Although our understanding of the clinical epidemiology of delirium has advanced considerably over the past decade, greater phenomenological study should allow more targeted studies of underlying mechanisms and therapeutic response. Delirium involves a constellation of symptoms reflecting widespread disruption of higher cortical functions that characteristically occur with an acute onset and fluctuating course. However, the interrelationship of delirium symptoms and their relevance to aetiology, treatment experience and outcome are poorly understood. Moreover, there is a dearth of research using validated instruments designed to assess the phenomenological breadth and complexity of this disorder (Reference Turkel, Trzepacz and TavareTurkel et al, 2006).
Two validated tools open the way for more detailed phenomenological study of delirium. The Cognitive Test for Delirium (CTD; Reference Hart, Levenson and SesslerHart et al, 1996) measures five cognitive domains using standard neuropsychological methods. The Delirium Rating Scale – Revised–98 (DRS–R98; Trzepacz et al, Reference Trzepacz, Mittal and Torres2001a ,Reference Trzepacz, Mittal and Torres b ) covers a broad range of delirium symptoms not measured by other delirium instruments, including language, thought process abnormalities, visuospatial ability and both short- and long-term memory. We report a 2-year study of the frequency and severity of symptoms in 100 cases of delirium occuring in a palliative care setting using the DRS–R98 and the CTD. We explored the interrelationship among delirium symptoms and, by measuring cognition carefully in conjunction with the DRS–R98, tested the primacy of inattention in delirium.
METHOD
Study design
We conducted a prospective cross-sectional study of delirium symptoms and cognitive performance in consecutive cases of DSM–IV delirium referred from a palliative care in-patient service. Patients assessed on daily ward rounds by the palliative care team as having altered mental state were screened with the Confusion Assessment Method (CAM; Reference Inouye, van Dyck and AlessiInouye et al, 1990) – a four-item instrument based on DSM–III–R criteria. Patients were not included if they were near death or if circumstances were too difficult to allow assessment (in the opinion of the treating medical team), which resulted in a small number (less than 10%) being excluded. During the study period there were 434 new admissions to the unit, of which 100 (23%) are described here.
Delirium according to DSM–IV criteria (American Psychiatric Association, 1994) was confirmed by a research physician – (either the principal investigator (D.J.M.) or one of three specialist registrars trained to establish acceptable interrater reliability. Each case was then assessed by completion of the DRS–R98 followed by the CTD. The DRS–R98 rated the preceding 24 h period, whereas the CTD measured cognition at the time of its administration. Responses to the CTD were not used to rate DRS–R98 items. Both the DRS–R98 and the CTD are well-validated instruments, highly structured and anchored for rating and scoring.
Consent
The procedures and rationale for the study were explained to all patients, but because of their delirium at entry into the study it was presumed that most were not capable of giving informed written consent. Because of the non-invasive nature of the study, ethics committee approval was given to augment patient assent with proxy consent from next of kin (where possible) or a responsible caregiver for all participants in accordance with the Helsinki guidelines for medical research involving human subjects (World Medical Association, 2004).
Assessments
Demographic data, psychotropic drug exposure and the possibility of underlying dementia (suggested by history or investigation) were collected. Nursing staff were interviewed to assist rating of symptoms over the previous 24 h.
Delirium Rating Scale – Revised–98
The original Delirium Rating Scale (Reference Trzepacz, Baker and GreenhouseTrzepacz et al, 1988) is widely used to measure symptom severity in delirium, but has the limitations of grouping cognitive disturbances into a single item, not distinguishing motoric disturbances and not assessing thought process or language disorder. It has therefore been substantially revised to allow broad phenomenological assessment and serial ratings. The DRS–R98 is a 16-item scale with 13 severity items and 3 diagnostic items and it has high interrater reliability, sensitivity and specificity for detecting delirium in mixed neuropsychiatric and other hospital populations (Reference Trzepacz, Mittal and TorresTrzepacz et al, 2001a ). It was validated both as a total scale (16 items) and a severity scale (13 items) for repeated measures. Each item is rated 0 (absent/normal) to 3 (severe impairment), with descriptions anchoring each severity level. Severity scale scores range from 0 to 39, with higher scores indicating more severe delirium. Delirium typically involves scores above 15 points (severity scale) or 18 points (total scale). For determination of item frequencies in this study, any item scoring at least 1 was considered present.
Cognitive Test for Delirium
The CTD (Reference Hart, Levenson and SesslerHart et al, 1996) was specifically designed to assess patients with delirium – in particular those who are intubated or unable to speak or write. It assesses 5 neuropsychological domains (orientation, attention, memory, comprehension and vigilance), emphasising non-verbal (visual and auditory) modalities. Each individual domain is scored 0–6 in 2-point increments, except for comprehension which is scored in single-point increments. Total scores range between 0 and 30, with higher scores indicating better cognitive function. This measure reliably differentiates delirium from other neuropsychiatric conditions including dementia, schizophrenia and depression (Reference Hart, Best and SesslerHart et al, 1997).
Performance on individual neuropsychological sub-tests (e.g. attention) can be scored on a 4-point scale (6 normal, 4 mild inattention, 2 moderate inattention, 0 severe inattention). Item severities were used to compare the relationship between individual items of the DRS–R98 to assess the relationship between cognitive and non-cognitive elements of delirium.
Aetiology
Attribution of aetiology based on all available clinical information was made by the palliative care physician according to a standardised delirium aetiology checklist (further information available from the authors upon request) with 12 categories: drug intoxication, drug withdrawal, metabolic/endocrine disturbance, traumatic brain injury, seizures, infection (intracranial), infection (systemic), neoplasm (intracranial), neoplasm (systemic), cerebrovascular, organ insufficiency, other central nervous system disorder and other systemic disorder. The presence and suspected role of multiple potential causes were documented for each case of delirium, rated on a 5-point scale for degree of attribution to the delirium episode, ranging from ‘ruled out/not present/not relevant’ (0) to ‘definite cause’ (4).
Statistical analyses
Statistical analysis was conducted using the Statistical Package for the Social Sciences version 10.1. Demographic and rating scale data were expressed as means plus standard deviation. Continuous variables were compared by one-way analysis of variance (ANOVA). The severity of categorical and/or quasi-continuous variables such as the individual items of the DRS–R98 and CTD was compared with chi-squared analyses. Pearson correlations were performed between some individual items and between scale total scores. Level of significance was determined with a cut-off of 0.05, except where multiple comparisons were made when a Bonferroni correction (P<0.001) was applied.
RESULTS
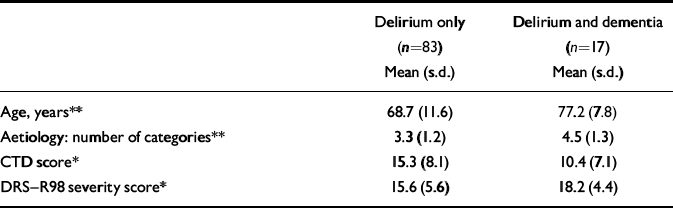
Half of the 100 patients in the study were men, and the mean age of the group was 70.1 years (s.d.=11.5). A mean of 3.5 (s.d.=1.3) aetiological categories were noted per case, with neoplasm (67%), systemic infection (63%), metabolic–endocrine disorder (45%), organ failure (32%), drug intoxication (27%) and central nervous system lesions (26%) being the most common contributing causes. Patients had a mean DRS–R98 total score of 21.1 (s.d.=5.5) and severity score of 16.6 (s.d.=5.5), and a mean CTD score of 14.5 (s.d.=8.1). The characteristics of patients with delirium only are compared with those of patients with comorbid dementia in Table 1.
Table 1 Characteristics of patients with delirium v. patients with comorbid delirium and dementia
| Delirium only (n=83) Mean (s.d.) | Delirium and dementia (n=17) Mean (s.d.) | |
|---|---|---|
| Age, years** | 68.7 (11.6) | 77.2 (7.8) |
| Aetiology: number of categories** | 3.3 (1.2) | 4.5 (1.3) |
| CTD score* | 15.3 (8.1) | 10.4 (7.1) |
| DRS—R98 severity score* | 15.6 (5.6) | 18.2 (4.4) |
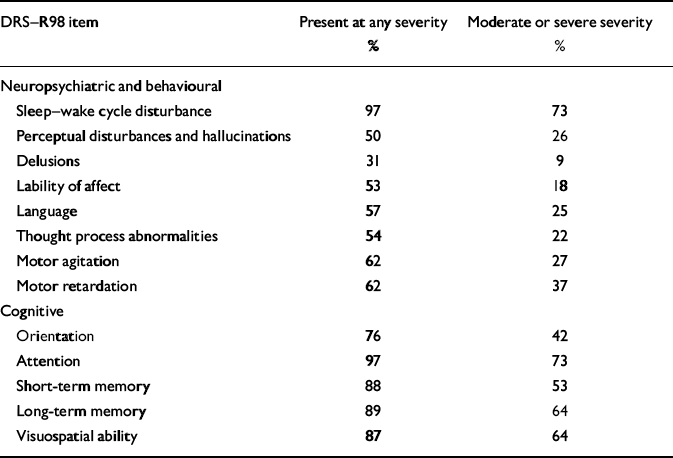
Table 2 summarises the cognitive and non-cognitive disturbances assessed with the DRS–R98. Inattention (diagnostic criterion A of DSM–IV) was present in 97% of patients; other cognitive deficits were also common (76–89%), disorientation being the least frequent. Among the non-cognitive items, sleep disturbance (97%) and motoric disturbance (62% each for hypoactive and hyperactive items, with 31 patients having evidence of both) were common, such that 94 patients had evidence of at least some degree of motoric disturbance (items 7 and 8 of DRS–R98). Language and thought process abnormalities were each present in over half the group but were less common than cognitive symptoms. Even when only more severe degrees of impairment were considered, attention and sleep–wake cycle deficits remained the most common, each at 73%.
Table 2 Frequency of delirium symptoms rated with the Dementia Rating Score–Revised–98 and recorded if present at different levels of severity (n=100)
| DRS—R98 item | Present at any severity % | Moderate or severe severity % |
|---|---|---|
| Neuropsychiatric and behavioural | ||
| Sleep—wake cycle disturbance | 97 | 73 |
| Perceptual disturbances and hallucinations | 50 | 26 |
| Delusions | 31 | 9 |
| Lability of affect | 53 | 18 |
| Language | 57 | 25 |
| Thought process abnormalities | 54 | 22 |
| Motor agitation | 62 | 27 |
| Motor retardation | 62 | 37 |
| Cognitive | ||
| Orientation | 76 | 42 |
| Attention | 97 | 73 |
| Short-term memory | 88 | 53 |
| Long-term memory | 89 | 64 |
| Visuospatial ability | 87 | 64 |
Forty-nine patients had evidence of psychosis, as defined by a score of ≥2 on item 2 (perceptual disturbances), item 3 (delusions) or item 6 (thought disturbance) on the DRS–R98. Eighteen of these patients scored 3 on one of these three items, indicating florid psychosis. The 49 patients with psychosis were not significantly different from the other 51 patients regarding motoric profile (DRS–R98 items 7 and 8) and overall severity of cognitive disturbance (measured by the CTD). They were younger (t=1.9, P=0.05) with higher total DRS–R98 scores (t=–3.8; P<0.001) and more severe affective lability (χ2=16.1, d.f.=2, P<0.001).
Patients with psychosis tended to have disturbance of a single psychotic component, with only 6 of these 49 patients scoring ≥2 on more than one item. For the whole cohort, DRS–R98 items 2 (perceptual disturbance) and 3 (delusions) were not significantly correlated (r=0.16); item 6 (thought disturbance) was not significantly correlated with item 2 (r=0.15) or item 3 (r=0.01). Moreover, when the analysis was restricted to patients with psychosis (n=49), thought disturbance and perceptual disturbances were inversely correlated (r–0.49, P=0.001) and both delusions (r=0.59, P=0.001) and thought disturbance (r=0.35, P=0.01) correlated positively with affective lability, whereas perceptual disturbance was negatively correlated with affective lability (r=–0.41, P=0.003).
Although neither delusions nor perceptual disturbances correlated significantly with any of the cognitive items of DRS–R98 or CTD, thought process disturbance correlated with impairments of attention (r=–0.46, P=0.001), memory (r–0.40, P<0.01), orientation (r=–0.30, P=0.03) and comprehension (r=–0.28, P=0.05) items on the CTD, and with attention (r=0.59, P<0.001), orientation (r=0.33, P=0.03) and long-term memory (r=0.34, P=0.03) items – but not short-term memory or visuospatial function items – on the DRS–R98.
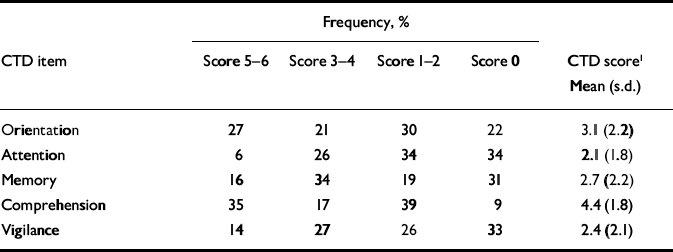
Cognitive dysfunction rated with the CTD is shown in Table 3. This shows wide-spread impairment of neuropsychological function, with the most frequent (94%) and severest impairments in attention and vigilance. This parallels the DRS–R98 impairments, of which attention was most often impaired and orientation least impaired, even though these scales were rated independently of one another and for different time frames – DRS–R98 for the previous 24 h and CTD for current performance. The DRS–R98 attention item includes distractibility and therefore encompasses both attention and vigilance as assessed in the CTD. Corresponding items on the CTD and the DRS–R98 correlated highly: DRS–R98 orientation and CTD orientation (r=–0.75), DRS–R98 attention and CTD attention (r=–0.73), DRS–R98 attention and CTD vigilance (r=–0.60), and CTD memory with DRS–R98 short-term memory (r=–0.47) and long-term memory (r=–0.61). Interestingly, CTD comprehension correlated with the DRS–R98 item for language (r=–0.42, P=0.001) but not with thought process abnormalities (r–0.09).
Table 3 Frequency of different severity levels of cognitive dysfunction and mean item scores assessed with the Cognitive Test for Delirium (n=100)
| Frequency, % | |||||
|---|---|---|---|---|---|
| CTD item | Score 5-6 | Score 3-4 | Score 1-2 | Score 0 | CTD score1 Mean (s.d.) |
| Orientation | 27 | 21 | 30 | 22 | 3.1 (2.2) |
| Attention | 6 | 26 | 34 | 34 | 2.1 (1.8) |
| Memory | 16 | 34 | 19 | 31 | 2.7 (2.2) |
| Comprehension | 35 | 17 | 39 | 9 | 4.4 (1.8) |
| Vigilance | 14 | 27 | 26 | 33 | 2.4 (2.1) |
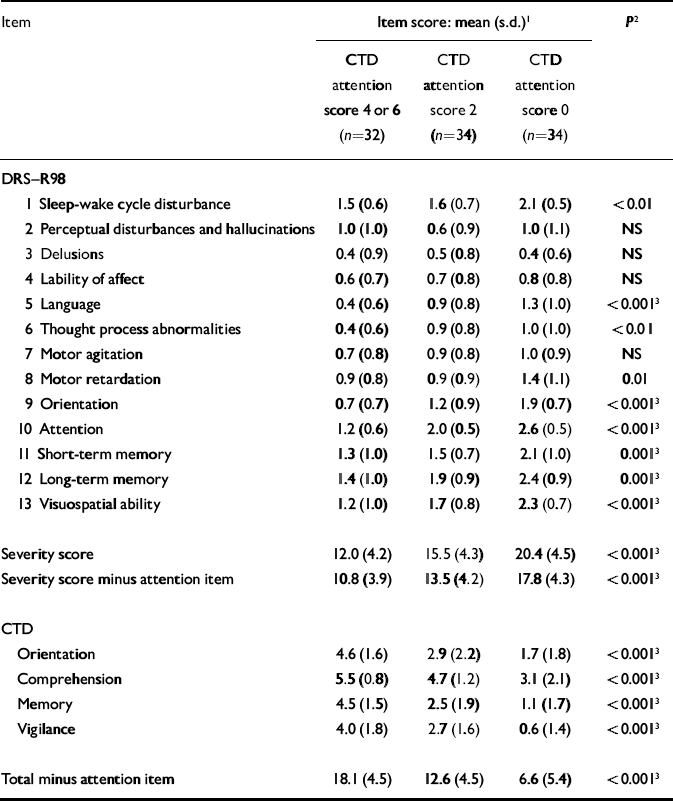
In view of the central role given to disturbed attention in current delirium descriptions, patients were divided into three categories according to the severity of attentional deficit measured using the CTD: score 4–6, (n=32), score 2 (n=34) and score 0 (n=34). These groups differed for many items (Table 4); however, when significance levels were corrected for multiple comparisons, the degree of inattention was associated with the level of impairment of other cognitive disturbances (rated on both CTD and DRS–R98) but not the non-cognitive DRS–R98 items, except for language (χ2=19.5, d.f.=6, P=0.001).
Table 4 Item scores for the two delirium scales according to degree of inattention on the Cognitive Test for Delirium
| Item | Item score: mean (s.d.)1 | P 2 | ||
|---|---|---|---|---|
| CTD attention score 4 or 6 (n=32) | CTD attention score 2 (n=34) | CTD attention score 0 (n=34) | ||
| DRS—R98 | ||||
| 1 Sleep-wake cycle disturbance | 1.5 (0.6) | 1.6 (0.7) | 2.1 (0.5) | <0.01 |
| 2 Perceptual disturbances and hallucinations | 1.0 (1.0) | 0.6 (0.9) | 1.0 (1.1) | NS |
| 3 Delusions | 0.4 (0.9) | 0.5 (0.8) | 0.4 (0.6) | NS |
| 4 Lability of affect | 0.6 (0.7) | 0.7 (0.8) | 0.8 (0.8) | NS |
| 5 Language | 0.4 (0.6) | 0.9 (0.8) | 1.3 (1.0) | <0.0013 |
| 6 Thought process abnormalities | 0.4 (0.6) | 0.9 (0.8) | 1.0 (1.0) | <0.01 |
| 7 Motor agitation | 0.7 (0.8) | 0.9 (0.8) | 1.0 (0.9) | NS |
| 8 Motor retardation | 0.9 (0.8) | 0.9 (0.9) | 1.4 (1.1) | 0.01 |
| 9 Orientation | 0.7 (0.7) | 1.2 (0.9) | 1.9 (0.7) | <0.0013 |
| 10 Attention | 1.2 (0.6) | 2.0 (0.5) | 2.6 (0.5) | <0.0013 |
| 11 Short-term memory | 1.3 (1.0) | 1.5 (0.7) | 2.1 (1.0) | 0.0013 |
| 12 Long-term memory | 1.4 (1.0) | 1.9 (0.9) | 2.4 (0.9) | 0.0013 |
| 13 Visuospatial ability | 1.2 (1.0) | 1.7 (0.8) | 2.3 (0.7) | <0.0013 |
| Severity score | 12.0 (4.2) | 15.5 (4.3) | 20.4 (4.5) | <0.0013 |
| Severity score minus attention item | 10.8 (3.9) | 13.5 (4.2) | 17.8 (4.3) | <0.0013 |
| CTD | ||||
| Orientation | 4.6 (1.6) | 2.9 (2.2) | 1.7 (1.8) | <0.0013 |
| Comprehension | 5.5 (0.8) | 4.7 (1.2) | 3.1 (2.1) | <0.0013 |
| Memory | 4.5 (1.5) | 2.5 (1.9) | 1.1 (1.7) | <0.0013 |
| Vigilance | 4.0 (1.8) | 2.7 (1.6) | 0.6 (1.4) | <0.0013 |
| Total minus attention item | 18.1 (4.5) | 12.6 (4.5) | 6.6 (5.4) | <0.0013 |
We further examined whether impairment on the other CTD items related to scores on DRS–R98 items as strongly as did CTD attention, to ascertain whether attention had a unique role. After corrections for multiple comparisons, the severity of vigilance impairment was closely related to all other aspects of cognition but not to non-cognitive items (except for language) and thus mirrored the findings with the CTD attention item. Orientation, memory and comprehension were less strongly associated with DRS–R98 cognitive items (Table 5). In contrast to attention, severity of comprehension disturbance was associated with the most non-cognitive DRS–R98 symptoms, including sleep–wake cycle disturbance, psychomotor retardation and language difficulties. These patterns suggest two different domains of delirium symptoms.
Table 5 Significance values for relationship between DRS—R98 items and severity levels for individual CTD items (other than attention)
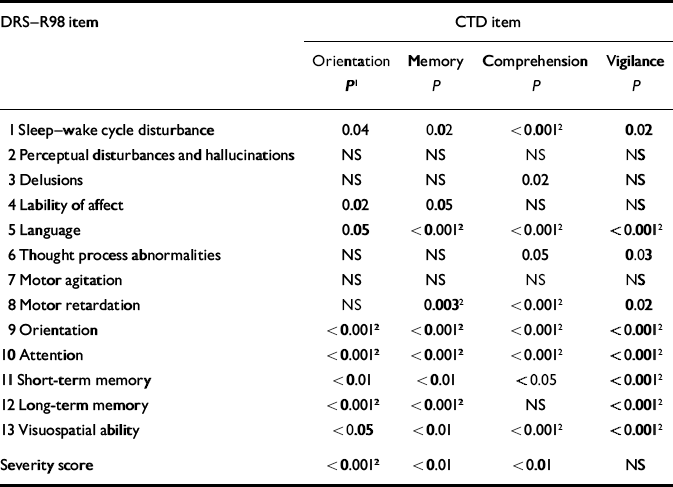
| DRS—R98 item | CTD item | |||
|---|---|---|---|---|
| Orientation P 1 | Memory P | Comprehension P | Vigilance P | |
| 1 Sleep—wake cycle disturbance | 0.04 | 0.02 | <0.0012 | 0.02 |
| 2 Perceptual disturbances and hallucinations | NS | NS | NS | NS |
| 3 Delusions | NS | NS | 0.02 | NS |
| 4 Lability of affect | 0.02 | 0.05 | NS | NS |
| 5 Language | 0.05 | <0.0012 | <0.0012 | <0.0012 |
| 6 Thought process abnormalities | NS | NS | 0.05 | 0.03 |
| 7 Motor agitation | NS | NS | NS | NS |
| 8 Motor retardation | NS | 0.0032 | <0.0012 | 0.02 |
| 9 Orientation | <0.0012 | <0.0012 | <0.0012 | <0.0012 |
| 10 Attention | <0.0012 | <0.0012 | <0.0012 | <0.0012 |
| 11 Short-term memory | <0.01 | <0.01 | <0.05 | <0.0012 |
| 12 Long-term memory | <0.0012 | <0.0012 | NS | <0.0012 |
| 13 Visuospatial ability | <0.05 | <0.01 | <0.0012 | <0.0012 |
| Severity score | <0.0012 | <0.01 | <0.01 | NS |
Seventeen patients had documented evidence of pre-existing cognitive deficits, suggesting their delirium co-occurred with chronic cognitive impairment. These patients were significantly older, had a greater aetiological burden of underlying diseases, and had more severe disturbances on the DRS–R98 and CTD than patients with delirium only (see Table 1). This difference in severity of DRS–R98 scores was accounted for by greater disturbance on the five DRS–R98 cognitive items (t=–2.8, P<0.01) rather than the eight DRS–R98 neuropsychiatric and behavioural items.
Out of concern that the inclusion of patients (n=17) with comorbid pre-existing cognitive impairment might have influenced findings, analyses were repeated for the study population with delirium only (n=83). The findings regarding DRS–R98 item frequencies, patterns of psychosis and interrelationship of cognitive items on CTD and DRS–R98 phenomenology were essentially unaltered.
DISCUSSION
This work investigates a more comprehensive range and specificity of symptoms than previous studies of delirium. We assessed 100 consecutive cases of DSM–IV delirium using valid, sensitive and standardised instruments designed for detailed phenomenological and neuropsychological evaluation of delirium. We confirmed that delirium is a complex neuropsychiatric syndrome that includes a combination of cognitive, behavioural and psychopathological features. We assessed the frequency and severity of less studied symptoms including visuospatial impairment, disorganised thinking, language impairment and different components of attention, memory, and motoric presentations, as well as more detailed evaluation of characteristics of sleep–wake cycle abnormality, perceptual disturbances and thought process abnormality. Previous phenomenological work has generally classed symptoms as present or absent without proportioning severity. This can result in more minor disturbances (e.g. of sleep) that are common in all hospitalised patients being rated as equivalent to more significant major disturbances (e.g. sleep–wake cycle reversal) that occur in delirium.
Our findings support the concept of delirium as primarily a disorder of cognition with prominent disturbance of attention consistent with DSM–IV, but also highlight the frequency of non-cognitive disturbances. Notably, the frequency of sleep and motoric disturbances were higher than previously described using the original Delirium Rating Scale (Reference Meagher and TrzepaczMeagher & Trzepacz, 1998). This may be related to sampling bias in the current study in the hospice setting or to methodological differences between the original scale and its revised version, or both.
Delirium symptoms can be divided into ‘core’ features that are almost invariably present (disturbances of attention, memory, orientation, language, thought processes and sleep–wake cycle) and ‘associated’ features that are more variable in presentation (e.g. psychotic symptoms, affective disturbances, different motoric profiles) (American Psychiatric Association, 1999; Reference TrzepaczTrzepacz, 1999). Disturbance of attention is a cardinal symptom of delirium and in our analysis associated strongly with all other cognitive deficits and language, but not with most of the non-cognitive features. Some neurologists have viewed delirium as a disorder of attention. However, the frequency of non-cognitive symptoms and their lack of association with the severity of objectively measured attentional impairment strongly support the view of delirium being a broader neuropsychiatric disorder. Unfortunately, DSM–IV criteria do not adequately reflect the importance of these other symptoms, for example, sleep–wake cycle disturbance, altered motoric behaviours, and thought content and process abnormalities. Sleep–wake cycle disturbance may underlie the fluctuating nature of delirium severity over a 24 h period (Reference Balan, Leibowitz and ZilaBalan et al, 2003).
Pattern of cognitive disruption in delirium
This study confirms delirium as a disorder of global cognition characterised by a prominent disturbance of attention and vigilance. Disorientation was the least frequent cognitive symptom, even though many non-psychiatric physicians rely on bedside tests of orientation to time, place and person as their principal mental status evaluation. Almost a quarter of our delirious patients had no evidence of disorientation on the DRS–R98 and only 52% had evidence of greater than mild disturbance of orientation on the CTD. The use of disorientation as a key indicator of delirium is thus fraught with the likelihood of missed cases, and the use of other, more consistent symptoms (such as inattention) would be a more reliable way of screening for suspected delirium. The use of instruments such as the Mini-Mental State Examination (Reference Folstein, Folstein and McHughFolstein et al, 1975), which are heavily weighted towards orientation, to detect or monitor delirium is therefore not supported by these findings.
The cognitive impairment of delirium may represent a single construct or a constellation of elements with differing under-pinnings. Poor performance on CTD attention and vigilance items was significantly related to the degree of disturbance on all other cognitive items on both the CTD and DRS–R98, but much less so for non-cognitive items. Because intact attention is required to recall new information, it is unclear whether the short-term memory deficits measured on the DRS–R98 (tested in verbal modality) and the visual memory deficits measured on the CTD are truly primary memory dysfunctions or secondary to attentional deficits. The DRS–R98 long-term memory impairments may be more related to retrieval problems and perhaps less affected by inattention than short-term memory for new material.
Performance on CTD orientation, memory and comprehension items was significantly related to fewer cognitive items compared with CTD attention. The CTD comprehension item (comprising a combination of language and executive function) was associated with more non-cognitive DRS–R98 items than the other CTD items and may denote a different domain of delirium symptoms than does attention. The combination of disturbed attention and comprehension may best represent the underlying disturbances central to overall delirium phenomenology.
Visuospatial abnormalities are not usually measured in delirium assessments even though they may underlie problems of wandering and poor environmental interactions. Mean visuospatial ability scores were almost as impaired as attention, and CTD attention is measured in a visuospatial modality. This overlap may reflect the shared role of the non-dominant posterior parietal cortex in both attention and visuospatial functions (Reference TrzepaczTrzepacz, 1999).
Despite an enduring emphasis on the characteristic fluctuating nature of delirium, this has not been directly studied. Ratings of equivalent cognitive items on the DRS–R98 and CTD were highly correlated (inversely as expected), despite one being a symptom rating scale evaluating a 24 h period and the other a cognitive test measuring current status. This suggests that certain delirium symptoms – cognition and language – are not as fluctuant as previously described, although this requires further scrutiny with serial measurement over relatively short periods.
Psychotic symptoms
The significance of psychotic symptoms in delirium remains unclear. It is not known whether patients develop these features due to specific physiological causes, cognitive impairment with misunderstanding of the external environment, misperceptions, as part of mood disturbances, or through some other aspect of individual patient vulnerability (Reference FrancisFrancis, 1992). We found that thought process abnormalities – but not delusions or perceptual disturbances – correlated with overall cognitive impairment. Both delusions and thought disorder correlated with affective lability, although perceptual disturbance was inversely correlated to both thought disorder and affective lability. Previous work comparing the psychosis of delirium with that of schizophrenia found that in delirium thought content disturbances tended to involve themes from the immediate environment and circumstances, hallucinations were frequently visual rather than auditory, and formal thought disorder typically comprised poverty of thinking and illogicality (Reference CuttingCutting, 1987). We found little relationship among the three elements of psychosis in delirium, as suggested by previous work (Reference Trzepacz and DewTrzepacz & Dew, 1995). This contrasts with functional psychotic illness, in which closer relationships have been identified (Reference O'Leary, Flaum and KeslerO'Leary et al, 2000; Reference Meagher, Quinn and BourkeMeagher et al, 2004). The psychosis of delirium also differs from dementia, in which psychotic symptoms are less common despite the shared generalised nature of brain impairment, and psychosis is associated with degree and rate of decline in cognition (Reference Levy, Cummings and FairbanksLevy et al, 1996; Reference Aalten, de Vugt and JaspersAalten et al, 2005). These differences may have important implications for delirium neuropathophysiology.
Psychotic symptoms are considered particularly common in hyperactive delirium, such as delirium tremens, but also occur in hypoactive presentations. We did not find a relationship between psychosis and motoric items, highlighting the fact that patients with quieter presentations also experience disturbing psychotic symptoms.
Advancing the concept of delirium
The concept of delirium has evolved considerably over the past 25 years. This is reflected in recent studies comparing diagnostic frequency when DSM–III, DSM–III–R, DSM–IV and ICD–10 criteria are applied to single populations (Reference Laurila, Pitkala and StrandbergLaurila et al, 2003; Reference Cole, Dendukuri and McCuskerCole et al, 2003). Future descriptions will allow further refinement of the syndrome in keeping with emerging evidence and need to account for key phenomenological issues, including the following:
-
(a) delirium detection and diagnosis are confounded by inadequate appreciation of variations in presentation and breadth of symptoms;
-
(b) core features used to define delirium should be readily detectable and occur with consistency; over-reliance on less common symptoms contributes to non-detection, which in turn hampers clinical and research efforts;
-
(c) core defining features should differentiate delirium from other neuropsychiatric disorders, especially dementia.
Study limitations
Studies with cross-sectional designs do not examine symptom evolution or whether domains of symptoms vary as overall severity changes. Longitudinal studies suggest that early delirium is characterised by psychomotor disturbances and a disrupted sleep–wake cycle (Reference Fann, Alfano and BuringtonFann et al, 2005), and that orientation difficulties, inattention, poor memory, emotional lability and sleep disturbances are more persistent symptoms (Reference Levkoff, Liptzin and EvansLevkoff et al, 1994; Reference McCusker, Cole and DendukuriMcCusker et al, 2003).
Second, the inclusion of patients with dementia might affect the clinical profile but there was little discernible effect when our study analyses were repeated for the pure-delirium study population. It appears that delirium phenomenology is altered little by the presence of dementia (Reference Trzepacz, Mulsant and DewTrzepacz et al, 1998), such that delirium symptoms tend to overshadow dementia when they co-exist although these symptoms do occur in the context of greater overall cognitive impairment. Equally, it should be recognised that in order to be truly representative of delirium, studies need to include patients who also have dementia, in recognition of the substantial comorbidity between the two conditions.
This study describes delirium phenomenology in a palliative care population, which may restrict its generalisability to other groups with this condition. Delirium is considered a unitary syndrome with a stereotyped constellation of symptoms thought to reflect disturbance of a final common neural pathway (Reference TrzepaczTrzepacz, 1999). Moreover, the term has subsumed the many synonyms that have been used to denote acute generalised cognitive disturbances in various settings but were not based on scientific evidence. Nonetheless, clinical profile may be influenced by factors that characterise different aetiological or treatment settings, but single studies have not compared symptom profiles across patient groups. Delirium occurring in cancer patients tends to be particularly multifactorial in causation, with hypoactive motoric presentations especially common (Reference Morita, Tei and TsunodaMorita et al, 2001; Reference Centeno, Sanz and BrueraCenteno et al, 2004; Reference Spiller and KeenSpiller & Keen, 2006). Our sample included patients with a broad range of relevant aetiologies and medications, many with significant psychotropic effects that could alter clinical presentation. Further studies are needed to explore the impact of aetiological, treatment and other individual patient factors on the clinical presentation of delirium.








eLetters
No eLetters have been published for this article.