Many older adults with chronic illnesses have depression, which worsens their outcomes and undermines treatment adherence. Reference Lebowitz, Pearson, Schneider, Reynolds, Alexopoulos and Bruce1 Chronic obstructive pulmonary disease (COPD) with co-occurring depression exemplifies the challenge in managing patients with chronic illnesses that require patient participation in care and can serve as a model for intervention development. Depression compounds the demands of COPD rehabilitation, which consists of strengthening, breathing and endurance exercises. We developed a personalised intervention for depression and COPD (PID-C), which focuses on both conditions. It draws from the ‘theory of reasoned action’, according to which patients weigh treatment risks and benefits Reference Ajzden, PM and JA2 aimed at shifting the balance in favour of treatment participation. The PID-C is administered by care managers who work with: (a) each patient to identify treatment barriers, and help them to work on their rehabilitation and take their prescribed antidepressants; and (b) the physicians in monitoring their patients' treatment and progress. This study (ClinicalTrials.gov: NCT00151372) tested the hypothesis that PID-C offered in the community is more effective than treatment as usual (TAU) in inducing remission of depression and reducing depressive symptoms and dyspnoea-related disability over 28 weeks.
Method
Participants were recruited from consecutive admissions to an acute in-patient pulmonary rehabilitation unit. They signed consent approved by the Weill-Cornell Institutional Review Board. The diagnosis of COPD was made by a pulmonologist according to American Thoracic Society Guidelines 3 after examination, spirometry and other tests. Participants met criteria for unipolar major depression (SCID/DSM-IV), Reference First, Spitzer, Gibbon and Williams4 and had a score of ⩾14 on the 17-item Hamilton Rating Scale for Depression (HRSD). Reference Hamilton5 Patients with other psychiatric diagnoses (except anxiety disorders) or severe cognitive impairment (i.e. Mini-Mental State Examination Reference Folstein, Folstein and McHugh6 score ⩽20) were excluded. Participants were randomised into PID-C or TAU in blocks of five.
Participants in the PID-C group had their first session of PID-C (30 min) at discharge and the remainder in their own homes at weeks 3, 4, 8, 12, 16, 20, 24, and 26 (see online supplement for a brief outline of the intervention). The PID-C care managers were social workers trained on the PID-C manual in three practice cases. The intervention targets patient-specific barriers to non-adherence in seven domains, i.e. misconceptions about COPD and depression, misunderstanding about their regimen, misattribution of depressive symptoms, hopelessness, overestimation of exercise effort, dissatisfaction with care and practical barriers. The care managers telephoned the patients' physicians and informed them of the patients' status and adherence. Physicians' recommendations for depression and COPD were not influenced by PID-C managers. For participants in the TAU group, at discharge their own physicians received a letter informing them of the diagnosis of depression.
The primary outcomes were: (a) remission of depression (HRSD ⩽7); (b) reduction of depressive symptoms; and (c) dyspnoea-related disability quantified with the Pulmonary Functional Status and Dyspnea Questionnaire – Modified (PFSDQ-M), with questions on degree of performance and frequency in ten activities influenced by dyspnoea. The PFSDQ-M has significant correlations with pulmonary function tests and partial pressure of oxygen (PO2). Reference Lareau, Meek and Roos7,Reference Lareau, Meek and Roos8 These instruments were administered by trained raters and not the care managers. Intent-to-treat analyses were performed. Cox's proportional hazards survival analysis was used to study remission. To study depressive symptoms and dyspnoea, profiles of HRSD and PFSDQ-M from discharge to 28 and from 28 to 52 weeks were compared between PID-C and TAU using mixed-effects models. The models included time-trend parameter(s), group, and time× intervention interaction. The robustness of these models was tested by comparing them with the same models after multiple imputation, using a Markov Chain Monte Carlo approach.
Results
Pulmonary patients (n = 898) were screened and 138 met criteria and provided signed consent (see online Fig. DS1 for CONSORT diagram). There were no significant clinical differences among participants randomised to PID-C or TAU. During the intervention phase 12 (18%) in the PID-C and 12 (17%) in the TAU group died. Other attrition was 25% (n = 17) in the PID-C and 17% (n = 12) in the TAU group. There were no significant differences in demographics, depression and disability between those who dropped out and those who completed the study.
By the time of discharge, 88 participants had failed to remit (HRSD >7). The PID-C group had a higher remission rate (HRSD ⩽7) than TAU participants (Wald χ2 = 5.78, d.f. = 1, P = 0.016, hazard ratio (HR) = 2.18; number needed to treat: 3.83, Fig. 1). Mixed-effects modelling showed that the PID-C group had a greater decline in HRSD than those in the TAU group between discharge and 28 weeks (treatment×time: F (1,396) = 5.40; P = 0.021); effect size at 28 weeks was 0.53 (95% CI 0.09–0.97). The PID-C group experienced greater reduction in dyspnoea-related disability (PFSDQ-M) than the TAU group (treatment× time: F (1,197) = 4.11; P = 0.044); effect size at 28 weeks was 0.40 (95% CI −0.01 to 0.87) (Fig. 1).
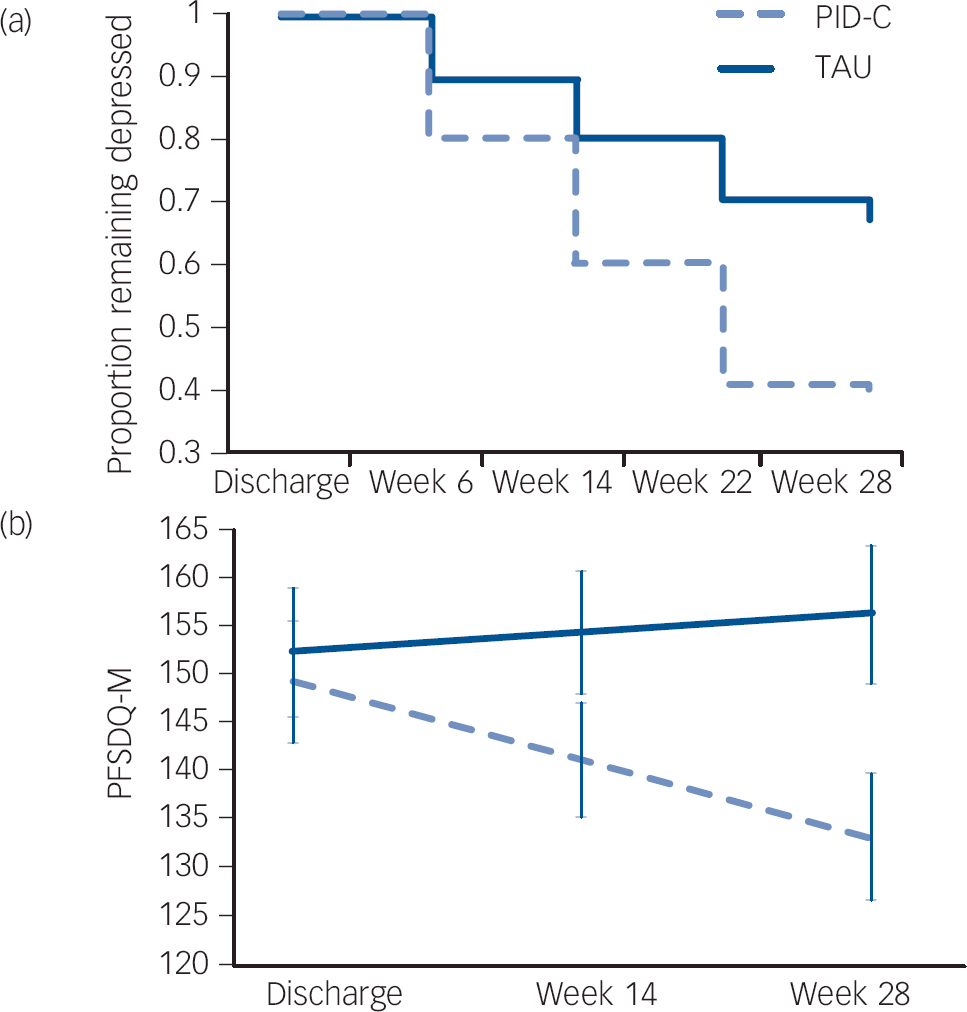
Fig. 1 (a) Remission of depression after discharge from rehabilitation hospital and (b) course of dyspnoea-related disability in older adults with major depression and chronic obstructive pulmonary disease (COPD) randomised to personalised intervention for depression and COPD (PID-C) or treatment as usual (TAU).
Remission of depression: 17-item Hamilton Rating Scale for Depression (HRSD) ⩽7. PFSDQ-M, Pulmonary Functional Status and Dyspnea Questionnaire – Modified.
Mixed-effects modelling showed that the PID-C group had greater decline in HRSD than the TAU group during the follow-up phase (treatment: F (1,105) = −2.41; P = 0.018); PID-C: least square mean at 28 weeks 9.12 (s.e. = 0.96) and at 52 weeks 9.44 (s.e. = 1.06); TAU: least square mean at 28 weeks 12.24 (s.e. = 0.87) and at 52 weeks 11.43 (s.e. = 1.02). The advantage of PID-C over TAU on the PFSDQ-M was maintained even after the intervention ended (treatment×time: t = −1.61, d.f. = 57.5, P = 0.113, Fig. 1). Comparison of least square means at 52 weeks favoured PID-C (t 128 = −3.11; P = 0.002). All comparisons cited above were similar to imputed model analyses.
Discussion
We found that PID-C led to a higher remission rate of depression (1 more remission every 3.83 patients), and also reduced depressive symptoms and dyspnoea-related disability more than TAU in community residing patients with major depression and severe COPD. These benefits lasted 6 months after the intervention end. Even though COPD has a deteriorating course, dyspnoea-related disability did not worsen in the PID-C group over 1 year. If replicated, PID-C may serve as a management model for the increasing numbers of people with both depression and a medical illness that requires active patient participation in care. Care management models for primary care patients with depression have been found effective Reference Alexopoulos, Reynolds, Bruce, Katz, Raue and Mulsant9 and cost-effective. Reference Schoenbaum, Unutzer, Sherbourne, Duan, Rubenstein and Miranda10 However, most trials targeted patients in stable medical health and have not addressed the complex needs of patients with depression who also have severe illnesses and burdensome treatment requirements. This study is unique because it targeted patients with depression at the most severe end of a deteriorating medical illness with a bleak prognosis evidenced by their high mortality (23% in 52 weeks). As PID-C requires only brief training it could be implemented by organisations serving patients with COPD such as home healthcare and rehabilitation programmes.
Study limitations include brief assessments and high attrition, both influenced by the severity of COPD. Concerns about burden limited the number of assessments. People who are severely ill with COPD may refuse follow-up because of fatigue. Nonetheless, both arms had similar attrition. Although PID-C focused on treatment adherence, other factors, including increased physician attention, may have mediated its benefits.
In conclusion, a personalised intervention offered by care managers over a period of 28 weeks increased remission rates, and improved depressive symptoms and dyspnoea-related disability more than TAU. These benefits were sustained for an additional 6 months. A next step may be to deliver and study PID-C in a group format and facilitate its dissemination. This intervention may serve as a care model for people with both depression and a medical illness with a deteriorating course, who often neglect their own care.
Acknowledgements
We thank Timothy E. Clark and Susan Friedman, LCSW for their contributions to this study.




eLetters
No eLetters have been published for this article.