Dysregulation of the hypothalamic–pituitary–adrenal (HPA) axis is believed to play a key role in the pathophysiology of depression. Reference Carroll, Cassidy, Naftolowitz, Tatham, Wilson and Iranmanesh1 Moreover, depression and its course may be partly genetically determined. Reference Bet, Penninx, Bochdanovits, Uitterlinden, Beekman and van Schoor2–Reference Brouwer, Appelhof, van Rossum, Koper, Fliers and Huyser5 We previously found a higher cortisol awakening response in people with a current or a remitted major depressive disorder. Reference Vreeburg, Hoogendijk, van Pelt, Derijk, Verhagen and van Dyck6 Together with other reports, this may indicate that HPA axis dysregulation represents a trait rather than a state marker. Reference Bhagwagar and Cowen7 A similarly elevated cortisol awakening response was observed for current panic disorder with agoraphobia. Reference Vreeburg, Zitman, van Pelt, Derijk, Verhagen and van Dyck8 To further investigate whether this dysregulation of the cortisol morning curve represents a trait marker and whether it reflects a biological vulnerability for, rather than a consequence (i.e. a biological ‘scar’) of, depression or anxiety disorders it is essential to know whether it is present before the onset of the disorder. Family inheritance is one of the most reliable risk factors of depression. Reference Sullivan, Neale and Kendler3 Recent studies have examined parental history of depression and cortisol morning levels in people without depression, and showed higher Reference Mannie, Harmer and Cowen9 or similar levels of morning cortisol Reference Vinberg, Bennike, Kyvik, Andersen and Kessing10,Reference Young, Vazquez, Jiang and Pfeffer11 compared with people without parental history. However, these studies had a limited sample size, used limited morning cortisol samples, did not include anxiety disorders or adjust for important confounders, such as smoking or sleep duration. In addition, these studies did not compare cortisol levels of unaffected people with those of people with depression or anxiety disorders. Therefore, we examined differences in 1-h awakening cortisol between unaffected people with and without parental history of depression and/or anxiety. We included an additional comparison group of participants with depression or anxiety disorders and accounted for important covariates, subthreshold symptoms, adverse childhood experiences, life events and neuroticism.
Method
Data are from the baseline assessment (September 2004 to February 2007) of the Netherlands Study of Depression and Anxiety (NESDA). Reference Penninx, Beekman, Smit, Zitman, Nolen and Spinhoven12 Respondents (aged 18–65) were recruited from the community, general practice and specialised mental healthcare services, and included people with and without psychopathology. General exclusion criteria were: a primary diagnosis of psychotic disorder, obsessive–compulsive disorder, bipolar disorder or severe addiction disorder and not being fluent in Dutch. The research protocol was approved by the Ethical Committee of participating universities and all respondents provided written informed consent.
Within the participants with no lifetime history of panic disorder, generalised anxiety disorder, agoraphobia, social phobia, major depressive disorder or dysthymia as assessed by the DSM–IV Composite International Diagnostic Interview (CIDI version 2.1) 13 and no subthreshold symptoms (Beck Anxiety Inventory (BAI)≤20, Reference Beck, Epstein, Brown and Steer14 Inventory of Depressive Symptoms (IDS)≤14) Reference Rush, Gullion, Basco, Jarret and Trivedi15 or use of antidepressants or benzodiazepines, three groups were compared.
Participants without parental history
These participants reported no parental history of depression or anxiety disorder, as assessed by the family history inventory (n = 238) on which the respondent reported on the presence of an anxiety or depressive disorder in all individual first-degree relatives.
Participants with self-reported parental history
These participants reported at least one biological parent with depression and/or anxiety (n = 173).
Participants with diagnosed parental history
These participants (n = 122) had previously participated in the Adolescents at Risk of Anxiety and Depression (ARIADNE) study Reference Landman-Peeters, Hartman, van der Pompe, den Boer, Minderaa and Ormel16 into the development of depression and anxiety disorders among offspring (aged 13–25) of people with psychiatric disorders, approximately 4 years before participating in NESDA. Parents were treated for depression or panic disorder in specialised mental healthcare. Both offspring and parent were interviewed with the CIDI. Reference Kessler and Üstün17
Comparison group
A comparison group of participants consisted of 2010 people who were diagnosed with a remitted or current major depressive disorder and/or current panic disorder with agoraphobia as assessed by the CIDI, for whom we observed the highest cortisol levels as compared to people without psychopathology. Reference Vreeburg, Hoogendijk, van Pelt, Derijk, Verhagen and van Dyck6,Reference Vreeburg, Zitman, van Pelt, Derijk, Verhagen and van Dyck8
Exclusions and final sample
We subsequently excluded 22 pregnant or breastfeeding women and 140 participants on corticosteroids, leaving 2381 respondents. Of these, 1630 (68.5%) returned sufficient saliva samples: 180 (81.8%) without parental history; 114 (70.4%) with self-reported parental history; 74 (61.7%) with diagnosed parental history and 1262 (67.2%) with a psychiatric diagnosis, P<0.001).
Salivary cortisol
A minimally intrusive way to measure basal cortisol level is through saliva sampling, reflecting the active unbound form of cortisol. Reference Kirschbaum and Hellhammer18 As described in more detail elsewhere, Reference Vreeburg, Kruijtzer, van Pelt, van Dyck, DeRijk and Hoogendijk19 participants were instructed to collect saliva samples at home on a regular (working) day shortly after the interview. Instructions concerning saliva sampling prohibited smoking, eating, drinking tea or coffee or brushing teeth within 15 min before sampling. Furthermore, no dental work 24 h prior to sampling was allowed. Saliva samples were obtained using Salivettes (Sarstedt, Germany) at four time points; upon awakening (T 1) and 30 (T 2), 45 (T 3) and 60 (T 4) min later. After return by mail, samples were stored at –80 °C. Cortisol analysis was performed by competitive electrochemiluminescence immunoassay (E170 Roche, Switzerland). The functional detection limit was 2.0 nmol/l and the intra- and interassay variability coefficients in the measuring range were less than 10%. Data cleaning excluded values >2 standard deviations above the mean.
In addition to conducting linear mixed models analyses (see statistical analyses section) using all four morning saliva samples, we calculated the area under the curve with respect to the increase (AUCi) and with respect to the ground (AUCg) using Pruessner's formulas. Reference Pruessner, Kirschbaum, Meinlschmid and Hellhammer20 The AUCg is an estimate of the total cortisol secretion over the first hour after awakening, whereas the AUCi is a measure of the dynamic of the cortisol awakening response, more related to the sensitivity of the system, emphasising changes over time after awakening. Reference Edwards, Clow, Evans and Hucklebridge21–Reference Schmidt-Reinwald, Pruessner, Hellhammer, Federenko, Rohleder and Schürmeyer23 If samples were collected outside of a margin of 5 min around the time protocol, values were assigned missing. Morning cortisol analyses included all participants with at least two valid morning cortisol values (n = 1630), since linear mixed models analyses can adequately interpolate for missing data. Reference Gueorguieva and Krystal24 For 1441 participants all four morning samples were available and could therefore be included in the AUC analyses (158 without parental history, 101 with self-reported parental history, 68 with diagnosed parental history and 1114 with a psychiatric diagnosis).
Covariates
Previously, we described effects of gender, age, time of awakening, working status, season, sleep duration, physical activity and smoking on salivary cortisol levels, Reference Vreeburg, Kruijtzer, van Pelt, van Dyck, DeRijk and Hoogendijk19 which were considered as covariates. Respondents reported time of awakening and working status on the sampling day. Season was categorised into less (October through to February) and more daylight (March through to September) months. Average sleep duration during the past 4 weeks was dichotomised as ≤6 or >6 h/night, Reference Levine, Kripke, Kaplan, Lewis, Naughton and Bowen25 and smoking status as current versus non-smoker. Physical activity was assessed using the International Physical Activity Questionnaire and expressed in 1000 MET-min (metabolic energy turnover per min) a week. Reference Craig, Marshall, Sjöström, Bauman, Booth and Ainsworth26 Menstrual cycle phase, menopausal status or use of oral contraceptives were not associated with salivary cortisol and were therefore not included as covariates. Reference Vreeburg, Kruijtzer, van Pelt, van Dyck, DeRijk and Hoogendijk19
Explanatory factors
To examine whether found effects were influenced by neuroticism, childhood trauma and life events we adjusted all analyses for these factors. Neuroticism was measured with the 12-item subscale of the NEO Five-Factor Inventory (NEO–FFI) Questionnaire Reference Costa and McCrae27 ranging from 0 (low neuroticism) to 48 (high neuroticism). In order to examine the role of earlier childhood trauma, we constructed a cumulative childhood trauma index using the NEMESIS childhood trauma interview, Reference Vreeburg, Hoogendijk, van Pelt, Derijk, Verhagen and van Dyck6 which summarises the frequency of four reported traumas before the age of 16 – emotional neglect, psychological abuse, physical abuse and sexual abuse – resulting in an index score between 0 and 8. Finally, negative life events in the past year were assessed by the Brugha questionnaire Reference Brugha, Bebbington, Tennant and Hurry28 and included 12 specific events and one ‘other’ category asking about another serious (not specified) negative life event. Since symptom severity scores were not associated with salivary cortisol levels in our previous studies, these were not included as covariates. Reference Vreeburg, Hoogendijk, van Pelt, Derijk, Verhagen and van Dyck6,Reference Vreeburg, Zitman, van Pelt, Derijk, Verhagen and van Dyck8
Statistical analyses
Baseline characteristics were compared using χ2 and ANOVA statistics. Area under the curves showed normal distributions, 1-h awakening cortisol levels were slightly skewed and therefore log-transformed for linear mixed models analyses. Back-transformed values were used in the figure. To analyse differences in 1-h awakening cortisol across groups, ANCOVA analyses with AUCi and AUCg were conducted. First, we compared the three unaffected parental history groups. Additionally, parental history groups were compared with the group of participants with a psychiatric diagnosis. Random coefficient analysis of the four morning cortisol levels was performed using linear mixed models, which keeps original values on all four data points, can accommodate for incomplete cases, and takes correlation between repeated measurements into account. Reference Gueorguieva and Krystal24 Parental history groups, time point (T 1, T 2, T 3, T 4) and all covariates were entered as fixed factors, participants were treated as a random effect and a random intercept was estimated. To examine whether the course of cortisol level after awakening was different across groups, we added a group × time interaction term. For significant findings, effect sizes were calculated with Cohen's d. All analyses were conducted using SPSS version 15.0 for Windows.
Results
Sample characteristics are presented in Table 1. Participants with diagnosed parental history were younger, had later awakening times, less often slept ≤6 h/night, had a lower trauma index score and higher neuroticism scores than other unaffected participants (Table 1). Participants with self-reported parental history reported more childhood trauma and had higher neuroticism scores than unaffected participants without parental history. Compared with parents in the diagnosed parental history group, parents in the self-reported parental history group more often had only anxiety disorders. Diagnosed parents mostly had depression with or without comorbid anxiety disorder.
Table 1 Sample characteristics
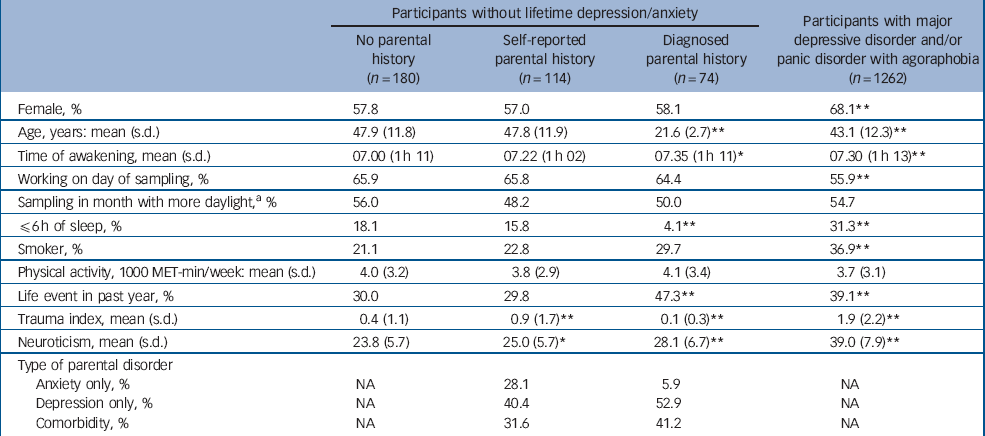
| Participants without lifetime depression/anxiety | Participants with major depressive disorder and/or panic disorder with agoraphobia (n = 1262) | |||
|---|---|---|---|---|
| No parental history (n = 180) | Self-reported parental history (n = 114) | Diagnosed parental history (n = 74) | ||
| Female, % | 57.8 | 57.0 | 58.1 | 68.1** |
| Age, years: mean (s.d.) | 47.9 (11.8) | 47.8 (11.9) | 21.6 (2.7)** | 43.1 (12.3)** |
| Time of awakening, mean (s.d.) | 07.00 (1 h 11) | 07.22 (1 h 02) | 07.35 (1 h 11)* | 07.30 (1 h 13)** |
| Working on day of sampling, % | 65.9 | 65.8 | 64.4 | 55.9** |
| Sampling in month with more daylight,a % | 56.0 | 48.2 | 50.0 | 54.7 |
| ≤6 h of sleep, % | 18.1 | 15.8 | 4.1** | 31.3** |
| Smoker, % | 21.1 | 22.8 | 29.7 | 36.9** |
| Physical activity, 1000 MET-min/week: mean (s.d.) | 4.0 (3.2) | 3.8 (2.9) | 4.1 (3.4) | 3.7 (3.1) |
| Life event in past year, % | 30.0 | 29.8 | 47.3** | 39.1** |
| Trauma index, mean (s.d.) | 0.4 (1.1) | 0.9 (1.7)** | 0.1 (0.3)** | 1.9 (2.2)** |
| Neuroticism, mean (s.d.) | 23.8 (5.7) | 25.0 (5.7)* | 28.1 (6.7)** | 39.0 (7.9)** |
| Type of parental disorder | ||||
| Anxiety only, % | NA | 28.1 | 5.9 | NA |
| Depression only, % | NA | 40.4 | 52.9 | NA |
| Comorbidity, % | NA | 31.6 | 41.2 | NA |
In total 68.5% of the respondents showed an increase in cortisol levels within 1 h of waking up (65.0% in the group without parental history, 68.4% in the self-reported parental history group, 77.0% in the diagnosed parental history group, P = 0.17). Parental history groups showed significant differences in AUCg (d.f. = 2/316, F = 3.47, P = 0.03), but not in AUCi (d.f. = 2/316, F = 1.73, P = 0.18). The group with diagnosed parental history showed higher overall adjusted cortisol levels than participants without parental history, reflected by a larger AUCg (P = 0.01, effect size (Cohen's d) = 0.50, Table 2) and confirmed by a borderline significant direct effect in linear mixed models analyses (F = 3.39, P = 0.07, data not shown). There was a trend towards a larger AUCi (P = 0.08, d = 0.35, Table 2) for the diagnosed parental history group, but the interaction with time was not significant (F = 1.09, P = 0.35). The group with self-reported parental history did not show significant differences in morning cortisol compared with the group without parental history (Table 2, linear mixed models analyses: direct effect: F = 0.05, P = 0.82; interaction with time: F = 0.85, P = 0.47).
Table 2 Results of age-adjusted and fully adjusted ANCOVA analyses associating parental history with the cortisol awakening curvea

| No parental history (n = 183) Mean (s.e.) | Self-reported parental history (n = 120) Mean (s.e.) | Self-reported v. no parental history P | Diagnosed parental history (n = 73) Mean (s.e.) | Diagnosed v. no parental history P | |
|---|---|---|---|---|---|
| AUCg, nmol/l/hb | 17.5 (0.5) | 17.6 (0.7) | 0.91 | 19.9 (1.0) | 0.07 |
| AUCg, nmol/l/hc | 17.4 (0.5) | 17.5 (0.6) | 0.90 | 20.6 (1.0) | 0.01 |
| AUCi, nmol/l/hb | 1.0 (0.5) | 1.7 (0.6) | 0.38 | 3.1 (1.0) | 0.09 |
| AUCi, nmol/l/hc | 1.0 (0.5) | 1.8 (0.6) | 0.30 | 3.1 (1.0) | 0.08 |
Additional analyses
When comparing unaffected people with diagnosed parental history with participants with a psychiatric diagnosis, no difference was observed (mean AUCg 20.0 (s.e. = 0.9) v. 19.5 (s.e. = 0.2) nmol/l/h, P = 0.62, mean AUCi 2.6 (s.e. = 0.8) v. 2.6 (s.e. = 0.2) nmol/l/h, P = 0.46, respectively, after full adjustment). However, this group of participants with a psychiatric diagnosis did differ from the group without parental history (mean AUCg 17.7 (s.e. = 0.6) nmol/l/h, P = 0.002, mean AUCi 1.4 (s.e. = 0.5) nmol/l/h, P = 0.03) and participants with self-reported parental history (mean AUCg 17.8 (s.e. = 0.7) nmol/l/h, P = 0.02, mean AUCi 2.1 (s.e. = 0.6) nmol/l/h, P = 0.46). These results are graphically represented in Fig. 1.
Additional adjustment for childhood trauma, neuroticism and life events in the past year did not essentially change results. In order to conduct analyses in which age groups are more equal, we repeated the analyses excluding all participants older than 30 years. Mean AUCg was higher for diagnosed parental history (18.6 (s.e. = 0.8) nmol/l/h, P = 0.03, d = 0.65, n = 68) and also for self-reported parental history (17.7 (s.e. = 1.7) nmol/l/h, P = 0.13, d = 0.50, n = 13) compared with participants without parental history (14.5 (s.e. = 1.5) nmol/l/h, n = 20).
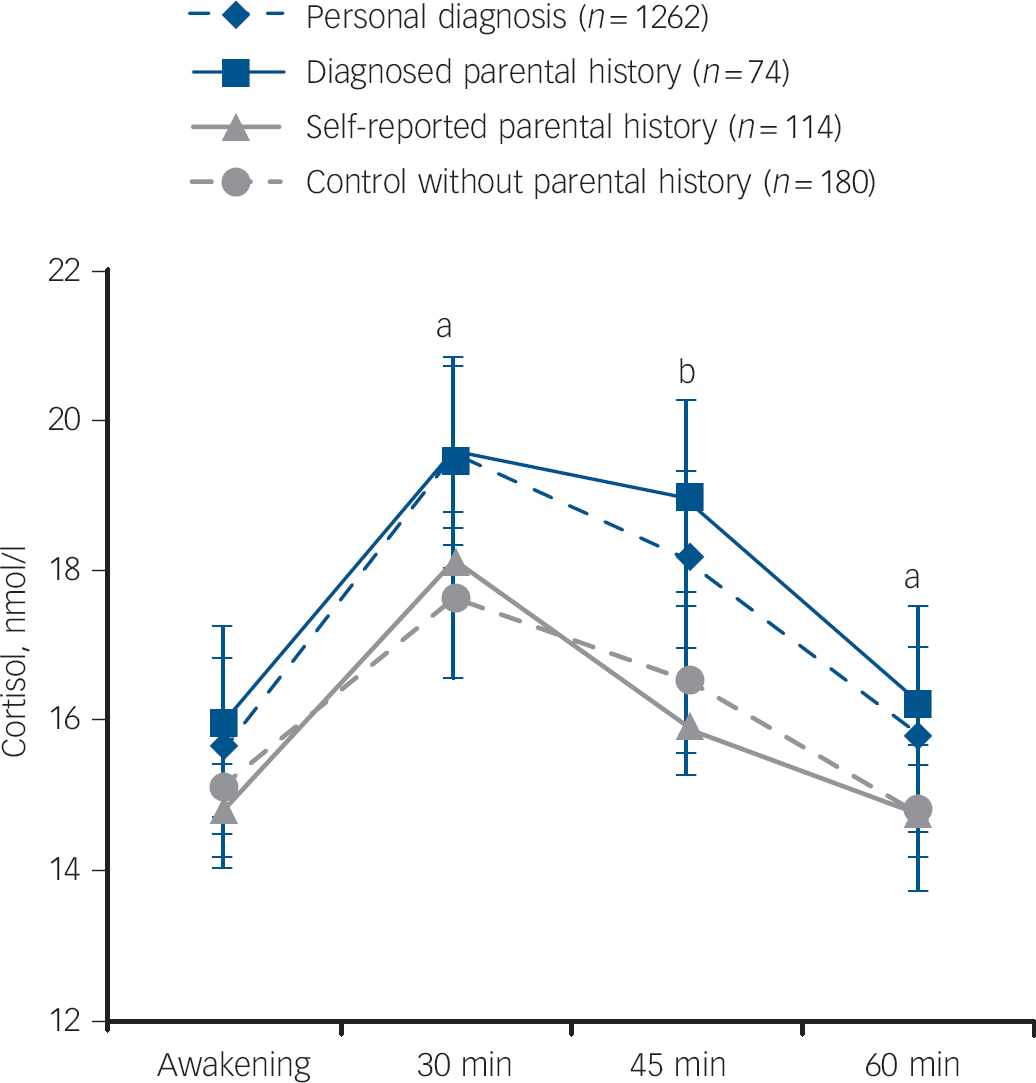
Fig. 1 Mean salivary cortisol levels for unaffected participants without parental history, with self-reported parental history and with diagnosed parental history and for participants with depression or panic disorder.
Adjusted for gender, age, working status, time of awakening, sleep duration, season, physical activity and smoking. Results of linear mixed models analyses (compared with controls without parental history): self-reported parental history: P = 0.92, interaction with time: P = 0.48. Diagnosed parental history: P = 0.07 and interaction with time: P = 0.62. Personal diagnosis: P = 0.01 and interaction with time: P = 0.25. Error bars illustrate standard errors. (a) P<0.05 for group with personal diagnosis compared with controls; (b) P<0.05 for personal diagnosis and diagnosed parental history compared with controls.
Since the self-reported parental history group had relatively more parents with anxiety compared with depression or comorbidity, we reanalysed differences only including participants with self-reported parental depression or comorbid disorders. Results showed a higher AUCg in the diagnosed parental history group (mean AUCg = 20.5 (s.e. = 1.0), P = 0.01) and a similar AUCg in the self-reported parental history group (mean AUCg = 17.3 (s.e. = 0.8), P = 0.96) as compared with controls (mean AUCg = 17.3 (s.e. = 0.5) nmol/l/h).
Discussion
Main findings
This study shows that cortisol awakening levels in unaffected participants with diagnosed parental history of depression or anxiety were significantly higher than in unaffected participants without parental history, and similar to those in participants with major depressive disorder or panic disorder with agoraphobia. Reference Vreeburg, Hoogendijk, van Pelt, Derijk, Verhagen and van Dyck6,Reference Vreeburg, Zitman, van Pelt, Derijk, Verhagen and van Dyck8 In fact, the effect size for unaffected participants with diagnosed parental history was medium (d = 0.50) and higher than the effect sizes (d = 0.15–0.32) previously described for major depressive disorder and panic disorder with agoraphobia. Reference Vreeburg, Hoogendijk, van Pelt, Derijk, Verhagen and van Dyck6,Reference Vreeburg, Zitman, van Pelt, Derijk, Verhagen and van Dyck8 Our results suggest that a higher cortisol awakening curve represents a trait factor and reflects an underlying biological vulnerability marker.
The higher cortisol levels were only found in offspring of patients with a CIDI diagnosis treated in specialised mental healthcare and not in offspring with affected parents based on self-report. Although often used to assess parental history, our findings may indicate that self-report of parental psychopathology is of insufficient reliability. Alternatively, the parents reported on by their offspring may represent the milder cases and be associated with lower familial loading, especially since it was not asked whether parents were treated, in contrast to the diagnosed parents of the ARIADNE sample for whom referral status was required for enrolment in the study. Higher familial loading in the diagnosed parental history group might also be reflected by the higher neuroticism scores in this group relative to the self-reported parental history group. In addition, when excluding participants older than 30 years, effect sizes were higher in both parental history groups relative to the group without parental history, also possibly reflecting the importance of familial loading. Presumably, and consistent with the idea that cortisol elevation represents a liability for later onset of anxiety and depression, part of the younger parental history group may not have developed depression or anxiety yet. However, the older group, being still unaffected, may never develop depression or anxiety disorder. The larger proportion of anxiety disorder without comorbid depression in the self-reported parental history group did not explain the results, since additional analysis excluding anxiety disorder without comorbid depression generated similar results.
Our results are in line with one study reporting higher morning cortisol, Reference Mannie, Harmer and Cowen9 but in contrast with studies reporting no association with morning cortisol in unaffected people with parental history. Reference Vinberg, Bennike, Kyvik, Andersen and Kessing10,Reference Young, Vazquez, Jiang and Pfeffer11 However, the latter two studies measured the morning curve with only one and two saliva samples, respectively. Participants with diagnosed parental history showed overall higher cortisol levels (AUCg) and a trend towards a higher increase (AUCi), indicating that especially the total cortisol secretion within the first hour after awakening is elevated. This emphasises the importance of collecting multiple morning samples. The increased AUCg is possibly reflective of basal hyperactivity of the HPA axis, since it is related to cortisol levels during the rest of the day, whereas the AUCi is less dependent on the diurnal cortisol rhythm and is regarded an indicator of reactivity of the HPA axis. Reference Edwards, Clow, Evans and Hucklebridge21–Reference Schmidt-Reinwald, Pruessner, Hellhammer, Federenko, Rohleder and Schürmeyer23 It has been hypothesised that the AUCg is particularly suited to measure trait effects, whereas the AUCi is better suited for state effects. Reference Hellhammer, Fries, Schweisthal, Schlotz, Stone and Hagemann29
An important finding was that cortisol levels were comparable with those found in participants with a psychiatric diagnosis. Previous studies did not compare morning cortisol levels of individuals with familial history with people with depression/anxiety. However, reports on cortisol release on the dexamethasone/corticotropin-releasing hormone test showed cortisol levels in between controls and patients. Reference Holsboer, Lauer, Schreiber and Krieg30
Genetic factors
Genetic factors could account for our findings. Reference Sullivan, Neale and Kendler3,Reference Wüst, Federenko, Hellhammer and Kirschbaum31 The cortisol awakening response has a heritability of 32–48% Reference Wüst, Federenko, Hellhammer and Kirschbaum31,Reference Kupper, de Geus, van den Berg, Kirschbaum, Boomsma and Willemsen32 and depression has a heritability of 37%. Reference Sullivan, Neale and Kendler3 It could be that the same genes underlie both depression and morning cortisol regulation, for example the serotonin transporter gene, Reference Wüst, Kumsta, Treutlein, Frank, Entringer and Schulze33 mineralocorticoid or glucocorticoid receptor genes. Reference Bet, Penninx, Bochdanovits, Uitterlinden, Beekman and van Schoor2 Epigenetic factors such as histone modification or methylation of deoxyribonucleic acid (DNA) as a result of early life stress could also play a role. Reference Murgatroyd, Patchev, Wu, Micale, Bockmühl and Fischer34
Also, early childhood trauma and adversity could be more prevalent in people growing up in families with psychiatric problems, which may exert direct effects on inflammatory and glucocorticoid signalling. Reference Danese, Pariante, Caspi, Taylor and Poulton35,Reference Miller, Chen, Kok, Walker, Lim and Nicholls36 However, adjustment for childhood trauma, including emotional abuse, did not change our results, since these variables were not associated with saliva cortisol levels. Also subthreshold symptoms, as measured using the IDS or BAI score, were not associated with salivary cortisol levels. Reference Vreeburg, Hoogendijk, van Pelt, Derijk, Verhagen and van Dyck6 Therefore, it is unlikely that subthreshold symptoms or past traumatic events can completely account for the relationship found in our study. Possibly, a differential home environment due to parental depression could have played a role, for example less structure provided by parents Reference Ellenbogen and Hodgins37 and chronic family stress. Reference Ronsaville, Municchi, Laney, Cizza, Meyer and Haim38 Exposure to parental depression could also have resulted in more stress among the children, even when the depression is in remission, because of possible personality deviances and role function problems of the parents. Reference Ormel, Oldehinkel, Nolen and Vollebergh39,Reference Ormel, Oldehinkel and Vollebergh40
Strengths and limitations
Our study had several strengths, including offspring from parents with confirmed diagnoses, the use of four morning cortisol samples and an additional comparison group of participants with a psychiatric diagnosis. Moreover, we studied an adult sample, whereas most studies included children, showing that HPA axis dysregulations persist into adulthood. Some limitations have to be acknowledged. First, non-adherence to the sampling instructions could have resulted in a blunted cortisol response after awakening. Reference Kudielka, Broderick and Kirschbaum41 However, even when awakening is closely monitored at least 15% of all participants still do not respond with a cortisol rise. Reference Dockray, Bhattacharyya, Molloy and Steptoe42 Moreover, we have no reason to believe that possible non-adherence is unevenly distributed among our groups. Second, since the majority of parents with a diagnosis had a depressive disorder, our results are mostly restricted to depression with or without comorbid anxiety. Future studies are warranted to specifically examine HPA axis activity and parental history of anxiety disorders.
Implications
Our study adds to the evidence that HPA axis alterations in depression and anxiety represent a trait factor that may indicate a biological vulnerability for the development of these disorders. Therefore, the cortisol awakening curve may constitute an important endophenotype of depression (and anxiety) for genetic research. Although the clinical relevance of the difference in 1-hour awakening cortisol needs to be explored in further large-scale research, there is some evidence that morning cortisol levels predict unfavourable metabolic Reference Dekker, Koper, van Aken, Pols, Hofman and de Jong43 and mental health outcomes. Reference Harris, Borsanyi, Messari, Stanford, Cleary and Shiers44






eLetters
No eLetters have been published for this article.