercigonja, Remeta, Orehovac and Brkljacic32 suggested a higher frequency of hormone use among the mothers of individuals with autism than among the mothers of controls with intellectual disability (mental retardation). Maimburg & Vaeth Reference Maimburg and Vaeth38 found a 50% increased risk of autism associated with maternal use of medicine in a population-based case–control study using Danish national registries. Although they observed no significant association for anti-epileptics, antihypertensives, cardiovascular drugs, tocolytics, nor use of steroids, a significant 60% increased risk of autism was observed in relation to use of psychoactive drugs. The association with maternal use of psychoactive drugs may reflect either an effect of the medication exposure, an adverse effect of the actual treated condition itself on fetal development (confounding by indication) or transmission of genetic traits possibly shared between autism and other psychiatric disorders.
Non-causal hypotheses
Investigators have questioned the causal nature of the observed relationship between prenatal complications and autism. Confounding by birth order has been suggested, as an increased risk of autism and obstetrical complications are often observed in first-, fourth- and later-born offspring. Reference Bolton, Murphy, Macdonald, Whitlock, Pickles and Rutter52,Reference Zwaigenbaum, Szatmari, Jones, Bryson, MacLean and Mahoney73 Although some studies have shown that associations were attenuated and no longer significant after adjusting for parity, Reference Piven, Simon, Chase, Wzorek, Landa and Gayle41,Reference Lord, Mulloy, Wendelboe and Schopler61 other studies have shown that the positive relationship persists. Reference Bolton, Murphy, Macdonald, Whitlock, Pickles and Rutter52,Reference Zwaigenbaum, Szatmari, Jones, Bryson, MacLean and Mahoney73 A second non-causal hypothesis is that obstetrical complications occur as a result of the autistic condition in the offspring or as a consequence of other factors (e.g. genetic factors) that are the true causal determinants of autism. Reference Bolton, Murphy, Macdonald, Whitlock, Pickles and Rutter52 In this epiphenomena explanation, pregnancy complications simply reflect the abnormalities of autistic fetal development, or the same familial factors cause both autism and obstetrical complications. The study conducted by Bolton et al Reference Bolton, Murphy, Macdonald, Whitlock, Pickles and Rutter52 provided strong evidence in support of the shared risk hypothesis, as there was an association between obstetric suboptimality and measures of autism severity and familiality and the obstetric suboptimality scores in the individuals with autism were highly correlated with that of their affected siblings. In addition, probands with increased obstetric complications had more extended family members with the broader autism phenotype, although this finding was not replicated in a second study by Zwaigenbaum et al. Reference Zwaigenbaum, Szatmari, Jones, Bryson, MacLean and Mahoney73 The shared risk hypothesis was also supported by the findings in the Zwaigenbaum et al study that indicated more obstetric adversity among unaffected siblings of children with pervasive developmental disorders that had high familial loading for the broader autism phenotype. Reference Zwaigenbaum, Szatmari, Jones, Bryson, MacLean and Mahoney73
Limitations
Methodological limitations that have impaired the precision and validity of results include small sample size, otherwise affected control groups (e.g. Down syndrome), broad disease definition, and retrospective parental recall of exposures. Of the 64 studies included in the review, only 19 had over 80% power to detect a relative risk of 2 for an exposure with 10% prevalence. Nineteen of the studies used broad diagnostic criteria resulting in the possible inclusion of individuals with other autism-spectrum disorders, which may limit the ability to detect associations due to aetiological heterogeneity. Twenty-one studies assessed the exposure variables retrospectively resulting in the high possibility of recall bias. However, the use of medical records also has the limitation of being incomplete. Lastly, the majority of studies included only univariate analyses and did not assess potential confounding. These methodological weaknesses were also likely sources of heterogeneity of effects across studies. Although significant heterogeneity was observed for few factors, the test of heterogeneity lacked power because the majority of the meta-analyses conducted were able to include fewer than six studies and therefore variability in study characteristics was lacking.
This meta-analysis has a few limitations. First, only published data were used. Second, of the 64 studies reviewed, only 40 reported the data necessary for inclusion in the meta-analysis. Within these 40 studies the investigators did not report the necessary data for a meta-analysis on all factors examined. Although 40 studies were included in the meta-analysis overall, for each factor there were generally fewer than six studies included, limiting the statistical power to detect heterogeneity across studies and potential effect modification by study characteristics. Third, as a result of the rarity of many of the exposures examined and the small sample sizes in many studies, there were instances of zero cell counts within studies. The relatively small addition of 0.5 to the cell counts may have had an impact on the overall results because of the small sample sizes. Fourth, a few studies only reported an effect estimate and an indication of whether the results were statistically significant. In these cases, the confidence intervals were estimated based on assumptions regarding the actual P-value (P = 0.05 if significant, P = 0.50 if not significant). In the case of statistically significant findings, these assumptions resulted in conservative estimates of the true confidence intervals. Fifth, the tests of publication bias were underpowered because of the limited number of studies in each meta-analysis. Lastly, many studies simply examined all available prenatal data using designs with methodological weaknesses and without a priori hypotheses or knowledge about reproductive epidemiology. As a result, significant associations observed because of chance are possible in this meta-analysis.
The current review and meta-analysis was not restricted to studies with particular methodological strengths. In addition, individual study characteristics were examined in meta-regressions rather than assigning studies aggregate quality scores. These strategies are consistent with the recommendations proposed by the ‘Meta-Analysis of Observational Studies in Epidemiology Group’ that advocated the use of broad inclusion criteria for studies along with regression analyses to relate specific study design characteristics to outcome. Reference Stroup, Berlin, Morton, Olkin, Williamson and Rennie97 This maximises the amount of data available for review. In addition, different methodological considerations are relevant for each exposure. However, the increased probability for heterogeneity of results using the broad inclusion criteria is important to note.
Twin studies and family aggregation studies have provided clear evidence for the important role of genetics in autism aetiology. Reference Newschaffer, Fallin and Lee6 The difficulty in identifying environmental risk factors is likely a result of the complex interactions between these factors and genetics in determining disease susceptibility and the methodological considerations detailed above. Future investigations of prenatal exposures should also collect DNA to study potential gene–environment interactions.
Autism is a devastating condition with no known cure. The rising prevalence, coupled with the severe emotional and financial impact on the families, underscores the need for large, prospective, population-based studies with the goal of elucidating the modifiable risk factors, particularly those during the prenatal period.
Acknowledgements
We thank Ruifeng Li, MS (Harvard School of Public Health, Department of Biostatistics) for providing additional statistical and programming support. We also thank Alberto Ascherio, MD, DrPH (Harvard School of Public Health, Departments of Epidemiology and Nutrition; Channing Laboratory, Department of Medicine, Brigham and Women's Hospital and Harvard Medical School) and Janet Rich-Edwards, ScD (Harvard School of Public Health Department of Epidemiology; Harvard Medical School) for their help and guidance in reviewing the results and manuscript. We thank Dr. Tatjana Rundek, Yueh-Hsiu Mathilda Chiu, and Handan Wand for their translations of articles published in languages other than English.






eLetters
No eLetters have been published for this article.