Smoking is common in patients with schizophrenia, with one meta-analysis estimating prevalence at 62%, nearly three times higher than in the general population. Reference deLeon and Diaz1 Smoking is one of the contributory factors to the starkly increased standardised mortality ratio in schizophrenia (approximately double that of the general population). Even within this population, smoking doubles the risk of cardiac-related death. Reference Kelly, McMahon, Wehring, Liu, Mackowick and Boggs2 Long-term smoking cessation rates are relatively low, and people with schizophrenia have more difficulty in quitting smoking compared with the general population. Reference Kelly, McMahon, Wehring, Liu, Mackowick and Boggs2
Prevalence studies examining the association between symptom domains and smoking have found conflicting results, with some studies suggesting increased positive and negative symptoms, Reference Kao, Liu, Cheng and Chou3 some suggesting no difference in symptomatology between smokers and non-smokers, Reference Kotov, Guey, Bromet and Schwartz4,Reference Barnes, Lawford, Burton, Heslop, Noble and Hausdorf5 and a few suggesting links to either positive or negative symptoms. Reference Aguilar, Gurpegui, Diaz and De Leon6,Reference Patkar, Gopalakrishnan, Lundy, Leone, Certa and Weinstein7 The small minority of studies that have measured ‘nicotine dependence’ Reference Kao, Liu, Cheng and Chou3,Reference Aguilar, Gurpegui, Diaz and De Leon6–Reference Herran, deSantiago, Sandoya, Fernandez, Dez-Manrique and Vazquez-Barquero8 have included mainly in-patients or consecutive out-patients receiving routine care. As far as we are aware, no study has attempted to study the association between clinical variables in patients with schizophrenia and nicotine dependence in an epidemiologically defined geographical area. We therefore conducted a cross-sectional survey of clinical measures and measures of nicotine dependence in Nithsdale, Scotland.
Method
Sample
The study was approved by the Dumfries and Galloway research ethics committee. On 1 February 2006, all those with a clinical diagnosis of schizophrenia according to ICD-10 9 , and with a home address in Nithsdale, Scotland, were identified. Diagnosis was ascertained on the basis of the ‘key informant’ method, which involved identifying all patients with a diagnosis of schizophrenia known to Crichton Royal Hospital. This method has been described in detail elsewhere. Reference Telfer, Shivashankar, Krishnadas, McCreadie and Kirkpatrick10
Measures
Participants underwent a clinical interview by two of the authors (S.S. and S.T.), within 15 months of the patient census of 1 February 2006. Symptoms of schizophrenia were assessed using the Positive and Negative Syndrome Scale (PANSS). Reference Kay, Fiszbein and Opler11 Total scores on individual items and each subscale were used as continuous measures in determining severity of symptoms. All participants who smoked were administered the Fagerström Test for Nicotine Dependence (FTND), and classified with high/severe dependence (FTND >6) or mild–moderate dependence (FTND <6), as defined in previous studies. Reference Aguilar, Gurpegui, Diaz and De Leon6,Reference de Leon, Diaz, Becona, Gurpegui, Jurado and Gonzalez-Pinto12,Reference Fagerstrom, Kunze, Schoberberger, Breslau, Hughes and Hurt13
Participants were assessed for the presence of tardive dyskinesia using the Abnormal Involuntary Movement Scale (AIMS), Reference Lane, Glazer, Hansen, Berman and Kramer14 and for akathisia and extrapyramidal symptoms using the Barnes Akathisia Scale Reference Barnes15 and Simpson–Angus Scale (SAS) Reference Simpson and Angus16 respectively. Individuals were also classified into those with and without the deficit syndrome of schizophrenia, using the Proxy for the Deficit Syndrome criteria. Reference Kirkpatrick, Buchanan, Breier and Carpenter17
Social adjustment in the community was assessed using the Social Adjustment Scale-Self Report (SAS-SR). Reference Weissman and Bothwell18 It has 52 items, covering ‘patient's role performance, interpersonal relationships, friction, feelings and satisfaction in work, and social and leisure activities with the extended family, as a spouse, parent, and member of a family unit’. Reference Weissman and Bothwell18 The questionnaire was completed with the supervision of a psychiatrist (S.S. or S.T.). Greater scores suggest poorer social adjustment. We used the overall SAS-SR score to see whether there was an association between social adjustment, PANSS and FTND scores. Prescriptions were converted to defined daily dose (DDD) units in order to calculate quantity of use across formulations. The DDD system is promoted by the World Health Organization, 19 allowing the quantity of medications received to be expressed as number of days’ supply of medication received at a standardised maintenance dose. Participants also completed a semi-structured questionnaire, asking for details of their smoking use, whether smoking helped them in any way, and whether they had intentions of quitting smoking. This study was part of a larger project, and the raters were unaware of the hypothesis during the study period.
Statistical analysis
Analysis was performed using SPSS version 17 for Windows. Differences between groups on categorical variables were tested using either the chi-squared test or Fisher's exact test. According to a priori hypotheses, we planned to examine the relationship between smoking and psychopathology (PANSS) using multivariate general linear model approaches. We covaried for age, gender, SAS-SR, DDD and type of antipsychotic, with family-wise error multiple testing corrections where appropriate.
Mediation is the process where the observed association between an ‘explanatory’ variable and ‘outcome’ variable occurs via a third ‘mediator’ variable. In our analysis, we found that greater nicotine dependence was associated with greater positive symptoms. Greater positive symptoms were also associated with poorer social adjustment (SAS-SR scores). We therefore wished to examine whether the relationship between nicotine dependence and SAS-SR scores was explained indirectly by greater positive symptoms in patients with severe nicotine dependence. In order to examine this indirect effect, we conducted a mediation analysis. The mediation analysis tests the hypothesis that a proportion of variance in the dependent variable (social adjustment) that is predicted by variance in an independent variable (nicotine dependence) can be accounted for by the mediator variable (positive symptoms). In other words, ‘the mediation analysis partitions the variance explained by the predictor into a part that is independent of the mediating variable, and a part that is accounted for via the mediating variable’. Reference Palaniyappan and Liddle20
We conducted a bootstrap analysis, using Preacher and Hayes’ indirect macro for SPSS, to examine the indirect effect of nicotine dependence (independent variable) and SAS-SR scores (dependent variable) through positive symptoms scores (mediator). For each analysis, 10 000 random samples of the original size are taken from the obtained data, replacing each value as it was sampled, and the indirect effect (a*b) is computed in each sample (bootstrapping). The point estimate (mean a*b) of the indirect effect is computed over the samples. Confidence intervals are then derived from the standard errors obtained (with Z-score based and accelerated corrections for bias due to the underlying distribution). This analysis requires no assumption regarding the underlying distributions, since the statistical significance level is determined non-parametrically. Reference Preacher and Hayes21
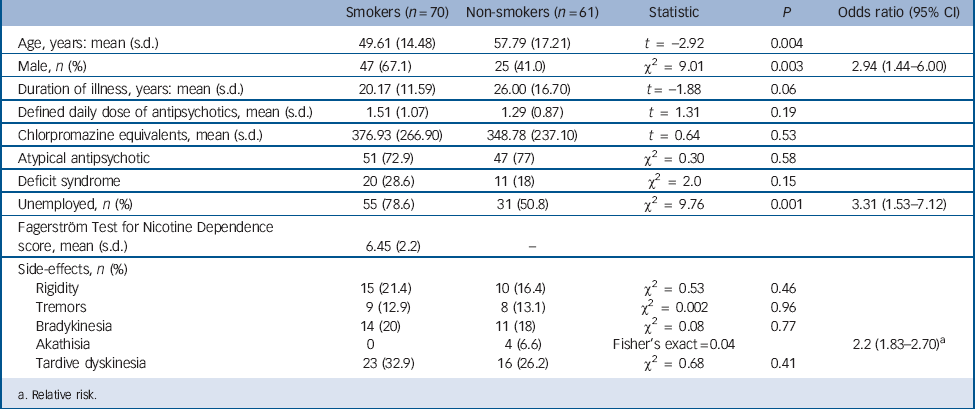
TABLE 1 Demographic and clinical details
| Smokers (n = 70) | Non-smokers (n = 61) | Statistic | P | Odds ratio (95% CI) | |
|---|---|---|---|---|---|
| Age, years: mean (s.d.) | 49.61 (14.48) | 57.79 (17.21) | t = –2.92 | 0.004 | |
| Male, n (%) | 47 (67.1) | 25 (41.0) | χ2 = 9.01 | 0.003 | 2.94 (1.44–6.00) |
| Duration of illness, years: mean (s.d.) | 20.17 (11.59) | 26.00 (16.70) | t = –1.88 | 0.06 | |
| Defined daily dose of antipsychotics, mean (s.d.) | 1.51 (1.07) | 1.29 (0.87) | t = 1.31 | 0.19 | |
| Chlorpromazine equivalents, mean (s.d.) | 376.93 (266.90) | 348.78 (237.10) | t = 0.64 | 0.53 | |
| Atypical antipsychotic | 51 (72.9) | 47 (77) | χ2 = 0.30 | 0.58 | |
| Deficit syndrome | 20 (28.6) | 11 (18) | χ2 = 2.0 | 0.15 | |
| Unemployed, n (%) | 55 (78.6) | 31 (50.8) | χ2 = 9.76 | 0.001 | 3.31 (1.53–7.12) |
| Fagerström Test for Nicotine Dependence score, mean (s.d.) | 6.45 (2.2) | – | |||
| Side-effects, n (%) | |||||
| Rigidity | 15 (21.4) | 10 (16.4) | χ2 = 0.53 | 0.46 | |
| Tremors | 9 (12.9) | 8 (13.1) | χ2 = 0.002 | 0.96 | |
| Bradykinesia | 14 (20) | 11 (18) | χ2 = 0.08 | 0.77 | |
| Akathisia | 0 | 4 (6.6) | Fisher's exact = 0.04 | 2.2 (1.83–2.70)Footnote a | |
| Tardive dyskinesia | 23 (32.9) | 16 (26.2) | χ2 = 0.68 | 0.41 |
a. Relative risk.
Results
Demographics and clinical measures
Demographic details, according to smoking status, are shown in Table 1. Two hundred and five people with a diagnosis of schizophrenia were identified, giving a point prevalence of 3.59/1000 general population. Two people (1%) were unable to give valid consent because of cognitive decline. Although they were living in the Nithsdale area on the census day, 4 individuals (2%) had died by the time of clinical interview, and another 6 (3%) had moved to a different region. Sixty-two people (30%) refused to take part in the study. A total of 131 individuals (64%) were therefore interviewed, and of these, 70 (53.4%) were current smokers. Of the 61 who did not smoke, 21 had a history of smoking. In smokers, using the FTND cut-off score of >6to diagnose severe nicotine dependence, 50 (71%) fulfilled criteria for severe dependence and 20 (29%) for mild–moderate dependence.
Smokers were significantly younger than non-smokers (t = –2.92; P = 0.004), more likely to be male (odds ratio (OR) = 2.94; 95% CI 1.44–6.00) and unemployed (OR = 3.31; 95% CI 1.53–7.12). Mean duration of smoking was 34.35 years (s.d. = 15.15). There was no significant difference in mean DDD, chlorpromazine equivalent dose, duration of illness or presence of the deficit syndrome between smokers and non-smokers. The PANSS positive scores correlated negatively with age (r = –0.31; P<0.001). There was no difference in PANSS scores between males and females. Significantly more non-smokers reported akathisia (OR = 2.22; 95% CI 1.83–2.7), with no difference in other extrapyramidal symptoms or between the two groups.
Nicotine dependence
Defined daily dose of medication was greater in the severely dependent group compared with the mild–moderate group (mean difference –0.7; P = 0.019), although the difference compared with non-smokers did not reach statistical significance (mean difference –0.42; P = 0.06) (Table 2). There was no difference in duration of illness and antipsychotic type among the three groups.
TABLE 2 Difference between the groups on PANSS scoresFootnote a

| Non-smoker (n = 61) |
Mild dependence (n = 20) |
Severe dependence (n = 50) |
F | P | N–M Mean difference (P) |
N–S Mean difference (P) |
M–S Mean difference (P) |
|
|---|---|---|---|---|---|---|---|---|
| Age, years: mean (s.d.) | 57.79 (17.20) | 54.65 (17.86) | 47.60 (12.54) | 4.73 | 0.01 | 2.44 (0.90) | 9.17 (0.01) | 6.7 (0.29) |
| Defined daily dose of antipsychotic, mean (s.d.) | 1.29 (0.86) | 1.017 (0.76) | 1.71 (1.11) | 4.72 | 0.01 | 0.27 (0.60) | –0.42 (0.06) | –0.7 (0.02) |
| Duration of illness, years: mean (s.d.) | 26.00 (16.70) | 22.00 (12.69) | 19.68 (11.42) | 1.96 | 0.14 | 4 (0.81) | 6.31 (0.15) | 2.3 (0.95) |
| Duration of smoking, years: mean (s.d.) | 38.50 (17.3) | 32.06 (12.08) | t = 1.7 | 0.10 | ||||
| People taking atypical antipsychotic, n (%) | 47 (77) | 14 (70) | 37 (74) | χ2 = 0.43 | 0.81 | |||
| SAS-SR overall score | 2.10 (0.56) | 2.3 (0.52) | 2.13 (0.51) | 1.10 | 0.334 | |||
| PANSS Positive scoreFootnote b | 10.38 (0.53) | 10.01 (0.92) | 12.46 (0.60) | 3.96 | 0.02 | 0.37 | –2.07Footnote * | –2.45 |
| PANSS Negative scoreFootnote b | 13.32 (0.65) | 16.56 (1.13) | 14.10 (0.73) | 3.08 | 0.04 | –3.22Footnote * | –0.78 | 2.47 |
N, non-smoker; M, mild dependence; S, severe dependence; SAS-SR, Social Adjustment Scale – Self Report; PANSS, Positive and Negative Syndrome Scale.
a. Only items with a significant difference are shown.
b. Multivariate analysis of covariance with PANSS subscores as dependent variables; covariates appearing in the model are evaluated at the following values: SAS-SR overall = 2.14, gender = 0.45, defined daily dose = 1.41, type of antipsychotic = 0.21, age = 53.22. Post-hoc tests are based on estimated marginal means and adjusted for multiple comparisons using Bonferroni family-wise error correction.
* Significant at P<0.05.
PANSS subscale scores and nicotine dependence
Multivariate analysis of covariance was used to examine the relationship between non-smokers (n = 61), smokers with severe dependence (>6 on FTND, n = 50), mild dependence (<6on FTND, n = 20) and PANSS scores. Nicotine dependence status significantly predicted PANSS scores (Wilk's lambda 0.875; F(6,242) = 2.78; P = 0.01). On tests of between-participants effects, dependence status significantly predicted total positive scores (F(2,123) = 3.96; P = 0.02) and total negative scores (F(2,123) = 3.08; P = 0.04), but not general psychopathology scores (F(2,123) = 0.092; P = 0.9).
We conducted post hoc pair-wise comparisons of the total scores between non-smokers, those with mild dependence and those with severe dependence, using Bonferroni family-wise error multiple testing corrections. Those with severe dependence had greater positive scores compared with non-smokers (t = 2.72; P = 0.001). Those with mild dependence had greater total negative symptoms scores compared with non-smokers (t = 3.72; P = 0.007) (Table 2). This was strengthened by the finding that 35% of those with mild dependence had deficit syndrome compared with 18% of non-smokers and 26% of those with severe nicotine dependence. This difference did not reach statistical significance.
Smoking severity, PANSS scores and social adjustment
There was a significant association between SAS-SR overall scores and PANSS positive (r = 0.25; P = 0.004), negative (r = 0.34; P<0.001) and general psychopathology (r = 0.47; P<0.001) scores. There was no direct statistically significant association between SAS-SR scores and smoking dependency (r = 0.18; P = 0.84). However, there was an indirect effect, where greater PANSS positive symptom score mediated the relationship between greater nicotine dependence and worse overall SAS-SR scores (effect = 0.01; Z = 2.10; s.e. = 0.0043; 95% CI 0.003–0.02).
We compared quitters and never-smokers (21 out of 61 non-smokers had a history of nicotine use). Quitters did not differ from never-smokers in terms of positive symptoms (mean difference 0.51; P = 0.9), negative symptoms (mean difference 2.6; P = 0.11) or SAS-SR scores (mean difference 0.16; P = 0.76) and DDD (mean difference 0.14; P = 0.9).
Among smokers, 42 (60%) stated that it helped them to relax, 11 (16%) stated that they smoked because they felt lonely, 10 (14%) that it helped them to socialise better and 22 (31%) stated that they smoked because they felt anxious or depressed. Fifty-six smokers (80%) said that they enjoyed smoking. In total, 22 (31%) claimed that they tried to give up smoking in the past 12 months, 21 (30%) had a desire to give up and 13 (19%) said they had an intention to give up.
Discussion
In an epidemiologically defined sample of patients with schizophrenia, we found that smoking rates in patients (53.4%) were twice that seen in the general population in Dumfries and Galloway (26.4% in men and 24.9% in women). Reference Whyte, Gordon, Haw, Fischerbacher, Scott and Harrison22 Smoking rates in this population do not appear to have changed significantly since they were last measured over 20 years ago (58%), Reference Kelly and McCreadie23 a finding echoed in a recent prospective study. Reference Kotov, Guey, Bromet and Schwartz4
Patients with severe nicotine dependence had greater positive symptoms and were prescribed higher doses of antipsychotic medication. Negative symptoms were greater in those with mild dependence (compared with non-smokers and those with severe dependence), a finding that was confirmed by increased rates of deficit syndrome in this group. This is in contrast to previous findings from Nithsdale, which did not find a relationship between symptomatology and smoking status. However, that study used the Manchester Scale to identify psychopathology, and did not measure nicotine dependence. Reference Kelly and McCreadie23
Greater positive and negative symptoms were associated with poorer social adjustment. We also found an indirect effect of nicotine dependence on overall social adjustment, suggesting that nicotine dependence may be associated with poorer social adjustment indirectly through its association with greater positive symptoms. This is further supported by our finding that patients with severe nicotine dependence were more likely to be unemployed and receiving greater doses of antipsychotic medication. The higher dosages of medications in patients with severe dependence may reflect either increased rate of antipsychotic metabolism and/or the need for more aggressive treatment of positive symptoms. Furthermore, quitters and never-smokers did not differ on measures of psychopathology, DDD or social adjustment.
These results, however, should be interpreted with caution. Although classic mediation analysis tests causal relationships between variables, such conclusions cannot be made owing to the cross-sectional nature of our data. Most patients in our sample started smoking before the onset of their illness. However, we do not know the temporal relationship between nicotine dependence, symptomatology and social adjustment. It is possible that greater dependence, greater medication dosage and poorer social adjustment are epiphenomena associated with greater symptomatology in this group.
Similar to previous findings from Nithsdale, those who smoked in the present cohort were more likely to be male and significantly younger. Younger people also had greater PANSS positive scores. As there is some evidence suggesting that positive symptoms become less prominent with age, it is tempting to argue that the presence of younger patients in the sample was the reason for the greater positive symptoms scores in the smoking group. Reference Tandon, Nasrallah and Keshavan24 However, the relationship between nicotine dependence and positive symptoms remained after controlling for age. Duration of illness between smokers and non-smokers did not differ in our population. This finding replicates those of a recent meta-analysis, which suggests that the age at onset of psychosis did not differ significantly between smokers and non-smokers. Reference Myles, Newall, Compton, Curtis, Nielssen and Large25
Strengths and limitations
This clinical study was conducted in a defined population of people with schizophrenia, with an attempt to minimise selection bias. Self-report of smoking status is viewed as a reliable measure, Reference Pierce, Dwyer, DiGiusto, Carpenter, Hannam and Amin26 and efforts were made to measure not only smoking status, but also a measure of nicotine dependence (FTND), acknowledging that dependence is independent of the number of cigarettes smoked, level of nicotine within cigarettes or duration of smoking. Reference Panday, Reddy, Ruiter, Bergstrom and de Vries27 Furthermore, another study has found the FTND to have a strong association with DSM-IV diagnosed nicotine dependence (r = 0.89, P<0.001). Reference Patkar, Gopalakrishnan, Lundy, Leone, Certa and Weinstein7 Although some authors have pointed to poor psychometric properties of the FTND in patients with schizophrenia, Reference Levander, Eberhard and Lindstrom28 the study most often quoted in this regard found an internal consistency of 0.74 in smokers with schizophrenia (higher than in smoking controls). Reference Weinberger, Reutenauer, Allen, Termine, Vessicchio and Sacco29 Limitations of our study include its cross-sectional nature, and therefore the direction of causality cannot be ascertained. Regarding antipsychotic medication, we did not measure concordance through use of other measures (e.g. pill count, corroborative history), although measures of adherence are not routinely used in clinical studies. Another limitation is the absence of clinical measures (other than the PANSS) measuring psychiatric comorbidity in more depth (e.g. anxiety and depression) or cognitive functioning. A substantial proportion of people refused to take part in the study, another source of selection bias that is inherent in clinical studies in a ‘real world’ setting. Examining the differences between those who participated and those who did not, revealed that individuals living in supported accommodation were more accessible and were more willing to participate, while the relatively ‘higher functioning’ group who were employed and living with family, with lesser duration of illness, refused to take part in the study.
Measuring nicotine dependence in patients with schizophrenia
The majority of the clinical literature on smoking and schizophrenia has focused solely on smoking status, with conflicting results, that may reflect differences in sample selection.
To our knowledge, only four other studies have measured nicotine dependence (each utilising the FTND), although only one of these studies analysed differences in groups, based on a categorical measure of nicotine dependence, Reference Aguilar, Gurpegui, Diaz and De Leon6 finding lower total PANSS and positive PANSS scores in mildly dependent smokers compared with severely dependent smokers and non-smokers. In that study, individuals with severe dependence had worse outcome, and in one of the other studies, increasing FTND scores correlated with poorer Clinical Global Impression score. Reference Herran, deSantiago, Sandoya, Fernandez, Dez-Manrique and Vazquez-Barquero8 It is worth noting that an almost consistent feature of clinical studies, including the present study, examining smoking in schizophrenia is the poorer outcome in smoking groups. Reference Kao, Liu, Cheng and Chou3,Reference Aguilar, Gurpegui, Diaz and De Leon6 Our study found that patients who smoked were three times more likely to be unemployed, and those with greater dependence were also prescribed greater doses of antipsychotic medication. The finding of increased antipsychotic dose in smokers is well recognised; for example, Salokangas et al found an increased antipsychotic dosage in older patients with schizophrenia who smoked, with higher plasma levels of unmetabolised fractions of antipsychotic detected. Reference Salokangas, Saarijarvi, Taiminen, Lehto, Niemi and Ahola30 Parkinsonian symptoms did not differ among the groups in our study. However, compared with smokers, patients who did not smoke were twice as likely to develop akathisia. Lower scores on the Barnes Akathisia Scale in smokers with schizophrenia have been reported elsewhere, Reference Barnes, Lawford, Burton, Heslop, Noble and Hausdorf5 and were also found in a placebo-controlled trial of nicotine transdermal patches applied to patients with schizophrenia treated with haloperidol. Reference Yang, Nelson, Kamaraju, Wilson and McEvoy31 Our findings are similar to those of Zhang et al who found no association between tardive dyskinesia and smoking status. Reference Zhang, Yu, Sun, Zhang, Li and Xiu32
Examining the relationship between nicotine dependence and schizophrenia
Various theories have been put forward for the association between smoking and schizophrenia. Reference Winterer33 The two that are most often cited are the self-medication and shared vulnerability hypotheses.
Nicotine as self-medication
Originally proposed over 25 years ago as a way of understanding substance dependence, the self-medication hypothesis has been increasingly used to explain the high association between substance misuse and severe mental illness. With regard to smoking, the mechanisms include possible regulation of central dopamine availability by pharmacodynamic and pharmacokinetic processes (e.g. enzyme induction of the hepatic enzyme system, lowering antipsychotic effectiveness). These processes have also been implicated in reducing the side-effects of dopamine blockade of typical antipsychotics. More recently, based on a number of preclinical and clinical studies, this theory proposes that patients with schizophrenia smoke in order to ‘treat’ underlying negative symptoms and cognitive deficits. Reference Matthews, Wilson and Mitchell34,Reference Kumari and Postma35
Nicotinic receptors are present on ventral tegmental dopaminergic cell bodies, with nicotine inducing dopamine release in the ventral tegmental system. Nicotine-induced release of dopamine in the mesocortical system results in improved cognitive performance and a decrease in negative symptoms. Reference George36 The effects of smoking on the mesolimbic system are more complex. Evidence from human positron emission tomography studies suggests it increases the release of dopamine in the dorsal/ventral striatum (to a similar degree as other drugs of misuse). Reference Brody, Olmstead, London, Farahi, Meyer and Grossman37 This, along with increased metabolism of antipsychotics may be a plausible explanation for our finding of increased positive symptoms in our population, given the dopamine hypothesis that links positive psychotic symptoms to increased release of dopamine in the limbic system. Reference Howes and Kapur38 Greater negative symptoms in those with mild dependence compared with severe dependence, could suggest a trajectory where those with severe dependence have successfully overcome negative symptoms by increasing their level of nicotine dependence. This seems to come at a price: increased positive symptoms, treatment with greater antipsychotic dose and poorer social adjustment. Whether a dose–response relationship exists between nicotine and psychopathology, cognitive symptoms or side-effects of medication is not clear. In our study, a significant majority of patients said they enjoyed smoking, that it helped them relax and socialise better. This suggests that a significant proportion of people perceive some benefit from smoking.
A number of neurophysiological studies have provided evidence for the self-medication hypothesis. Normalisation of the auditory sensory gating (P50) abnormalities, prepulse inhibition abnormalities and eye-tracking deficits following nicotine administration in patients with schizophrenia, and in relatives of patients, have all pointed towards a biological basis underlying the self-medication hypothesis of schizophrenia. Reference Winterer33 Similarly, Jacobsen et al using functional magnetic resonance imaging showed that nicotine enhances functional connectivity between regions of the brain that mediate a working memory task. Reference Jacobsen, D'Souza, Mencl, Pugh, Skudlarski and Krystal39 However, the relationship between smoking, negative and cognitive symptoms is complex. In a recent case–control study, Zhang et al found that although smokers had lower negative symptoms compared with non-smokers, they performed poorly on cognitive functioning, as measured by the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS). Reference Zhang, Chen da, Xiu, Haile, Sun and Lu40 It should be noted that RBANS is not the most robust measure of frontal/executive function. Reference Strauss, Sherman and Spreen41
Nicotine as shared vulnerability
In our study, smoking pre-dated the onset of illness. However, the relationship between nicotine dependence and duration of prodromal or subsyndromal symptoms preceding the onset of illness are not known. This finding suggests that self-medication may not be the only possible reason for the increased rates of nicotine dependence in this population. Previous studies have put forward a shared vulnerability hypothesis between nicotine and schizophrenia.

FIG. 1 Reasons for smoking and the development of dependence in schizophrenia.
Dashed arrows depict the self-medication hypothesis whereby smoking improves negative and cognitive symptoms and side-effects. The continuous arrows represent pathways that may lead to poor outcomes. Double-sided arrow suggests a shared vulnerability. EPS, extrapyramidal symptoms.
In this context, Esterberg et al found that first-degree relatives of patients with schizophrenia, with greater schizotypy, were more likely to smoke. Reference Esterberg, Jones, Compton and Walker42 There was no relationship between levels of schizotypy and smoking status in the control group, despite having similar levels of schizotypy. This finding is relevant, as the only difference between relatives and controls was the genetic vulnerability in the relatives. Esterberg et al therefore proposed that having a genetic vulnerability to schizophrenia may also place an individual at risk for smoking cigarettes. Furthermore, a number of studies have shown a shared genetic vulnerability to both schizophrenia and nicotine dependence. Reference Yoshimasu and Kiyohara43 Genetic and human post-mortem studies have shown that both high- and low-affinity nicotinic receptor expression is reduced in several brain regions in people with schizophrenia, even in those with a history of smoking (an effect opposite to that seen in normal smokers). The 15q14 locus near the coding region of the CHRNA7 gene (coding for the neuronal acetylcholine receptor α7 subunit, α7-nAChR protein) has been consistently linked to schizophrenia and sensory gating abnormalities, especially the deficient P50 suppression (see Wing et al for a recent review of the literature). Reference Wing, Wass, Soh and George44 A recent preclinical study found that reduction in α7-nAChR is associated with the motivation for nicotine self-administration. Together, these findings suggest that reduction in α7-nAChR function (a core deficit in patients with schizophrenia), promotes nicotine use in patients with schizophrenia. Reference Brunzell and McIntosh45
Whether nicotine use is a result of self-medication, or a shared vulnerability, outcomes appear worse in this group of patients. Our findings also suggest that reasons for smoking (and the development of dependence) in schizophrenia are possibly multifactorial (Fig. 1.).
Clinical implications
Rates of smoking in patients with schizophrenia were greater than in the general population and do not appear to have changed significantly over the past 20 years. A rather disconcerting finding in our study was that less than a quarter of the sample had an intention of giving up smoking. On a positive note, about 25% of those with a lifetime history of smoking had quit, and clinically they did not differ from lifetime non-smokers.
Nicotine dependence was found to be associated with symptom severity and outcome in people with schizophrenia. Although our study does not establish a causal relationship between these variables, identifying and treating nicotine dependence may have some value in reducing morbidity and mortality in schizophrenia. People with schizophrenia who smoke have been shown to benefit from pharmacological and psychosocial interventions that target smoking. When patients are assessed in view of quitting smoking, care should be taken to delineate the relationship between nicotine dependence and symptom severity, as this may potentially interfere with a person's successful attempt at quitting smoking. Further systematic longitudinal studies are required to establish whether a causal link exists between nicotine dependence and psychopathology.
Acknowledgements
We would like to thank all study participants.






eLetters
No eLetters have been published for this article.