‘The period of acute psychotic turmoil (or panic, or ego disintegration), which initiated the psychotic episodes,... was shown to be associated with very great elevations in cortico-steroid... excretion’ (Reference Sachar, Kanter and BuieSachar et al, 1970).
People who are in the acute phase of a psychotic disorder, with florid symptoms, newly hospitalised or unmedicated, show hypothalamic–pituitary–adrenal (HPA) axis hyperactivity, but the central mechanisms underlying this neuroendocrine abnormality are unclear (Reference Cotter and ParianteCotter & Pariante, 2002). In major depression, HPA axis hyperactivity has been associated with an increased volume of the pituitary gland measured using magnetic resonance imaging (MRI), which correlates with the circulating cortisol levels (Reference Krishnan, Doraiswamy and LurieKrishnan et al, 1991; Reference Axelson, Doraiswamy and BoykoAxelson et al, 1992). To investigate whether HPA axis activation in psychosis is also associated with increased pituitary volume, we measured the pituitary glands of patients experiencing their first episode of psychosis, when they are most likely to show HPA axis abnormalities. We also measured pituitary volumes in a group of people with established schizophrenia, to investigate whether this putative abnormality varies at different stages of the psychotic disorder.
METHOD
Participants
We studied 134 individuals: 24 people with first-episode psychosis, 51 with established schizophrenia and 59 healthy controls. These participants received a brain MRI scan as part of a large study investigating brain structural abnormalities in psychosis (Reference Velakoulis, Pantelis and McGorryVelakoulis et al, 1999). All were screened for comorbid medical and psychiatric conditions by clinical assessment and by physical and neurological examination. Exclusion criteria were a history of significant head injury or seizures, electroconvulsive therapy during the 6 months before the scan, polydipsia, neurological disease, impaired thyroid function, asthma, diabetes, steroid use or meeting DSM–IV (American Psychiatric Association, 1994) criteria for alcohol or substance abuse or dependence. Individuals with a metallic object in the body or other contra-indication to MRI, and control volunteers with a personal or family history of psychiatric illness, were also excluded. After complete description of the study, written informed consent was obtained from all participants. The local Internal Review Board (Research and Ethics Committee) approved the study.
First-episode group
Twenty-four in-patients with first-episode psychosis were recruited from the Early Psychosis Prevention and Intervention Centre (EPPIC) in Melbourne, Australia (Reference McGorry, Edwards and MihalopoulosMcGorry et al, 1996). Study inclusion criteria were age at onset 16–30 years and current psychosis, reflected by the presence of at least one of delusions, hallucinations, disorder of thinking or speech (other than simple accelerations or retardation) or disorganised, bizarre or markedly inappropriate behaviour. Patients’ DSM–IV diagnoses were based on chart review, the Structured Clinical Interview for DSM–IV Disorders (SCID; Reference First, Spitzer and GibbonFirst et al, 1997) and the Royal Park Multidiagnostic Instrument for Psychosis (RPMIP; Reference McGorry, Kaplan and DossestorMcGorry et al, 1989), administered during the initial treatment episode. Eleven patients had a diagnosis of schizophrenia or schizophreniform disorder, 9 of schizoaffective disorder, 2 of bipolar disorder with psychotic symptoms, 1 of psychosis not otherwise specified and 1 of delusional disorder. Members of this group were antipsychotic-naïve before their admission. At the time of the scan, 23 out of 24 patients were taking antipsychotic medication: 14 were receiving a typical antipsychotic agent (mean daily dosage 153 mg (s.d.=81) chlorpromazine equivalents) and 9 an atypical agent, of whom 7 were receiving risperidone (mean daily dosage 2.9 mg (s.d.=1.5)), 1 olanzapine and 1 clozapine. Thirteen patients were taking benzodiazepines, 6 were taking an anticholinergic, 4 lithium and 2 an antidepressant. We collected socio-demographic and clinical information including the date of first admission, medication data and level of compliance.
Established schizophrenia group
Fifty-one people with established schizophrenia were recruited from the rehabilitation unit of the Royal Park Hospital in Melbourne, Australia. Diagnoses were based on clinical and chart review using DSM–III–R criteria (American Psychiatric Association, 1987). All of these patients had been ill for at least 5 years from the time of their first admission. Of the 42 patients for whom complete medication data were available, 39 were receiving antipsychotic treatment at the time of scanning: 19 were taking a typical antipsychotic agent (mean daily dosage 682 mg (s.d.=554) chlorpromazine equivalents – a significantly higher dosage than that in the group with first-episode psychosis: analysis of variance, F=12.5, d.f.=1,31; P=0.001) and 20 were taking an atypical antipsychotic, of whom 16 were receiving clozapine (mean daily dosage 450 mg (s.d.=225)), 2 olanzapine, 1 quetiapine and 1 risperidone. Sixteen patients were taking benzodiazepines, 8 were taking an anticholinergic, 6 an antidepressant and 5 lithium. We collected socio-demographic and clinical information including the date of first admission, medication data and level of compliance.
Control group
Fifty-nine healthy volunteers were recruited by approaching ancillary hospital staff and through advertisements. They were drawn from socio-demographic backgrounds similar to those of the participants with psychotic disorders.
Scanning and data analysis
All participants were scanned using a GE Sigma 1.5 T scanner (GE Medical Systems, Milwaukee, USA) at the Royal Melbourne Hospital. Head movement was minimised by the use of foam padding and restraining straps across the forehead and chin. All patients received their normal medication on the day of scanning. A three-dimensional volumetric spoiled gradient recalled echo in the steady state sequence generated 124 contiguous 1.5 mm coronal slices. Imaging parameters were time to echo (3.3 ms, time to repetition (14.3 ms, flip angle 30°, matrix size 256 × 256, field of view 24 cm × 24 cm and voxel dimension 0.938 mm× 0.938 mm × 1.5 mm. The scanner was calibrated fortnightly using the same proprietary phantom to ensure stability and accuracy of measurements. Imaging data were transferred from digital audiotape to an SGI O2 workstation (SGI, Mountain View, California, USA) and coded to ensure patient confidentiality and masked rating of data. All volumes were estimated using ANALYZE 7.5 (Mayo Clinic). Methods for estimating whole brain volume and intracranial volume have been described by Velakoulis et al (Reference Velakoulis, Pantelis and McGorry1999) and Eritaia et al (Reference Eritaia, Wood and Stuart2000). Whole brain volume includes the hemispheres, cerebellum, brain-stem and the ventricles, but not the cisterns or sulcal cerebrospinal fluid. Intracranial volume represents the space within the following boundaries: dura mater, the undersurfaces of the frontal lobe, the dorsum sellae, the clivus and, at the craniovertebral junction, the attachment of the dura to the posterior, cutting across to the anterior arch of C1. Interrater and intrarater reliabilities were 0.99 for both measurements.
Pituitary measurement
Each pituitary gland was traced in all coronal slices where it could be visualised, using a method described by Sassi et al (Reference Sassi, Nicoletti and Brambilla2001). The mean number of coronal slices traced per case was 13 (range 9–16). The pituitary stalk was excluded from the tracings, but we included a posterior bright spot, corresponding to the posterior pituitary, the intensity of which is thought to reflect vasopressin concentrations (Reference Sassi, Nicoletti and BrambillaSassi et al, 2001). We traced around the usually well-defined borders of the anterior and posterior pituitary: the diaphragma sellae, superiorly; the sphenoid sinus, inferiorly; and the cavernous sinuses, bilaterally (Fig. 1) (Reference Lurie, Doraiswamy and HusainLurie et al, 1990; Reference ElsterElster, 1993; Reference Lum, Kucharczyk, Montanera and AtlasLum et al, 2002). Volume of the pituitary (in mm3) was calculated by summing volumes for all relevant slices. One rater (K.V.) traced all the pituitaries in the study. Before starting the study, she trained with a senior author involved in the development and optimisation of the technique (B.S.). The intrarater and interrater reliabilities were calculated by measuring the pituitary volumes in ten scans randomly selected from the original pool of MRI scans from all the study groups. The interrater reliability was 0.85, the intrarater reliability was 0.97 and the average variation between two measurements of the same pituitary was 5%.

Fig. 1 Pituitary gland (arrowed) viewed in sagittal (left) and coronal (right) magnetic resonance imaging scans. Coronal slices were used for tracing the gland.
Statistical analysis
Original data are presented as mean and standard deviation. Adjusted means are presented as mean and standard error of the mean (s.e.m.). Clinical and socio-demographic differences between groups were examined using one-way analysis of variance (ANOVA) followed by post hoc Student–Newman–Keuls test (Table 1). To limit the number of statistical comparisons, the differences in pituitary volumes between the control group and the two clinical groups were examined by conducting a single two-way analysis of covariance (ANCOVA) test, using the intracranial volume as a covariate, followed by pairwise comparisons of estimated means. By definition, it was impossible to have a single control group that was comparable with the young first-episode group as well as the older group with established schizophrenia, for both age and gender distribution. Therefore, to control for these potential confounders, gender was used as between-subject factor, and age (and whole brain volume) were included as covariates in a second set of confirmatory analyses. We have previously used this approach to demonstrate differences in hippocampal volumes between normal volunteers, people with a first psychotic episode and people with established schizophrenia (Reference Velakoulis, Pantelis and McGorryVelakoulis et al, 1999). Moreover, the results obtained from this analysis were corroborated by conducting two separate ANCOVA tests comparing each of the clinical groups with another control group that had a similar age and gender distribution. These two control subgroups were obtained from the original control sample by ranking males and females by age (masked to their pituitary volume) and excluding older controls (in the comparison with the first-episode group) or younger controls (in the comparison with the established schizophrenia group) until comparable age and gender distributions were achieved (see Results). The ANCOVA test was also used to compare pituitary volumes between the following subgroups:
Table 1 Characteristics of the study sample

| Control group (n=59) | First-episode group (n=24) | Established schizophrenia group (n=51) | Group comparisons | |
|---|---|---|---|---|
| Age, years: mean (s.d.) | 29.3 (10.5) | 21.9 (3.3) | 41.0 (9.3) | ANOVA, F=41.2, d.f.=2,131; P<0.001 |
| Gender | ||||
| Male/female, n/n | 33/26 | 18/6 | 41/10 | χ2=8.2, d.f.=2, P=0.017 |
| Male, % | 56% | 75% | 80% | |
| Age at first admission, years: mean (s.d.) | 21.8 (3.2) | 22.1 (7.2) | ANOVA, F=0.03, d.f.=1,73; P=0.97 | |
| Time after first admission, years: mean (s.d.) | 0.2 (0.4) | 19.1 (10.2) | ANOVA, F=40.4, d.f.=1,73; P=0.001 | |
| Intracranial volume, mm3: mean (s.d.) | 1 442 20 1 (142 599) | 1 407 197 (137 136) | 1 449 456 (142 599) | ANOVA, F=0.8, d.f.=2,131; P=0.5 |
| Whole brain volume, mm3: mean (s.d.) | 1 349 542 (150 004) | 1 349 440 (144 621) | 1 318 138 (125 094) | ANOVA, F=0.8, d.f.=2,131; P=0.5 |
-
(a) males and females, across the whole sample;
-
(b) first-episode patients with schizophrenia or schizophreniform psychosis, first-episode patients with other diagnoses, and controls;
-
(c) patients receiving typical antipsychotics and patients receiving atypical antipsychotics;
-
(d) patients receiving lithium and/or antidepressants and patients not receiving these drugs.
Partial correlations, covarying for intracranial volume, were conducted between pituitary volume and age.
RESULTS
Characteristics of the sample
The main demographic and clinical features of the three groups are presented in Table 1. As expected, there were significant differences in age and gender between them. Post hoc comparisons showed that the control group members were younger than the patients with established schizophrenia and older than the patients with first-episode psychosis, and that there were significantly more males in the established schizophrenia group than in the control group. By definition, patients with established schizophrenia had a much longer duration of illness at the time of the MRI scan than the first-episode group. Although there was some variability in the duration of illness at the time of the scan in the first-episode group, 21 out of 24 of these patients were scanned within 2 months of their first admission. Intracranial volume and whole brain volume did not differ between the groups to a statistically significant extent.
Pituitary volume
There was a significant difference in pituitary volume between the three groups (ANCOVA, F=18.0, d.f.=2,132; P<0.001). Figure 2 shows the pituitary volumes of all participants, and the estimated mean and s.e.m. values for each group after adjustment for intracranial volume and gender. Although examination of the individual data showed an overlap between the groups, the presence of group differences was suggested by the fact that 17 of the 24 patients in the first-episode group (71%) had pituitary volumes that were larger than the median of the control group, whereas 35 of the 51 patients in the established disease group (69%) had pituitary volumes that were smaller than the median of the control group. The ANCOVA analysis showed that pituitary volumes in the first-episode group were, on average, 10% larger than those of the control group (+52 mm3, s.d.=24, P=0.032), whereas pituitary volumes in the group with established schizophrenia were, on average, 17% smaller than controls (-91 mm3, s.d.=19, P<0.001). These differences remained significant when age and whole brain volume were used as covariates in the analysis. Furthermore, these differences remained significant when each clinical group was compared with a control group that had similar age and gender distribution (obtained as described in the statistical analysis section, above; 32 controls in the comparison with the first-episode group and 29 controls in the comparison with the established illness group; ANOVA for age differences, F<0.2, P>0.6, for both comparisons; chi-squared test for gender differences, w2<2.1, P>0.2, for both comparisons).
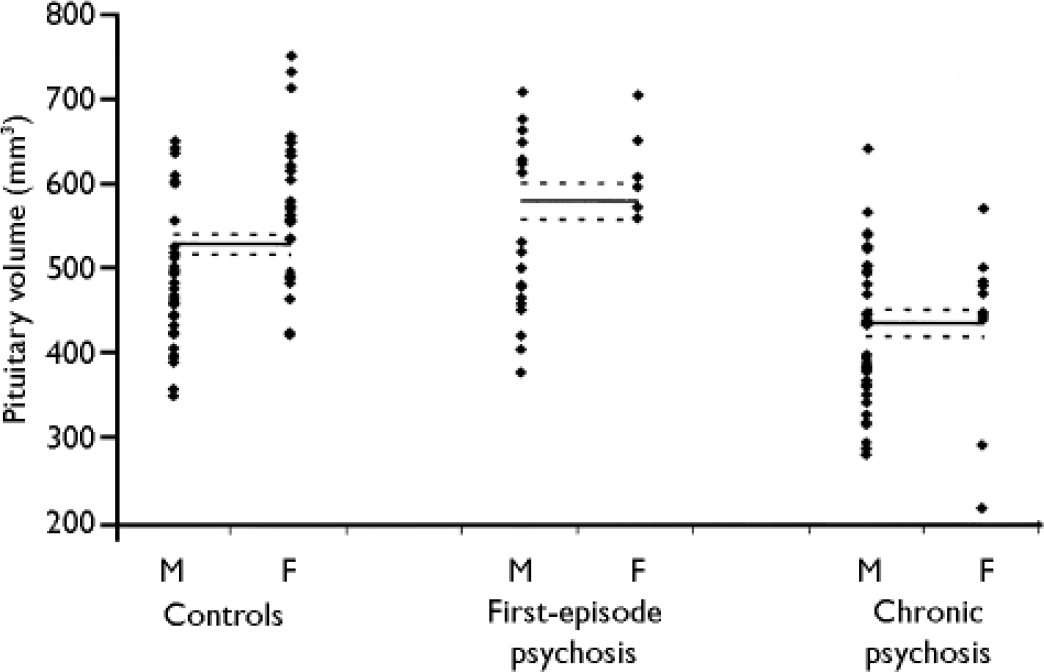
Fig. 2 Pituitary volumes in participants with first-episode psychosis, participants with established schizophrenia (chronic psychosis) and in healthy volunteers (controls). Each group is divided into males (M) and females (F). The figure also shows the estimated mean and standard error of the mean values for each group (controls, first-episode and chronic) after adjustment for intracranial volume and gender.
In the whole sample, women had larger pituitaries than men: 553 mm3 (s.d.=17) v. 484 mm3 (s.d.=10); ANCOVA, F=11.7, d.f.=1,133; P=0.001. There was a trend for a gender × group interaction (ANOVA, F=2.5, d.f.=2,132; P=0.085), which was due to a smaller gender effect in the group with established schizophrenia in comparison with the other groups (Fig. 2). In the whole sample, there was a negative correlation between age and pituitary volume (r=-0.36, d.f.=131; P<0.001), present in both males (r=-0.26, d.f.=89, P=0.012) and females (r=-0.56, d.f.=39; P<0.001).
Effects of clinical characteristics and subgroups
In the first-episode group, both patients with schizophrenia or schizophreniform psychosis (n=11) and patients with other diagnoses (n=13) had larger pituitary volumes than the control group; this subgroup analysis, however, did not reach statistical significance (ANCOVA, F=1.9, d.f.=2,54; P=0.17; schizophrenia/schizophreniform psychosis +48 mm3 (s.d.=30) v. controls, P=0.12; other diagnoses +50 mm3 (s.d.=36) v. controls, P=0.16).
There was no difference in pituitary volume between patients receiving typical and atypical antipsychotics, either in the first-episode group (ANCOVA, F=1.6, d.f.=1,22; P=0.2) or in the established schizophrenia group (ANCOVA, F=1.3, d.f.=1,38; P=0.3). Similarly, there was no difference in pituitary volume between the patients receiving antidepressants or lithium and the other patients, either in the first-episode group (ANCOVA, F<0.1, d.f.=1,23; P=0.97) or in the established illness group (ANCOVA, F=0.5, d.f.=1,41; P=0.5).
DISCUSSION
The pituitary gland regulates HPA axis activity by secreting adrenocorticotrophic hormone, which in turn stimulates the adrenal gland to produce cortisol. We found that patients with a first episode of psychosis had larger pituitary volumes than healthy volunteers, whereas patients with established schizophrenia (of at least 5 years’ duration) had smaller pituitary volumes than the controls.
Mechanisms leading to increased pituitary volume
We suggest that the increased pituitary volume in patients with first-episode psychosis is due to activation of the HPA axis and, in particular, to an increase in the size and number of corticotrophs (cells producing adrenocorticotrophic hormone). The few studies that have examined the HPA axis in people with a first episode of psychosis have found hyperactivity of this hormonal system (Reference Sachar, Kanter and BuieSachar et al, 1970; Reference Ryan, Collins and ThakoreRyan et al, 2003). Interestingly, both HPA axis hyperactivity and increased pituitary volume have been described in patients with severe major depression (Reference Krishnan, Doraiswamy and LurieKrishnan et al, 1991; Reference Axelson, Doraiswamy and BoykoAxelson et al, 1992), and have been interpreted in these patients as showing a lack of negative inhibitory feedback by circulating glucocorticoid hormones on the HPA axis, especially at the level of the pituitary (glucocorticoid resistance) (Reference Pariante and MillerPariante & Miller, 2001; Reference PariantePariante, 2003). Indeed, increased size and number of corticotrophs and increased pituitary volume are present also in people with a lack of negative inhibitory feedback by circulating glucocorticoid hormones due to Addison's disease (Reference Mineura, Goto and YoneyaMineura et al, 1987). Therefore, our findings suggest that glucocorticoid resistance may be present in people with first-episode psychosis. As glucocorticoid resistance is a common correlate of stress-induced HPA axis activation in animals and humans (Reference Raison and MillerRaison & Miller, 2003), our findings could be explained by an activation of the stress response. Such activation could be due to the distress caused by the first psychotic experience, to an increased biological susceptibility to daily life stress or to an increased level of independent stressors leading to the psychotic episode – or to all these causes (Reference Sachar, Kanter and BuieSachar et al, 1970; Reference Bebbington, Wilkins and JonesBebbington et al, 1993; Reference Myin-Germeys, van Os and SchwartzMyin-Germeys et al, 2001). Reassuringly, there is no evidence that HPA axis hyperactivity causes damage to the brain in people with mental disorders (Reference Muller, Lucassen and YassouridisMuller et al, 2001). We did not measure hormonal levels in our study participants, however, so our proposed relationship between pituitary hyperplasia and hyperactivity of the HPA axis remains speculative.
Mechanisms leading to decreased pituitary volume
The second major finding of our study was that the group of people with established schizophrenia had smaller pituitary volumes than the control group. This finding is remarkably consistent with other studies, which have shown reduced pituitary volumes in patients with an eating disorder (Reference Doraiswamy, Krishnan and FigielDoraiswamy et al, 1990) and in euthymic and depressed patients with bipolar disorder (Reference Sassi, Nicoletti and BrambillaSassi et al, 2001). Therefore, it is possible that common pathological mechanisms are present in people with mental disorders that lead to hypoplasia of the pituitary gland. Interestingly, based on the evidence that patients with bipolar disorder can present with HPA axis hyperactivity, Sassi et al (Reference Sassi, Nicoletti and Brambilla2001) have suggested that chronic activation of the HPA axis might decrease pituitary volume by reducing (through negative feedback) the function of cells producing other pituitary hormones. Because psychotic relapses are associated with activation of the HPA axis (Reference Tandon, Mazzara and De QuardoTandon et al, 1991), it is possible that repeated episodes of HPA axis activation associated with previous relapses in our group of patients with established schizophrenia led to the reductions in pituitary volume. Again, without having measured pituitary hormones, we do not know whether this volume reduction has any functional consequences. Previous studies have found normal HPA axis function in people with established psychosis, especially if they were clinically stable and receiving treatment (Reference Tandon, Mazzara and De QuardoTandon et al, 1991).
This is a cross-sectional study comparing different people at various stages of psychosis, not a prospective study; therefore, we can only speculate that the people in the first-episode group with large pituitary glands would ‘progress’ to having small pituitary glands 5 years later. It is also possible that these two groups are biologically distinct. The patients with established schizophrenia might have had a smaller pituitary volume even at the onset of their psychosis, for example, because of a neurodevelopmental problem (Reference Lum, Kucharczyk, Montanera and AtlasLum et al, 2002). Equally, patients with first-episode psychosis might have a normal pituitary volume if scanned in the future, as found in people with major depression in the euthymic phase (Reference Sassi, Nicoletti and BrambillaSassi et al, 2001).
Limitations of the study
As already stated, we did not measure hormonal levels in these samples. Moreover, the neuroimaging data were initially collected to examine brain structure, rather than neuroendocrine abnormalities. Future studies on this topic should include hormone measurements. A possible limitation of our study, common to all studies examining pituitary volume by imaging methods, is the difficulty in distinguishing between anterior and posterior pituitary volumes (Reference Doraiswamy, Krishnan and FigielDoraiswamy et al, 1990; Reference Lurie, Doraiswamy and HusainLurie et al, 1990; Reference Krishnan, Doraiswamy and LurieKrishnan et al, 1991; Reference Axelson, Doraiswamy and BoykoAxelson et al, 1992; Reference Sassi, Nicoletti and BrambillaSassi et al, 2001). However, the posterior pituitary, which releases vasopressin and oxytocin, constitutes less than 20% of the total pituitary volume and – in contrast to the anterior pituitary – there is no known condition associated with its enlargement, except tumour (Reference Krishnan, Doraiswamy and LurieKrishnan et al, 1991; Reference ElsterElster, 1993). Therefore, we believe that the changes in volume we have described are due to changes in the volume of the anterior pituitary.
Finally, we cannot exclude that pituitary hyperplasia in first-episode psychosis is due to increased function of pituitary cells secreting hormones other than adrenocorticotrophic hormone, such as growth hormone and prolactin; levels of these hormones are also elevated by stress. Furthermore, the patients in our first-episode group were receiving neuroleptic treatment, and antipsychotic drugs can induce proliferation of prolactin-secretingsecreting cells in animals (Reference Saiardi, Bozzi and BaikSaiardi et al, 1997). However, in these patients pituitary volume did not seem to be univocally correlated with antipsychotic administration. In fact, patients in both the first-episode group and the established schizophrenia group were receiving antipsychotic medication, but the differences in pituitary volume, compared with controls, were in opposite directions; moreover, there was no difference in pituitary volume between patients taking typical and atypical antipsychotics, even though the effects of these two kinds of drugs on prolactin secretion are quite different (Reference Halbreich and KahnHalbreich & Kahn, 2003).
Strengths of the study
Three lines of evidence support our conclusions. First, the effects of age and gender on pituitary volume in our sample are consistent with previously published studies (Reference Lurie, Doraiswamy and HusainLurie et al, 1990; Reference Sassi, Nicoletti and BrambillaSassi et al, 2001). Second, the most common causes of increased pituitary volume – administration of exogenous oestrogens, hypothalamic tumour, pregnancy, primary hypothyroidism and puberty (Reference ElsterElster, 1993) – were excluded in our study sample. Third, these results are not limited by the socio-demographic differences between the three groups, because variables that could regulate pituitary volumes (age, gender, intracranial volume and whole brain volume) are controlled, both in the main statistical analysis and in the two direct comparisons between each clinical group and the matched control groups.
Although the fact that the first-episode sample was clinically heterogeneous could be interpreted as a limitation, studies on such individuals usually include all diagnoses of psychosis. In fact, because of the temporal instability of the diagnosis in these patients, restricting the analysis to a single diagnostic group in the initial stages would imply missing or misdiagnosing a large number of individuals who would have qualified at a later stage (Reference Amin, Singh and BrewinAmin et al, 1999). Future studies on similar, larger samples would clarify whether there are differences in pituitary volume across psychiatric diagnoses. Moreover, prospective studies of HPA axis function and brain imaging in people at various stages of psychosis, including those at high risk of developing psychosis, will clarify whether these pituitary abnormalities are indeed related to HPA axis function and whether measurements of the stress response could be used to predict the development of psychosis.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ The first episode of a psychotic disorder is associated with enlargement of the pituitary. One possible interpretation of this finding is that it indicates activation of the hormonal stress response, and especially of the hypothalamic–pituitary–adrenal axis.
-
▪ The finding of a reduced pituitary volume in people with established psychosis is similar to that described in people with bipolar disorders and anorexia, and suggests that common pathological mechanisms are present in chronic mental disorders, leading to hypoplasia of the pituitary gland.
-
▪ Prospective studies will clarify whether measurements of the stress response can be used to predict the development of psychosis.
LIMITATIONS
-
▪ Because the participants in the first-episode group were receiving antipsychotics, we cannot exclude that pituitary hyperplasia could be related, at least in part, to increased function of prolactin-producing cells activated by neuroleptic treatment.
-
▪ It is difficult to distinguish between anterior and posterior pituitary volume using imaging methods.
-
▪ Without measurements of hormonal levels, our proposed relationship between pituitary hyperplasia and hyperproduction of pituitary hormones remains speculative.
Acknowledgements
This research was funded by project grants from the National Health and. Medical Research Council grant numbers 970598, 970391 and 981112, the. Victorian Health Promotion Foundation, the Stanley Foundation, the Ian Potter. Foundation, the Woods Family Trust, the Australian Communications and Computing Institute, the Percy Baxter Charitable Trust and the National. Alliance for Research on Schizophrenia and Depression (NARSAD). C.M.P. was funded by a travel award from the Wellcome Trust and two travel grants from Brain. P.D. was funded by a short-term travel grant from the Wellcome Trust. and a grant from the University of London. C.M.P. and P.D. have both received a NARSAD Young Investigator Award for their research in the UK.






eLetters
No eLetters have been published for this article.