Folate, vitamin B12, homocysteine and methylenetetrahydrofolate reductase (MTHFR) are involved in one-carbon transfer (methylation) reactions necessary for the production of monoamine neurotransmitters, phospholipids and nucleotides. Folate and vitamin B12 deficiency, hyperhomocysteinaemia and the T677 allele of the MTHFR gene, which cause impaired methylation reactions in the central nervous system, have been associated with depressive disorders. Reference Bjelland, Tell, Vollset, Refsum and Ueland1,Reference Bottiglieri, Laundy, Crellin, Toone, Carney and Reynolds2 However not all studies have found such associations. Reference Almeida, Flicker, Lautenschlager, Leedman, Vasikaran and van Bockxmeer3,Reference Tiemeier, van Tuijl, Hofman, Meijer, Kiliaan and Breteler4 Discrepant findings in previous studies may relate to their cross-sectional design. In particular, changes in appetite and diet associated with depressive states may affect nutritional status, so that the direction of cause and effect remains unclear. To address this limitation, we analysed data from a 2-year longitudinal study to investigate both cross-sectional and prospective associations between these factors and depression in late life.
Method
A secondary analysis was made of data from a community-based prospective survey of late-life psychiatric morbidity conducted in Kwangju, Republic of Korea, from 2001 to 2003, in collaboration with the 10/66 International Dementia Research Program in Developing Countries. Reference Prince, Acosta, Chiu, Scazufca and Varghese5 All participants gave written, formal informed consent at each examination. This study was approved by the Chonnam National University Hospital institutional review board.
Baseline sample and measurements
A cross-sectional survey of a geographically defined population was carried out in 2001. The sampling procedure and measurements have been described previously. Reference Kim, Stewart, Shin and Yoon6 In brief, 732 community residents aged 65 years or over within two catchment areas of Kwangju were recruited from national residents' registration lists (5% refusal rate). Examinations included a fully structured diagnostic interview for depression; blood samples taken for folate, vitamin B12, homocysteine and MTHFR genotype; and formal assessment of potential confounding factors.
Depression
Depression was assessed using the community version of the Geriatric Mental State (GMS) schedule. Reference Copeland, Dewey and Griffiths-Jones7 This is a fully structured diagnostic instrument in wide international use with an accompanying computerised algorithm which provides likelihoods of individual diagnoses on a scale of 0 to 5. The GMS was translated into Korean according to a formal standardisation process. Reference Kim, Stewart, Prince and Yoon8 As in other studies, a ‘stage one’ (non-hierarchical) confidence level of 3 or above from the Automated Geriatric Examination for Computer Assisted Taxonomy (AGECAT) algorithm was used to define depression. The instrument and algorithm are designed to define current depression present at a level of severity warranting clinical intervention, and focus on the month preceding the interview. Case-level depression encompasses both moderate and severe symptoms, and is therefore a broader syndrome than DSM–IV major depression. The 0–5 AGECAT confidence score at baseline was included as a covariate in secondary analysis to allow exploratory adjustment for depressive symptom severity as an ordinal rather than a binary measure.
Blood samples and biochemical analyses
Blood samples were collected from the participants in a fasting state and were taken in the morning where possible. The samples were drawn into tubes of ethylenediamine tetra-acetic acid (EDTA), centrifuged, separated into plasma aliquots and stored at −70°C within 2 h of collection. Biochemical assays were carried out after 3 years. Serum folate and vitamin B12 levels were determined using an immunoassay, and total plasma homocysteine level was measured by high-performance liquid chromatography. The MTHFR C677T genotype was determined by a polymerase chain reaction (PCR) and HinFI restriction enzyme digestion as described previously, Reference Frosst, Blom, Milos, Goyette, Sheppard, Matthews, Boers, den Heijer, Kluijtmans, van den Heuvel and Rozen9 with minor modification: HinFI digestion (1.5 U per 25 μl reaction mixture) was performed directly in the PCR tube at 37°C for 4 h before analysis of restriction fragments by polyacrylamide gel electrophoresis. Allele frequencies were estimated by gene counting and observed numbers of each genotype were compared with those expected under Hardy–Weinberg equilibrium.
Other measurements
Age, gender and education of the participants were recorded. Cognitive function was evaluated by the Korean version of the Mini-Mental State Examination (MMSE). Reference Park and Kwon10 Disability was assessed by means of the Korean version of the World Health Organization Disability Assessment Schedule II (WHODAS–II). Reference Kim, Stewart, Glozier, Prince, Kim, Yang, Shin and Yoon11 Smoking history and current smoking status were ascertained. A lifetime history of alcohol consumption was obtained from the participants, and corroboration from family members was sought. Problem drinking was defined on the basis of consumption over the previous 3 months of more than 14 alcoholic drinks per week for men or more than 7 drinks per week for women, in accordance with guidelines from the National Institute of Alcohol Abuse and Alcoholism. 12 Daily physical activity, taking into account both work and leisure activity, was ascertained and sedentary lifestyle was defined as a binary variable. For vascular risk factors and disorders a summary ‘vascular risk’ score was developed from summing self-reported disorders (stroke, heart disease, hypertension, diabetes), measured obesity (body mass index >25 kg/m2) and hypercholesterolaemia (fasting cholesterol >5.1 mmol). Serum creatinine level was also assayed, since impaired renal function may elevate serum metabolite levels independent of vitamin intake.
Follow-up evaluation
Follow-up was carried out in 2003. Reference Kim, Stewart, Kim, Yang, Shin and Yoon13 The mean follow-up period was 2.4 years (s.d.=0.3). Attempts were made to follow up all previous participants. Identical procedures were used to identify depression (GMS–AGECAT) and further blood samples for folate, vitamin B12 and homocysteine were collected, centrifuged within 1 h and stored at −70°C. Assays were done after 1 year. Vitamin supplementation was investigated in the context of an inventory taken of all prescription and non-prescription medication taken in the past month.
Statistical analysis
Statistical analyses were carried out using SPSS version 12.0 for Windows. Associations between baseline depression and baseline quintiles of folate, vitamin B12 and homocysteine levels were assessed by χ2-tests (linear trend). Associations with demographic characteristics, assessment scales (MMSE and WHODAS–II), lifestyle characteristics (smoking, problem drinking and physical activity), vascular risk or disease and serum creatinine level were investigated using t-, χ2- or Mann–Whitney U-tests as appropriate. Odds ratios and their 95% confidence intervals were calculated for associations between baseline depression and baseline quintiles of folate, vitamin B12 and homocysteine, and for MTHFR genotype, in logistic regression models after adjustment for the other independent variables. For all analyses, quintiles of folate, vitamin B12 and homocysteine were entered as ordinal variables with one degree of freedom, in accordance with an a priori hypothesis that that associations, if present, would show linearity across the distributions.
For investigating prospective associations, participants with case-level depression at baseline were excluded, and case-level depression at follow-up (incident depression) was treated as the dependent variable. Associations between incident depression and baseline quintiles of folate, vitamin B12 and homocysteine were estimated in logistic regression models both before and after adjustment for relevant factors including vitamin supplementation. In a secondary analysis, associations between baseline folate, vitamin B12 and homocysteine levels and depression at follow-up were recalculated for the total followed-up sample and then further adjusted for baseline depression scale score (AGECAT 0–5 scale). Further exploratory analyses for the incident depression analysis were carried out to investigate effect modification of the three exposure–outcome associations by gender and MTHFR genotype, particularly in view of previous findings that the impact of folate deficiency may be modified in this respect. Reference Bjelland, Tell, Vollset, Refsum and Ueland1,Reference Lewis, Lawlor, Davey Smith, Araya, Timpson, Day and Ebrahim14
In participants without depression at baseline, change in levels of folate, vitamin B12 and homocysteine over the follow-up were calculated and re-categorised by quintiles. Associations between changes in these levels and incident depression were calculated and investigated further in identical logistic regression models to the analyses of baseline levels as predictors.
Final analyses were carried out to investigate associations with standard categories of folate and vitamin B12 deficiency and hyperhomocysteinaemia. Reference Tiemeier, van Tuijl, Hofman, Meijer, Kiliaan and Breteler4,Reference Penninx, Guralnik, Ferrucci, Fried, Allen and Stabler15 Folate deficiency was defined on the basis of levels below 11.4 nmol/l and a homocysteine level higher than 13.9 μmol/l; vitamin B12 deficiency as a level less than 258 pmol/l; and hyperhomocysteinaemia as a plasma level above 15.0 μmol/l.
Results
Participants' characteristics at baseline
Of 732 participants at baseline, case-level depression was present in 101 (13.8%). Mean levels of folate, vitamin B12 and homocysteine for the total sample were 24.4 nmol/l (s.d.=12.5), 385.6 pmol/l (s.d.=168.3) and 12.8 μmol/l (s.d.=5.7) respectively. Frequencies of the MTHFR allele were C 0.45 and T 0.55, and the genotype distribution was C/C 18.7%, C/T 52.7% and T/T 28.6% (test for Hardy–Weinberg equilibrium: χ2=2.091, P>0.05). Folate level was correlated positively with vitamin B12 level (r=0.112, P=0.002) and negatively with homocysteine level (r=–0.310, P<0.001). Vitamin B12 level was negatively correlated with homocysteine level (r=–0.289, P<0.001). Homocysteine levels were significantly associated with MTHFR genotype, with mean levels of 12.1 μmol/l (s.d.=5.6), 12.4 μmol/l (s.d.=4.6) and 13.8 μmol/l (s.d.=7.2) for the C/C, C/T and T/T genotypes respectively (F=5.301, P=0.005). There was no association between MHTFR genotype and folate or vitamin B12 levels (all P values >0.1). Other characteristics of the sample and unadjusted associations with depression at baseline are summarised in Table 1.
Table 1 Baseline characteristics of the study sample

| Participants at baseline | Analysed participants at follow-up (n=521) | |||||||
|---|---|---|---|---|---|---|---|---|
| Total sample (n=732) | No depression (n=631) | Depression (n=101) | P a | |||||
| Demographic characteristics | ||||||||
| Age, years: mean (s.d.) | 72.8 (5.9) | 72.7 (5.8) | 73.7 (6.3) | 0.095 | 72.5 (5.5) | |||
| Female gender, n (%) | 432 (59.0) | 359 (56.9) | 73 (72.3) | 0.004 | 287 (55.1) | |||
| Education, years: median (IQR) | 1 (0–6) | 1 (0–6) | 0 (0–6) | 0.321 | 1 (0–6) | |||
| Assessment scales | ||||||||
| MMSE score: mean (s.d.) | 23.3 (5.0) | 23.5 (4.9) | 22.1 (5.5) | 0.006 | 23.7 (4.7) | |||
| WHODAS–II score: median (IQR) | 3.3 (0–9) | 2.8 (0–7) | 8.7 (2–20) | <0.001 | 2.4 (0–7) | |||
| Lifestyle characteristics | ||||||||
| Current smoking, n (%) | 294 (40.2) | 250 (39.6) | 44 (43.6) | 0.453 | 209 (40.1) | |||
| Current problem drinking, n (%) | 213 (29.1) | 185 (29.3) | 28 (27.7) | 0.743 | 154 (29.6) | |||
| Low physical activity, n (%) | 229 (31.3) | 176 (27.9) | 53 (52.5) | <0.001 | 139 (26.7) | |||
| Vascular risk score: median (IQR) | 1 (0–2) | 1 (0–2) | 2 (1–3) | <0.001 | 1 (0–2) | |||
| Serum creatinine, μmol/l: mean (s.d.) | 70.7 (26.5) | 70.7 (17.7) | 79.6 (61.9) | 0.075 | 70.7 (17.7) | |||
IQR, interquartile range; MMSE, Mini-Mental State Examination; WHODAS–II, World Health Organization Disability Assessment Schedule II
a. From t-test, χ2-test or Mann–Whitney U-test as appropriate
Of 631 participants without depression at baseline, 521 (83%) completed all evaluations at follow-up and formed the study sample. Of the remaining 110, contact could not be established with 58 (52%), 23 (21%) had died, 21 (19%) refused to participate and 8 (7%) were too unwell. Baseline characteristics of participants from the baseline non-depressed group who were followed up are displayed in the last column of Table 1. Between the participants and non-participants at follow-up, there was no substantial difference in any independent variable (all P values >0.06). Mean changes in levels from baseline to follow-up were as follows: folate −4.9 nmol/l (s.d.=12.1), vitamin B12 +48.0 pmol/l (s.d.= 139.7) and homocysteine +1.6 μmol/l (s.d.=5.0). Figure 1 summarises the prevalence and incidence of depression according to baseline levels of folate, vitamin B12 and homocysteine, and change in these levels over the follow-up period.

Fig. 1 Rates of prevalence (a) and incidence (b) of depression according to baseline levels of folate, vitamin B12 and homocysteine, and change in these levels over a 2-year follow-up period (c).
Baseline folate, vitamin B12 and homocysteine levels, and baseline depression
Depression at baseline was associated with lower levels of vitamin B12 (χ2=4.190, P=0.041) and higher levels of homocysteine (χ2=4.901, P=0.027), but was not significantly associated with folate levels (χ2=1.443, P=0.230) (Fig. 1). These findings persisted after adjustment for potential confounders (Table 2). Prevalence of depression by MTHFR genotype was 14.6% for C/C, 15.0% for C/T and 11.0% for T/T (χ2=1.191, P=0.275).
Table 2 Logistic regression models for the association between baseline folate, vitamin B12 and homocysteine levels and baseline depression (n=732)

| Odds ratio (95% CI) for depression per quintile change | |||||
|---|---|---|---|---|---|
| Folate (decrease) | Vitamin B12 (decrease) | Homocysteine (increase) | |||
| Unadjusted | 1.10 (0.95–1.27) | 1.17 (1.01–1.36) | 1.19 (1.02–1.38) | ||
| Model 1: adjusted for age, gender and education | 1.16 (0.99–1.37) | 1.21 (1.03–1.41) | 1.30 (1.10–1.53) | ||
| Model 2: model 1 plus MMSE and WHODAS–II | 1.18 (1.00–1.40) | 1.22 (1.04–1.44) | 1.33 (1.12–1.58) | ||
| Model 3: model 2 plus smoking, alcohol and activity | 1.15 (0.97–1.36) | 1.20 (1.02–1.42) | 1.31 (1.10–1.56) | ||
| Model 4: model 3 plus vascular risk score | 1.13 (0.95–1.34) | 1.21 (1.03–1.43) | 1.28 (1.07–1.52) | ||
| Model 5: model 4 plus serum creatinine | 1.12 (0.94–1.33) | 1.23 (1.04–1.45) | 1.25 (1.04–1.49) | ||
MMSE: Mini-Mental State Examination; WHODAS–II: World Health Organization Disability Assessment Schedule II
Baseline folate, vitamin B12 and homocysteine levels, and incident depression
Incident depression was associated with lower baseline levels of folate (χ2=6.701, P=0.010) and vitamin B12 (χ2=6.317, P=0.012) and higher baseline levels of homocysteine (χ2=5.335, P=0.021) (Fig. 1). Adjusted associations between these factors are displayed in Table 3. In summary, incident depression was associated with all three factors in the directions anticipated, with associations remaining significant after adjustment for other covariates (Table 3, model 6). When the three blood levels of interest were entered in combination (Table 3, models 7–9) the associations with lower folate and vitamin B12 were reduced only marginally when adjusted for each other, with larger reductions when adjusted for homocysteine. On the other hand, the association between raised baseline homocysteine level and incident depression was reduced substantially when adjusted for individual vitamin levels. Incident depression was not associated with MTHFR genotype (χ2=2.346, P=0.167). In a secondary analysis of the whole followed sample, the associations between folate, vitamin B12 and homocysteine, and depression at follow-up were not substantially changed when adjusted for baseline depression scale score (unadjusted odds ratios 1.24, 1.28 and 1.18 respectively, adjusted odds ratios 1.23, 1.27 and 1.18 respectively). In further exploratory stratified analyses, the association between descending folate and incident depression was significantly modified by MTHFR genotype: odds ratios for decreasing folate quintiles were 1.18 (95% CI 0.79–1.76), 1.22 (95 CI 0.86–1.73) and 1.85 (95% CI 1.14–3.00) within CC, CT and TT genotypes respectively (P=0.021 for statistical interaction). No significant interaction was found between MTHFR genotype and vitamin B12 or homocysteine as exposures, no significant gender interaction was found for any exposure and no significant two-way or three-way interactions were found between the three exposures of interest in predicting incident depression (data not shown). Among the 732 participants at baseline, a previous history of depression prior to age 60 years was reported by 16 (16%) of the 101 participants with current depression and by 17 (3%) of the remaining 631 participants. The findings of interest were not materially altered following restriction to those without a history of depression.
Table 3 Logistic regression models for the association between baseline folate, vitamin B12 and homocysteine levels, and incident depression over the 2-year follow-up period (n=521)
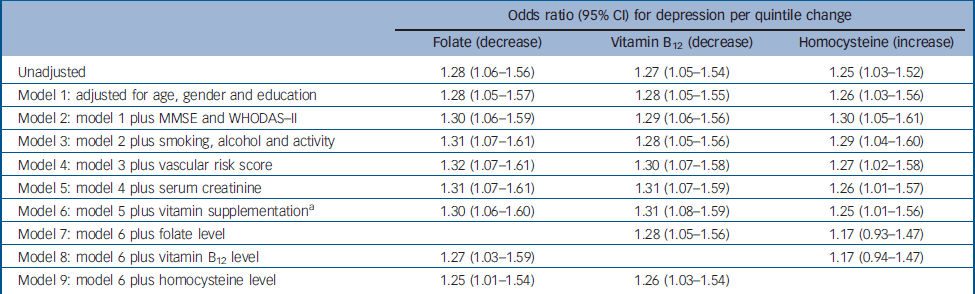
| Odds ratio (95% CI) for depression per quintile change | |||||
|---|---|---|---|---|---|
| Folate (decrease) | Vitamin B12 (decrease) | Homocysteine (increase) | |||
| Unadjusted | 1.28 (1.06–1.56) | 1.27 (1.05–1.54) | 1.25 (1.03–1.52) | ||
| Model 1: adjusted for age, gender and education | 1.28 (1.05–1.57) | 1.28 (1.05–1.55) | 1.26 (1.03–1.56) | ||
| Model 2: model 1 plus MMSE and WHODAS–II | 1.30 (1.06–1.59) | 1.29 (1.06–1.56) | 1.30 (1.05–1.61) | ||
| Model 3: model 2 plus smoking, alcohol and activity | 1.31 (1.07–1.61) | 1.28 (1.05–1.56) | 1.29 (1.04–1.60) | ||
| Model 4: model 3 plus vascular risk score | 1.32 (1.07–1.61) | 1.30 (1.07–1.58) | 1.27 (1.02–1.58) | ||
| Model 5: model 4 plus serum creatinine | 1.31 (1.07–1.61) | 1.31 (1.07–1.59) | 1.26 (1.01–1.57) | ||
| Model 6: model 5 plus vitamin supplementation a | 1.30 (1.06–1.60) | 1.31 (1.08–1.59) | 1.25 (1.01–1.56) | ||
| Model 7: model 6 plus folate level | 1.28 (1.05–1.56) | 1.17 (0.93–1.47) | |||
| Model 8: model 6 plus vitamin B12 level | 1.27 (1.03–1.59) | 1.17 (0.94–1.47) | |||
| Model 9: model 6 plus homocysteine level | 1.25 (1.01–1.54) | 1.26 (1.03–1.54) | |||
MMSE, Mini-Mental State Examination; WHODAS–II, World Health Organization Disability Assessment Schedule II
a. Vitamin supplementation ascertained at follow-up
Incident depression and co-occurring changes in folate, vitamin B12 and homocysteine levels
Incident depression was more frequent in people with a relative decline in vitamin B12 levels (χ2=5.735, P=0.017) and with a relative increase in homocysteine (χ2=6.594, P=0.010) (Fig. 1), whereas no association was found with change in folate levels (χ2=0.971, P=0.324). Adjusted associations between these factors are displayed in Table 4. The association between decline in vitamin B12 levels and incident depression remained strong after adjustment for other covariates, was increased in strength after adjustment for vitamin supplementation at follow-up, and was decreased in strength following adjustment for homocysteine change. The association between an increase in homocysteine levels and incident depression changed little following adjustment for all other covariates.
Table 4 Logistic regression models for the association between change in folate, vitamin B12 and homocysteine levels, and incident depression (n=521)
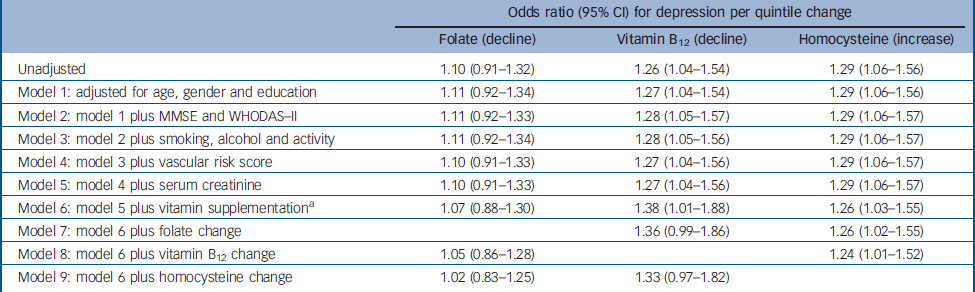
| Odds ratio (95% CI) for depression per quintile change | |||||
|---|---|---|---|---|---|
| Folate (decline) | Vitamin B12 (decline) | Homocysteine (increase) | |||
| Unadjusted | 1.10 (0.91–1.32) | 1.26 (1.04–1.54) | 1.29 (1.06–1.56) | ||
| Model 1: adjusted for age, gender and education | 1.11 (0.92–1.34) | 1.27 (1.04–1.54) | 1.29 (1.06–1.56) | ||
| Model 2: model 1 plus MMSE and WHODAS–II | 1.11 (0.92–1.33) | 1.28 (1.05–1.57) | 1.29 (1.06–1.57) | ||
| Model 3: model 2 plus smoking, alcohol and activity | 1.11 (0.92–1.34) | 1.28 (1.05–1.56) | 1.29 (1.06–1.57) | ||
| Model 4: model 3 plus vascular risk score | 1.10 (0.91–1.33) | 1.27 (1.04–1.56) | 1.29 (1.06–1.57) | ||
| Model 5: model 4 plus serum creatinine | 1.10 (0.91–1.33) | 1.27 (1.04–1.56) | 1.29 (1.06–1.57) | ||
| Model 6: model 5 plus vitamin supplementation a | 1.07 (0.88–1.30) | 1.38 (1.01–1.88) | 1.26 (1.03–1.55) | ||
| Model 7: model 6 plus folate change | 1.36 (0.99–1.86) | 1.26 (1.02–1.55) | |||
| Model 8: model 6 plus vitamin B12 change | 1.05 (0.86–1.28) | 1.24 (1.01–1.52) | |||
| Model 9: model 6 plus homocysteine change | 1.02 (0.83–1.25) | 1.33 (0.97–1.82) | |||
MMSE, Mini-Mental State Examination; WHODAS–II, World Health Organization Disability Assessment Schedule II
a. Vitamin supplementation ascertained at follow-up
Associations with clinical categories of folate and vitamin B12 deficiency and hyperhomocysteinaemia
The prevalence of baseline folate deficiency was 4.0%, vitamin B12 deficiency 16.8% and hyperhomocysteinaemia 22.1%. Odds ratios for associations with baseline depression were 1.32 (95% CI 0.49–3.54) for folate deficiency, 1.57 (95% CI 0.94–2.61) for vitamin B12 deficiency and 1.78 (95% CI 1.13–2.84) for hyperhomocysteinaemia. After adjustment for the other factors listed in Table 2, respective odds ratios were 1.86 (95% CI 0.59–5.80), 1.91 (95% CI 1.08–3.39) and 1.78 (95% CI 1.03–3.08). Respective odds ratios for incident depression adjusted for other covariates listed in Table 3 (model 6) were 1.94 (95% CI 0.58–6.47), 1.78 (95% CI 0.90–3.51) and 1.69 (95% CI 0.88–3.26).
Discussion
To our knowledge, this study is the first community-based, prospective investigation of associations between folate, vitamin B12, homocysteine and late-life depression. Principal findings were that incident depression was predicted by lower folate and vitamin B12 levels and higher homocysteine levels 2 years previously, and was associated with a decline in vitamin B12 levels and an increase in homocysteine levels over the intervening period. No direct association with MTHFR genotype was found, although the associations between folate levels and incident depression were modified by this factor. Associations between higher baseline homocysteine levels and incident depression were partly accounted for by vitamin B12 and folate levels.
Methodological issues
Previous community studies investigating the association between these factors and depression have been cross-sectional in design. Reference Bjelland, Tell, Vollset, Refsum and Ueland1,Reference Tiemeier, van Tuijl, Hofman, Meijer, Kiliaan and Breteler4,Reference Penninx, Guralnik, Ferrucci, Fried, Allen and Stabler15–Reference Tolmunen, Hintikka, Voutilainen, Ruusunen, Alfthan, Nyysonen, Viinamaki, Kaplan and Salonen19 This limits the extent to which causal relationships can be clarified, since measures of nutritional status such as vitamin levels may be affected by the emergence of depressed states and associated alterations in appetite and food intake. Relative deficiency may, in turn, account for associations with raised homocysteine levels. Most studies have also been limited in the use of brief screening instruments to define depression, Reference Bjelland, Tell, Vollset, Refsum and Ueland1,Reference Penninx, Guralnik, Ferrucci, Fried, Allen and Stabler15–Reference Tolmunen, Hintikka, Voutilainen, Ruusunen, Alfthan, Nyysonen, Viinamaki, Kaplan and Salonen19 and in numbers of potential confounding factors considered, or in the specific nature of the cohorts analysed. Strengths of our study were that prospective data on both depression and the blood assays of interest were obtained from a community population, that depression was ascertained using a widely validated diagnostic schedule, and that a large number of potential confounding factors were considered in the analyses. The follow-up rate was reasonable and not apparently differential with respect to risk factors of interest. The study sample was restricted to older age ranges, but it is this group who are likely to be most vulnerable to nutritional deficiency. Limitations of the study were that data on vitamin supplementation were not available at the baseline evaluation, and that at the follow-up evaluation the information on mental health was restricted to the previous month. Detailed constituents of vitamin preparations were also not available. The statistical models were constructed with the a priori assumption of linear associations between the three exposures and depression outcomes. Figure 1 indicates that this may not be a completely appropriate assumption. However, in the absence of obvious mechanistic explanations for non-linearity, the models were not changed.
The nature of the outcome should also be considered. Most ‘case’ participants will have had moderate levels of depression and the results may not be generalisable to secondary care clinical samples with more severe syndromes. Furthermore, the outcome was restricted to a single composite ‘diagnosis’, without specific analyses of clinical sub-types, comorbidity or particular symptom profiles. Finally, a prospective analysis was used treating depression as an ‘incident’ outcome which is unlikely to reflect fully complex symptom and syndrome trajectories (and which may have missed clinical episodes occurring and then recovering between the examination points). Depression at baseline was excluded as a binary variable; however, a secondary analysis carried out for the whole followed sample did not suggest a substantial contribution of syndrome prominence at baseline in accounting for the associations of interest.
Folate deficiency and depression
Previous case–control studies using clinical samples have reported significant associations between folate deficiency and prevalence, severity and duration of depressive disorders. Reference Levitt and Joffe20 This has been replicated in some community studies, Reference Ramos, Allen, Haan, Green and Miller18 but not in others. Reference Bjelland, Tell, Vollset, Refsum and Ueland1,Reference Tiemeier, van Tuijl, Hofman, Meijer, Kiliaan and Breteler4,Reference Penninx, Guralnik, Ferrucci, Fried, Allen and Stabler15 These discrepant results might be due to differences in sample characteristics, depression ascertainment or blood assays. In our study folate deficiency was not associated with depression in cross-sectional analyses, but lower folate levels were associated with a higher likelihood of incident depression 2 years later. The cross-sectional association between depression and folate deficiency might be obscured by selection bias if people with both depression and nutritional deficiency were less likely to participate, or if they were more prone to be hospitalised and therefore underrepresented in community samples. It is also possible that people with longer-lasting depressive states, who are over-represented in cross-sectional surveys, may regulate their diet in a way that might compensate for earlier deficiencies. The prevalence of folate deficiency at baseline was relatively low, but this is likely to be explained by the relatively high intake of folate-containing green vegetables in Korean populations, which has been previously recognised. Reference Park, Paik, Skinner, Spindler and Park21 Nevertheless, folate level remained negatively correlated with homocysteine level in our sample, as has been reported elsewhere. Reference Bjelland, Tell, Vollset, Refsum and Ueland1 The lower prevalence of folate deficiency might have obscured the association between the folate deficiency and depression at baseline in this particular population. The prospective association between lower folate levels and incident depression was not explained by other potential confounding factors (Table 3), and homocysteine, as a potential mediating factor, explained only a small proportion of this association. It is of interest that the association between lower folate and incident depression was significantly modified by MTHFR genotype, with strongest associations in those with the T/T genotype. A recent study suggested that, since the MTHFR gene influences the functioning of the folate metabolic pathway, folate or its derivatives might be causally related to risk of depression. Reference Lewis, Lawlor, Davey Smith, Araya, Timpson, Day and Ebrahim14
Vitamin B12 deficiency and depression
The cross-sectional significant association observed between lower vitamin B12 levels and depression is consistent with previous findings from both clinical samples Reference Mischoulon, Burger, Spillmann, Worthington, Fava and Alpert22 and community populations, Reference Tiemeier, van Tuijl, Hofman, Meijer, Kiliaan and Breteler4,Reference Penninx, Guralnik, Ferrucci, Fried, Allen and Stabler15 although not all studies have found this. Reference Bjelland, Tell, Vollset, Refsum and Ueland1 In prospective analyses, incident depression was associated both with lower baseline vitamin B12 levels and with a previous decline in vitamin B12 levels from baseline to follow-up. Vitamin B12 is required for the synthesis of S-adenosylmethionine, which is an important methyl donor in many important methylation reactions in the central nervous system. Inhibited synthesis of S-adenosylmethionine may reduce monoamine neurotransmitter synthesis, and S-adenosylmethionine has been suggested to have antidepressant activity. Reference Mischoulon and Fava23 A causal relationship between vitamin B12 levels and depression is supported by the prospective findings. Cross-sectional associations may also be explained by a depressive state adversely influencing dietary intake and resulting in lower circulating vitamin B12 levels. This is supported by the association between vitamin B12 decline and incident depression. However, inferences can only be tentative since the temporal relationship between vitamin B12 decline and affective state could not be established within the follow-up period. An important limitation with most research, including this study, is that circulating vitamin B12 is only a proxy marker of cobalamin deficiency at a cellular level. Methylmalonic acid is a more specific marker of functional vitamin B12 status, Reference Gultepe, Ozcan, Avsar, Cetin, Ozdemir and Gok24 but was not assayed in this study.
Hyperhomocysteinaemia and depression
Higher homocysteine levels have been associated with depressive symptoms in both middle-aged and older community populations. Reference Bjelland, Tell, Vollset, Refsum and Ueland1,Reference Almeida, Lautenschlager, Flicker, Leedman, Vasikaran, Gelavis and Ludlow17,Reference Tolmunen, Hintikka, Voutilainen, Ruusunen, Alfthan, Nyysonen, Viinamaki, Kaplan and Salonen19 Our findings were similar. Higher homocysteine levels have been found to be associated with disability Reference Marengoni, Cossi, Martinis, Calabrese, Orini and Grassi25 and with cerebrovascular diseases, 26 which are themselves potential risk factors for depression. However, we found little evidence of confounding by these factors to the extent to which they were measured in the study. Raised homocysteine levels have also been associated with cognitive impairment, Reference Stewart, Asonganyi and Sherwood27 which may be a potential confounder. However, although in the baseline sample raised homocysteine level was associated with lower MMSE score (data not shown), neither cross-sectional nor prospective associations between hyperhomocysteinaemia and depression were altered following adjustment for this. Incident depression was associated with a previous rise in homocysteine levels. This may indicate an effect of a depressive state. Although changes in vitamin B12 or folate levels did not appear to account for this association, these factors might not have been sufficiently accurate markers of bioavailability, limiting the inferences that can be drawn.
Interactions between folate, vitamin B12 and homocysteine levels
The correlation coefficients between the levels of folate, vitamin B12 and homocysteine were significant but only modest in strength, and lower than those found in previous studies. Reference Bjelland, Tell, Vollset, Refsum and Ueland1 In addition, there was no significant interaction between the folate, vitamin B12 and homocysteine levels on the prevalent or incident depression. Associations between baseline folate and vitamin B12 levels and incident depression were in part accounted for by homocysteine levels, suggesting that homocysteine might be a causal pathway factor between nutritional status and depression. The effect of adjusting the homocysteine–depression association for folate or vitamin B12 should, however, be viewed with caution, since the circulating levels assayed are only proxy markers for their function at a cellular level and confounding effects may be underestimated.
MTHFR genotype and depression
A direct association between MTHFR genotype and depression was not supported. This result was consistent with one Japanese case–control study Reference Kunugi, Fukuda, Hattori, Kato, Tatsumi, Sakai, Hirose and Nanko28 and an Australian community study, Reference Almeida, Flicker, Lautenschlager, Leedman, Vasikaran and van Bockxmeer3 although an association between MTHFR T/T homozygosity and depression was found in another Japanese case–control study Reference Arinami, Yamada, Yamakawa-Kobayashi, Hamaguchi and Toru29 and in a Norwegian community study. Reference Bjelland, Tell, Vollset, Refsum and Ueland1 MTHFR T/T genotype frequency in the sample reported here was 29%, higher than that in the Japanese samples (13–14%) and in those from Australia and Norway (12% and 8% respectively). In our study there was some evidence from an exploratory analysis that the T/T genotype might modify the association between low folate and depression. An overall association between MTHFR genotype and depression might conceivably have been reduced because of the relatively low prevalence of folate deficiency in this sample (due to the traditional Korean vegetable-rich diet).
Implications for public health and future research
Our findings in this prospective community study support roles for folate, vitamin B12 and homocysteine levels in the aetiology of late-life depression. From a public health perspective, there may be good arguments for focusing interventions for the prevention of depression on nutritionally deficient, frail populations. Although the use of vitamin supplements did not substantially modify the observed associations, further research is likely to be required as the ascertainment in this study might have been incomplete and obscured by dietary habits. Relationships with the dose, duration and (particularly) constituents of vitamin supplements should be investigated. However, it should be borne in mind that the results of observational research are often not confirmed by interventional studies. For example, a recent study reported that homocysteine reduction with B vitamins did not reduce the risk of recurrent cardiovascular disease after acute myocardial infarction, Reference Bonna, Njolstad, Ueland, Schirmer, Tverdal, Steigen, Wang, Nordrehaug, Arnesen and Rasmussen30 despite the fact that raised homocysteine levels had repeatedly been found to be associated with increased risk of cardiovascular disease in observational studies. In addition, although a role of MTHFR genotype was not supported in our study, gene–environment and gene–gene interactions require further evaluation.
Acknowledgements
The study was funded by a grant from the Korea Health 21 Research and Development Project, Ministry of Health and Welfare, Republic of Korea (A050047).








eLetters
No eLetters have been published for this article.