Major depressive disorder (MDD) is one of the leading causes of disability worldwide.1 Pharmacotherapy is an important evidence-based treatment.Reference Cuijpers, Noma, Karyotaki, Vinkers, Cipriani and Furukawa2 Although there are dozens of effective antidepressants available,Reference Cipriani, Furukawa, Salanti, Chaimani, Atkinson and Ogawa3 only about 35% of patients achieve symptomatic remission with the first antidepressant treatmentReference Rush, Trivedi, Wisniewski, Nierenberg, Stewart and Warden4,Reference Kato, Furukawa, Mantani, Kurata, Kubouchi and Hirota5 and treatment of those with inadequate response remains a critical clinical question. In case of inadequate response, clinical guidelines have traditionally recommended considering dose escalation of the first antidepressant, switching to another antidepressant, combining with another antidepressant, or augmenting with a second agent other than antidepressants.Reference MacQueen, Santaguida, Keshavarz, Jaworska, Levine and Beyene6 Recent meta-analyses showed no evidence of clinical benefits of dose escalation,Reference Rink, Braun, Bschor, Henssler, Franklin and Baethge7–Reference Furukawa, Cipriani, Cowen, Leucht, Egger and Salanti9 combinationReference Henssler, Bschor and Baethge10 or switching in general.Reference Bschor, Kern, Henssler and Baethge11 Across-class antidepressant combinationReference Henssler, Bschor and Baethge10 and switching,Reference Papakostas, Fava and Thase12 however, remain promising, and pharmacological augmentation with various non-antidepressant agents has been confirmed.Reference Carter, Strawbridge, Husain, Jones, Short and Cleare13 A systematic review of treatment guidelines for MDD published in the English language found that all the ten identified guidelines recommend augmentation with atypical antipsychotics such as aripiprazole, quetiapine, risperidone, olanzapine and brexpiprazole.Reference Taylor, Marwood, Oprea, DeAngel, Mather and Valentini14 Aripiprazole is the first atypical antipsychotic approved for augmentation treatment of adults with antidepressant-refractory depression in the USA and recommended by National Institute for Health and Care Excellence (NICE) guidelines in the UK.15 It is the only antipsychotic augmentation agent approved for MDD in Japan as of 2021. It remains one of the best-studied augmentation agents,Reference Carter, Strawbridge, Husain, Jones, Short and Cleare13 and several meta-analyses of randomised controlled trials have established its overall benefits.Reference Luan, Wan, Zhang and Zhao16–Reference Komossa, Depping, Gaudchau, Kissling and Leucht18
Of note, the dose range of aripiprazole recommended in the guidelines was very wide, ranging from 2 to 15 mg.Reference Taylor, Marwood, Oprea, DeAngel, Mather and Valentini14 Such a wide range would be confusing and unhelpful for clinicians considering initiating aripiprazole augmentation. It may also eventually result in some patients receiving too low a dose to be effective and some patients receiving too high a dose that only increases the risk of adverse events without additional benefits. Finding the optimal target dose enables clinicians to use aripiprazole augmentation effectively and safely. We aimed to summarise the currently available evidence with the use of dose–effect meta-analysis to inform this clinical question.
Objectives
To investigate the dose–effect relationship of aripiprazole as an augmentation agent for treating MDD with inadequate response to antidepressant therapy.
Method
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline.Reference Page, McKenzie, Bossuyt, Boutron, Hoffmann and Mulrow19 The protocol was registered in PROSPERO (CRD42021234782).
Criteria for considering studies for this review
Type of study
To examine dose–effect relationships, we included all assessor-masked trials that compared two or more doses of aripiprazole as augmentation of antidepressant therapy within a trial. We excluded quasi-randomised trials and studies where sequence generation was at high risk of bias, or allocation was clearly not concealed.
Type of participant
Participants were eligible if they were aged 18 years or older of either gender, with a primary diagnosis of MDD according to standard operationalised diagnostic criteria (Feighner criteria, Research Diagnostic Criteria, DSM-III, DSM-III-R, DSM-IV, DSM-5, ICD-10) with inadequate response to at least one trial of antidepressant therapy. People receiving psychotherapy were included, but those receiving electroconvulsive therapy were excluded. To mimic current practice,Reference Luo, Kataoka, Ostinelli, Cipriani and Furukawa20 we excluded trials with ≥20% of participants initially treated with tricyclic antidepressants. We excluded trials for people with depression who had a serious concomitant physical illness and trials of women with postpartum depression.
Type of intervention
We compared aripiprazole augmentation (either fixed or flexible dose) with the continuation of antidepressant treatment. We did not include active comparators, such as dose escalation of the initial antidepressant, switching to another antidepressant or adding another agent, because we were interested in the dose–effect relationship of aripiprazole augmentation. We included treatment groups within and outside the licensed dose range as defined by the international drug approval agencies or guidelines.
Search methods for identification of studies
Electronic searches and research registers
We systematically searched Cochrane CENTRAL and PubMed from inception to 16 February 2021. We used broad search terms for depression (depress* OR dysthymi* OR adjustment disorder* OR mood disorder* OR affective disorder OR affective symptoms) in conjunction with generic and commercial names (aripiprazole OR Abilify). We imposed no date, language or publication status restriction. No search filter was used. We searched ClinicalTrials.gov and the World Health Organization's International Clinical Trials Registry Platform (ICTRP) from inception to 16 February 2021 to identify unpublished or ongoing studies.
Drug approval agencies
We hand-searched the following drug approval agencies for additional published and unpublished data up to 20 February 2021: Food and Drug Administration (FDA) (USA), European Medicines Agency (EU), Medicines and Healthcare products Regulatory Agency (UK), Therapeutic Goods Administration (Australia) and Pharmaceuticals and Medical Devices Agency (Japan).
Reference lists and others
We checked the reference lists of all the included studies and review articles for additional references. We also contacted experts in the field to identify unpublished and on-going trials. We searched the website of Otsuka Pharmaceutical Co., Ltd. (Tokyo, Japan), which developed and is marketing aripiprazole, contacted the company and requested supplemental unpublished information about their pre-marketing and post-marketing trials (on 27 February 2021).
Data collection and extraction
Two review authors (Y.F., E.G.O.) independently screened and selected the included studies. Two review authors (Y.F., E.G.O.) extracted data independently from the included studies (up to 2 April 2021). We used the Cochrane Risk of Bias tool version 2 (RoB 2)Reference Sterne, Savović, Page, Elbers, Blencowe and Boutron21 to assess and summarise the risk of bias. Disagreements were resolved through discussion.
Primary outcomes
Our primary outcomes were efficacy (measured by the total number of responders, defined as ≥50% reduction on a standardised observer rating scale for depression), tolerability (measured by the total number of participants who dropped out because of adverse events) and acceptability (measured by the total number of participants who dropped out for any reason). When the number of responders was not reported, we calculated using a validated imputation method.Reference Furukawa, Cipriani, Barbui, Brambilla and Watanabe22 Those who had been randomised but not accounted for in the original study were assumed to have dropped out for some reason other than adverse events and without responding to treatment. We used the number of randomised participants as the denominator for all outcomes. We used the odds ratio of each outcome to synthesise data.Reference Bakbergenuly, Hoaglin and Kulinskaya23,Reference Doi, Furuya-Kanamori, Xu, Lin, Chivese and Thalib24
Statistical analysis
We used R (version 4.0.5; R Foundation, Vienna, Austria), meta (version 4.18-0) and dosresmeta (version 2.0.1) software packages on Mac (Big Sur).25–Reference Crippa and Orsini27
Assessment of heterogeneity and reporting biases
We investigated the heterogeneity between studies using the variance partition coefficient.Reference Crippa, Discacciati, Bottai, Spiegelman and Orsini28 This is the percentage of variation that is attributed to heterogeneity rather than sampling error and can be interpreted similarly to the I 2. To evaluate the possibility of small-study effects and their association with reporting bias, we drew contour-enhanced funnel plots for comparisons.Reference Balduzzi, Rücker and Schwarzer26
Dose–effect meta-analysis
Given the clinical and methodological heterogeneity likely present in the included studies, we used a random-effects model. We used the maximum dose of aripiprazole as the representative value for flexible-dose arms, because it is determined prior to randomisation and the result can be interpreted as the effect of starting the treatment with the intention to prescribe up to that maximum dose. We modelled the dose–effect relationship with restricted cubic splines with three knots. We used cubic splines with three knots as it is the smallest number required and to avoid overfitting, known to occur if there are few data points and many knots.Reference Harrell29 It was also used for a previous dose–effect meta-analysis of antidepressants for MDD.Reference Furukawa, Cipriani, Cowen, Leucht, Egger and Salanti9 Restricted cubic splices perform better when the knots are placed at doses where the outcome changes.Reference Hamza, Cipriani, Furukawa, Egger, Orsini and Salanti30 We chose 3 mg, the lowest dose we expected to be effective,Reference Kamijima, Higuchi, Ishigooka, Ohmori, Ozaki and Kanba31 and set the rest of the knots at its half dose (1.5 mg) and its double dose (6 mg). The last (6 mg) is close to the real-world dosing trend (6.9 mg in 2010, with a decreasing trend)Reference Jing, Guo, Kalsekar, Forbes, Hebden and Thase32 and therefore we believe the locations of knots cover the dose range where the outcome may change. We used the one-stage method to aggregate data.Reference Crippa, Discacciati, Bottai, Spiegelman and Orsini28 We used the dose–effect curve of the primary analysis to estimate the 50% effective dose (ED50) and 95% effective dose (ED95), as is customary in dose–effect analysis. The ED50 is the mean dose that produces 50% of the maximum effect in log-odds ratio compared with placebo augmentation, and the ED95 is the mean dose that produces 95% of the maximum effect in log-odds ratio. Y.F. wrote the code and T.H. double-checked the code.
Sensitivity analyses
To ascertain the robustness of the primary analyses, we conducted four sensitivity analyses using actual dose delivered and using different locations of knots.
Changes from the protocol
During systematic review, we found that the Thase & Rush staging modelReference Thase and Rush33 we planned to use to evaluate treatment resistance was not reported in the original reports. We therefore reported the definition of antidepressant-refractory depression descriptively. We initially planned to use only fixed-dose trials for the primary analyses, but we only found two fixed-dose arms at low doses. We therefore decided to include flexible-dose studies using their maximum target dose for the primary analyses and to include flexible-dose studies using their actually prescribed mean dose for sensitivity analyses. We evaluated the certainty of evidence using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework.
Results
We identified 2678 records via databases and registries, and 14 records through contact with the pharmaceutical company. We assessed 81 full-text records for eligibility and included 10 studies (7 published and 3 unpublished), giving a total of 2625 participants (Fig. 1).Reference Kamijima, Higuchi, Ishigooka, Ohmori, Ozaki and Kanba31,Reference Berman, Marcus, Swanink, McQuade, Carson and Corey-Lisle34–42 The list of excluded studies is provided in Supplementary Table 1, available at https://doi.org/10.1192/bjp.2021.165. The 10 studies included 9 two-armed studies and 1 three-armed study, consisting of 21 treatment arms in total: 2 fixed-dose arms, 9 flexible-dose arms and 10 placebo-arms. All three unpublished studies40–42 were prematurely terminated and we could obtain data only for tolerability and not for efficacy and acceptability, resulting in seven trials for efficacy analysis, ten for tolerability and seven for acceptability. The study year ranged between 2007 and 2018. Table 1 presents the characteristics of the included studies.
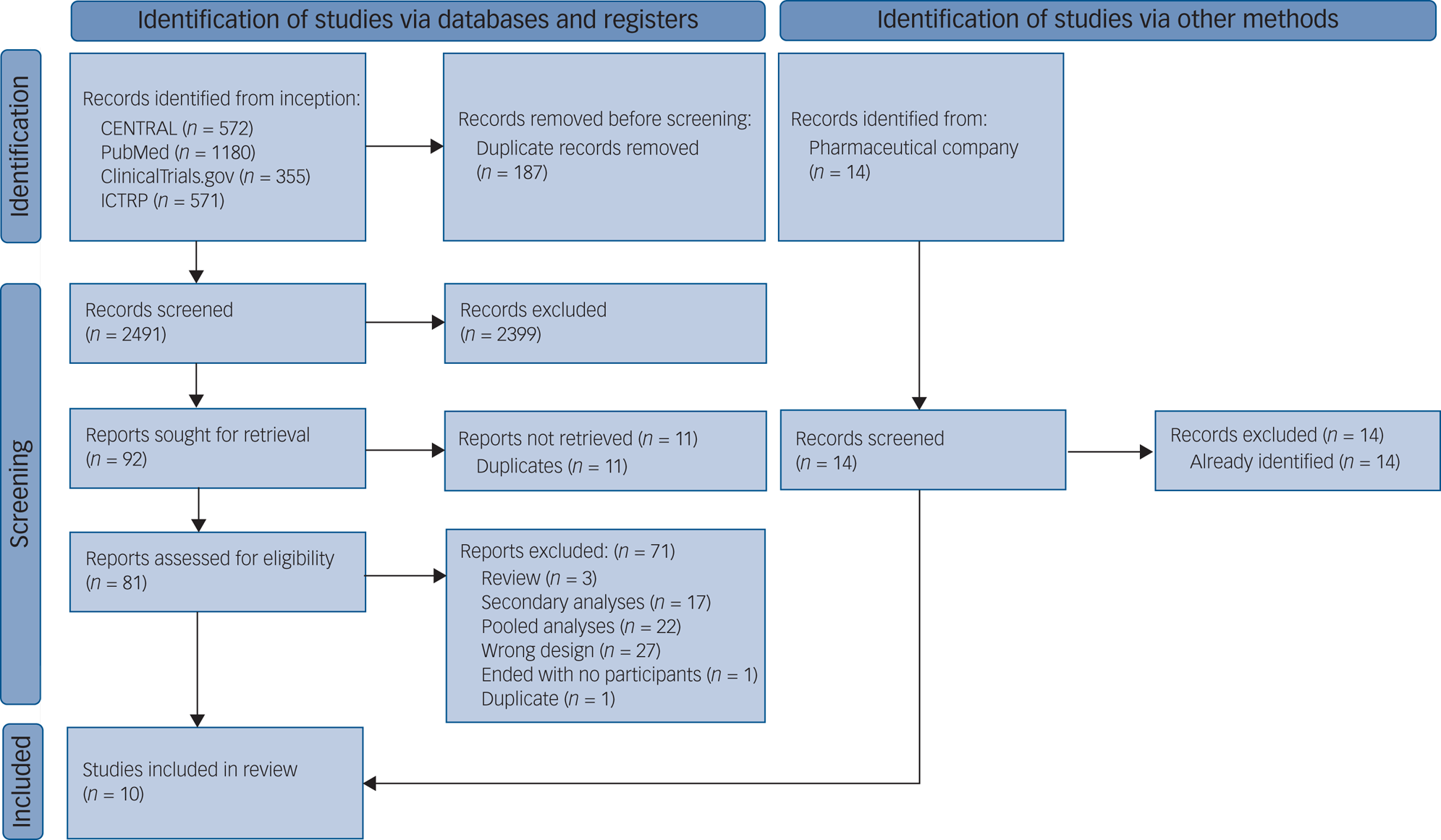
Fig. 1 PRISMA flow diagram. ICTRP, World Health Organization's International Clinical Trials Registry Platform.
Table 1 Characteristics of included studies

ADT, antidepressant drug therapy; Ari, aripiprazole; Cita, citalopram; CR, controlled release; Desv, desvenlafaxine; Dulo, duloxetine; Esci, escitalopram; Fluo, fluoxetine; Fluv, fluvoxamine; IQR, interquartile range; MADRS, Montgomery–Åsberg Depression Rating Scale; Miln, milnacipran; n.a., not applicable; Paro, paroxetine; Sert, sertraline; SNRI, serotonin–noradrenaline reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor; Venl, venlafaxine; XR, extended release.
The included studies were homogeneous by design, as all were double-blind, placebo-controlled, parallel-group, individually randomised, multi-centre trials using very similar inclusion and exclusion criteria. All trials took place in out-patient settings. In total, 1364 participants were randomly assigned to an active drug and 1261 were randomly assigned to placebo. The mean age was 42.3 years (s.d. = 11.1); 1380 (55.3%) of 2494 reported were women. The median duration of the acute treatment was 6 weeks (range 4.3–8). Most participants were recruited in North America (n = 1496, 57.0%) and Japan (n = 885, 33.7%). Mean reported baseline severity score on the Montgomery–Åsberg Depression Rating Scale (MADRS) was 26.3 (s.d. = 6.5). Baseline characteristics were similar among studies for the placebo arms as well as aripiprazole augmentation arms (Table 1). The response rate was imputed in one of the ten studies.Reference Lenze, Mulsant, Blumberger, Karp, Newcomer and Anderson38 Pharmaceutical companies funded all but one study.Reference Lenze, Mulsant, Blumberger, Karp, Newcomer and Anderson38 All studies used DSM for diagnostic criteria. All studies excluded patients with serious psychiatric comorbidities such as schizophrenia and bipolar disorder, alcohol or substance misuse and MDD with psychotic symptoms. Patients were excluded if they posed suicidal risk. All studies excluded people who had had electroconvulsive therapy in the past 10 years or who had received adjunctive antipsychotics for 3 weeks or more for the current episode. Antidepressant-refractory depression was defined as having inadequate response to 1–3 antidepressant trials of at least 6–12 weeks’ duration in the current episode. Continued antidepressants were all either selective serotonin reuptake inhibitors or serotonin–noradrenaline reuptake inhibitors. Primary outcomes used in the original studies were mean change in MADRS score (n = 8), MADRS response difference (n = 1) and MADRS remission rate (n = 1). The majority of studies reported some concern about the overall risk of bias (seven out of ten), and the three unpublished trials were at high overall risk of bias. (Supplementary Fig. 1).
Assessment of heterogeneity and reporting bias
We assessed heterogeneity using the efficacy outcome (seven studies). Although these assessments need to be carefully interpreted owing to the small number of studies included, we found no evidence of significant heterogeneity. The variance partition coefficient for efficacy was consistently low (<0.1) through the dose range investigated, which was not suggestive of significant heterogeneity (Supplementary Fig. 2). Visual evaluation of the contour-enhanced funnel plot did not suggest publication bias (Supplementary Fig. 3).
Dose–effect meta-analysis
We present maximum target dose–effect curves in Fig. 2 and the tabulation of results in Table 2. The maximum target dose–efficacy curve showed an increase up to doses between 2 and 5 mg, and then a non-increasing trend through the higher licensed doses up to 20 mg. The ED50 was 1.7 mg (OR = 1.39, 95% CI 1.13–1.72) and the ED95 was 4.0 mg (OR = 1.88, 95% CI 1.29–2.73). Tolerability also increased up to 5 mg and then there was a non-increasing trend through the higher licensed doses but the 95% CI of the spline curve remained very wide. There was no significant difference in acceptability between placebo and aripiprazole through the examined dose range. Sensitivity analyses using mean dose actually delivered or using knots at different doses confirmed the results (Supplementary Fig. 4). Owing to the small number of included studies, we were unable to test other models and use cross-validation for model selection. This is because models would be too unstable and results would be unreliable. According to the GRADE framework, the certainty of evidence for the dose–effect relationship was moderate for efficacy (owing to some concerns about risk of bias and imprecision) and low for tolerability and acceptability (owing to some concerns about risk of bias and serious concern about imprecision) (Supplementary Fig. 5).

Fig. 2 Dose–effect relationships for aripiprazole augmentation in antidepressant-refractory depression. (a) Response. (b) Drop-out owing to adverse events. (c) Drop-out for any reason. ED50, 50% effective dose; ED95 95% effective dose. The dotted lines represent 95% confidence intervals.
Given the average response rate at 8 weeks among the placebo augmentation arm of 23% (274/1197), the rate of drop-out because of adverse events of 1% (16/1261) and the rate of drop-out for any reason of 9% (110/1197), aripiprazole augmentation with a maximum target dose of 4.0 mg (ED95) would translate into a response rate of 36% (95% CI 28–45%), a rate of drop-out because of adverse events of 4% (95% CI 1–12%) and a rate of drop-out for any reason of 10% (95% CI 5–17%). Titrating up further while balancing side-effects seems safe, but does not seem to add more benefit.
Discussion
To our knowledge, this is the first systematic review and dose–effect meta-analysis of aripiprazole as augmentation strategy for antidepressant-refractory depression. The results showed that aripiprazole augmentation may achieve most of its efficacy at the lower range of its licensed dose (2–5 mg) in the acute treatment of major depression with inadequate response to initial antidepressant therapy, and that no additional benefits may be likely beyond 10 mg.
These results are in line with a previous three-armed trial,Reference Kamijima, Higuchi, Ishigooka, Ohmori, Ozaki and Kanba31 where the low-dose fixed-dose (3 mg/day) arm showed improvement similar to that in the flexible-dose arm (3–15 mg/day, mean actually delivered dose 9.8 mg) (mean change in score on the MADRS was −10.5 and −9.6 respectively). The finding that flexibly up-titrating above the lowest of the licensed dose ranges while balancing the benefits and side-effects may not bring more benefit is the same as what was found for antidepressant monotherapy for MDD.Reference Furukawa, Salanti, Cowen, Leucht and Cipriani8 Our findings are in contrast with an earlier review examining the clinical dose–efficacy relationship of aripiprazole augmentation, which concluded that low-dose aripiprazole was not effective.Reference Zhou, Keitner, Qin, Ravindran, Bauer and Giovane43 The arbitrary categorisation of doses (low dose and standard dose) and the relatively fewer number of trials for low-dose aripiprazole are methodological concerns that may have biased the results of the previous review. To overcome these limitations, we carried out a dose–effect meta-analysis, which allows synthesising not only data in the limited dose range, as the previous network meta-analysis did, but all the data via the dose–effect relationship and which realises greater resolution of change points. The relatively fewer number of trials in the low-dose range is reflected in the relatively wider confidence intervals in the dose range. The very wide dose range currently recommended (2–15 mg)Reference Taylor, Marwood, Oprea, DeAngel, Mather and Valentini14 in international guidelines may reflect the dose range used for the trials for FDA approval (2–20 mg),Reference Berman, Marcus, Swanink, McQuade, Carson and Corey-Lisle34–Reference Berman, Fava, Thase, Trivedi, Swanink and McQuade36 not the dose–effect relationship. Such a wide range could be misleading, potentially harmful and not cost-effective, as it may lead clinicians to prescribe too high a dose that only increases the risk of side-effects without additional benefits. Dose–effect meta-analysis can synthesise the knowledge based on extant trials and provide evidence-based recommendations for the optimum dosages. The international clinical guidelines for MDD recommend atypical antipsychotics augmentation with caution owing to the potential side-effects.Reference Taylor, Marwood, Oprea, DeAngel, Mather and Valentini14 Our study showed that the rate of drop-out because of adverse events might increase only up to around 5 mg but then showed non-increasing trends up to 20 mg and that the rate of drop-out for any reason might not differ between aripiprazole and placebo through the licensed dose range, if the drug was administered in a flexible manner for higher doses. However, the studies included in our review lasted up to 8 weeks at maximum and provide no information as to potential side-effects beyond the acute-phase treatment.
Limitations
Our study has several limitations. First, all the included studies presented with moderate to high overall risk of bias. This renders certainty of the evidence of the identified dose–effect relationships low, owing to study limitations. Second, the number of studies was small, leaving confidence intervals for tolerability and acceptability wide. The limited amount of data at the low-dose end of the spectrum does not allow for definitive conclusions about the shape of the dose–effect curve in the low-dose range. We reflected these limitations in GRADE evaluation. Third, original studies excluded patients with other serious psychiatric comorbidities or MDD with psychotic symptoms. It is therefore unknown whether the result of this study can be generalised to those patient groups. Fourth, we could not evaluate effect modifiers such as age, gender, sociodemographic or clinical characteristics, or other combined therapies. For example, none of the included studies had included protocolised psychotherapies for depression. The efficacy of aripiprazole augmentation and its dose–effect relationship may be moderated when active psychotherapies are concurrently administered. Future studies should consider evaluating possible effect modifiers by using individual participant data.Reference Tomlinson, Furukawa, Efthimiou, Salanti, Crescenzo and Singh44 Fifth, inclusion of flexible-dose studies made handling data and interpreting results complicated. Using only fixed-dose studies would have been more straightforward but was not possible. We decided to use the maximum dose rather than the achieved dose, because the former is determined prior to randomisation whereas the latter is not, and therefore the latter can identify only observational associations and should not be used for making causal claims. Also, when we compare lower range with higher range, we need to be aware that the fixed-dose arms (2 mg and 3 mg) are in the lower dose range whereas the flexible-dose arms (maximum dose 12–20 mg) are clustered in the higher dose range. The non-increasing trend of tolerability (drop-out owing to adverse events) beyond 5 mg might not mean that side-effects do not increase with higher doses, but rather that careful observation of side-effects and flexible dose adjustments could prevent drop-out even when adverse events occur. Last, the duration of the included studies was limited to acute-phase treatment, and we therefore remain uncertain of the benefits and the harms of longer-term treatment by aripiprazole augmentation.
Strengths
By contrast, the strengths of the current study may be as follows. First, we did the most comprehensive systematic review to date and found three unpublished studies that were not identified in the previous systematic reviews and meta-analyses.Reference Luan, Wan, Zhang and Zhao16,Reference Komossa, Depping, Gaudchau, Kissling and Leucht18,Reference Zhou, Keitner, Qin, Ravindran, Bauer and Giovane43 Second, we treated dose as a continuous variable, thus avoiding arbitrary categorisation of doses, which could lead to spurious dose–effect relationships. Third, we examined dose dependency not only for efficacy but also for tolerability and acceptability.
Future research
Further research is required to investigate the benefits of low versus high dose of aripiprazole augmentation. Future studies of prescribing recommendations can take advantage of dose–effect meta-analysis to obtain more precise and clinically more helpful recommendations.Reference Crescenzo, Garriga, Tomlinson, Coupland, Efthimiou and Fazel45
Supplementary material
Supplementary material for this article is available online at https://doi.org/10.1192/bjp.2021.165.
Data availability
Data and code used for analyses are available from the corresponding author on request.
Author contributions
Y.F. contributed to the conceputualisation, methodology, project administration, software, formal analysis, investigation, data curation, writing original draft, writing review and editing, visualisation. T.H. contributed to the methodology, software, validation, formal analysis, writing review and editing. A.C. contributed to the methodology, writing review and editing, supervision. T.A.F. contributed to the conceptualisation, methodology, writing review and editing, supervision. G.S. contributed to the methodology, writing review and editing, supervision. E.G.O. contributed to the conceptualisation, methodology, validation, investigation, data curation, writing review and editing, supervision. Y.F. and E.G.O. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
This study was supported in part by Japan Society for the Promotion of Science Grant-in-Aid for Scientific Research (grant 19K10661) to T.A.F.
Declaration of interest
A.C. has received research and consultancy fees from INCiPiT (Italian Network for Paediatric Trials), CARIPLO Foundation and Angelini Pharma; he is supported by the National Institute for Health Research (NIHR) Oxford Cognitive Health Clinical Research Facility, by an NIHR Research Professorship (grant RP-2017-08-ST2-006), by the NIHR Oxford and Thames Valley Applied Research Collaboration and by the NIHR Oxford Health Biomedical Research Centre (grant BRC-1215-20005). T.A.F. reports grants and personal fees from Mitsubishi-Tanabe, personal fees from MSD, personal fees from Shionogi, outside the submitted work. E.G.O. has received research and consultancy fees from Angelini Pharma and is supported by the NIHR Research Professorship to Professor Andrea Cipriani (grant RP-2017-08-ST2-006), by the NIHR Applied Research Collaboration (ARC) Oxford and Thames Valley, by the NIHR Oxford Cognitive Health Clinical Research Facility and by the NIHR Oxford Health Biomedical Research Centre (grant BRC-1215-20005). The views expressed are those of the authors and not necessarily those of the UK National Health Service, the NIHR, the UK Department of Health, or other affiliated organisations.









eLetters
No eLetters have been published for this article.