Intellectual impairment is a reliable correlate of schizophrenia, but it also determines, and frequently confounds, neuropsychological function in the disorder. Reference Heinrichs1–Reference Kremen, Seidman, Faraone and Tsuang7 Affected individuals with different IQ levels and trajectories are distinguishable in terms of neuropsychological performance, although abnormalities in certain cognitive processes, particularly executive functions and processing speed, may overlap across IQ categories, and coexist with intact intellect. Reference Weickert, Goldberg, Gold, Bigelow, Egan and Weinberger3–Reference Kremen, Seidman, Faraone and Tsuang6,Reference Reichenberg, Weiser, Rapp, Rabinowitz, Caspi and Schmeidler8–Reference Palmer, Heaton, Paulsen, Kuck, Braff and Harris10 Establishing neurocognitive commonalities across levels and trajectories of IQ is important for characterising abnormalities that are prototypical of schizophrenia, Reference Kremen, Seidman, Faraone and Tsuang6,Reference Allen, Goldstein and Warnick9,Reference Palmer, Heaton, Paulsen, Kuck, Braff and Harris10 and identifying intellectual or neuropsychological subtypes may help establish neurobiologically meaningful disease subtypes. Reference Reichenberg, Weiser, Rapp, Rabinowitz, Caspi and Schmeidler8,Reference Turetsky, Moberg, Mozley, Moelter, Agrin and Gur11,Reference Rabinowitz, De Smedt, Harvey and Davidson12 Earlier investigations of people with schizophrenia with different intellectual characteristics had several methodological limitations. Most studies used clinical rather than epidemiologically based samples, Reference Weickert, Goldberg, Gold, Bigelow, Egan and Weinberger3,Reference Badcock, Dragovic, Waters and Jablensky4,Reference Kremen, Seidman, Faraone and Tsuang6,Reference van Winkel, Myin-Germeys, Delespaul, Peuskens, De and van13 and included individuals with chronic schizophrenia with long-term exposure to antipsychotic medication. Reference Weickert, Goldberg, Gold, Bigelow, Egan and Weinberger3,Reference Badcock, Dragovic, Waters and Jablensky4,Reference Kremen, Seidman, Faraone and Tsuang6 All studies defined subgroups on the basis of either current IQ, Reference Kremen, Seidman, Faraone and Tsuang6 or a combination of estimated premorbid IQ and degree of decline from premorbid levels, Reference Weickert, Goldberg, Gold, Bigelow, Egan and Weinberger3–Reference Joyce, Hutton, Mutsatsa and Barnes5 but not both criteria combined. Finally, no study compared subgroups of people with schizophrenia, defined using both of the above criteria, with intellectually matched controls. Reference Kremen, Seidman, Faraone and Tsuang7 To address this gap in the literature, the present study explored neuropsychological function in intellectually different subgroups of individuals with first-episode schizophrenia/schizoaffective disorder and controls with no history of psychosis, derived from an epidemiological sample. Intellectual categories were generated using similar methods to those employed in previous studies, but were more refined. In contrast to earlier studies, group formation was based on both current IQ and degree of discrepancy from estimated premorbid levels, and participants with schizophrenia and controls were matched on both of the latter two criteria (in the case of controls, ‘premorbid’ denotes ‘prior’). We hypothesised that impairment in executive function and processing speed would be detected in all participant groups with schizophrenia compared with intellectually matched controls.
Method
The ÆSOP study
The data were derived from the baseline ÆSOP (Aetiology and Ethnicity in Schizophrenia and Other Psychoses) study, a major population-based, case–control study of first-episode psychosis. The study identified all individuals aged 16–65 years with a first episode of psychosis (F10–F29 and F30–F33 in ICD–10) 14 who presented to specialist mental health services in tightly defined catchment areas in south-east London, Nottingham and Bristol between September 1997 and August 1999. Local ethical committees at all three centres approved the study. Exclusion criteria were previous contact with health services for psychosis, organic causes of psychotic symptoms, transient psychosis due to acute intoxication (as defined by ICD–10) and IQ less than 50. The study further included a random sample of controls with no past or present psychotic disorder. Community controls were mainly recruited using a sampling method that matched them with participants with schizophrenia by area of residence. A detailed overview of the ÆSOP study has been published elsewhere. Reference Morgan, Dazzan, Morgan, Jones, Harrison and Leff15
Participants
One hundred and one people with schizophrenia or schizoaffective disorder (F20 or F25 in ICD–10; n = 85 and n = 16, respectively) and 317 community controls were investigated to establish whether they met criteria for allocation to one of six a priori defined intellectual categories (see Generation of IQ categories below). In addition to satisfying the general ÆSOP inclusion criteria, all participants were selected if they: had IQ measurements on the National Adult Reading Test – Revised (NART–R) Reference Nelson16 and a short form of the Wechsler Adult Intelligence Scale – Revised (WAIS–R), Reference Wechsler17 as well as at least one other score on the ÆSOP neuropsychological battery; and were native speakers of English or had migrated to the UK by the age of 11. The latter strategy ensured that participants with a good command of English as a non-native language had completed at least their secondary education in the UK, thus reducing linguistic or cultural effects on the performance of a multi-ethnic sample. After providing the participants with a complete description of the study written informed consent was obtained.
There were no differences in age, gender or years of education between individuals with schizophrenia/schizoaffective disorder who did not meet the language/migration criteria or had no neuropsychological/IQ data (n = 41) and those participating (all P>0.1), although the former showed a significantly higher Black African to White ratio (Fisher's exact P<0.001).
Diagnostic procedures
Clinical data were collected using the Schedules for Clinical Assessment in Neuropsychiatry (SCAN). 18 The Present State Examination – Version 10 (incorporated in the SCAN) was used to elicit symptom-related data at presentation. Where a patient interview was not possible, case notes were used to complete the SCAN Item Group Checklist. Patients' ICD–10 diagnoses were determined using the SCAN data on the basis of consensus meetings involving a principal investigator and other members of the research team. Interrater reliability was estimated by asking each principal investigator to independently formulate a diagnosis for 20 individuals based on the same SCAN information (kappa values ranged from 0.63 to 0.75, P<0.001).
Controls were screened for psychosis using the Psychosis Screening Questionnaire. Reference Bebbington and Nayani19 Those with a positive rating on this instrument were further assessed using the SCAN.
Sociodemographic characteristics
Data on age, gender, ethnicity and education were collected by interviews with the participants using the Medical Research Council Sociodemographic Schedule. Reference Mallett20 Information gaps were filled using additional data sources, including case notes and other informants. In cases of uncertain ethnicity, a consensus rating was made by members of the research team having long-standing expertise in the study of ethnicity and mental health.
Generation of IQ categories
Premorbid and current IQs were derived, respectively, using the NART–R Reference Nelson16 and a short form of the WAIS–R, Reference Wechsler17 comprising Vocabulary, Comprehension, Digit symbol and Block design.
Following the criticism by Kremen et al, Reference Kremen, Seidman, Faraone and Tsuang7 we used an adaptation of the Weickert et al Reference Weickert, Goldberg, Gold, Bigelow, Egan and Weinberger3 classification: all participants were characterised as intellectually ‘good’ or ‘poor’ based on current IQ (in line with Wechsler's classification Reference Wechsler17 a score of 90, the cut-off for ‘average’ IQ, was chosen as the cut-off for ‘good’ functioning), and as ‘improved’, ‘stable’ or ‘deteriorated’ based on degree of discrepancy between NART–R IQ and WAIS–R IQ. A NART–R minus WAIS–R discrepancy of 10 IQ points was used as the cut-off for a meaningful change (gain or decline) from estimated premorbid levels. This choice was based on previous studies, which showed that a 10-point discrepancy between current and premorbid IQ applied to less than 25% of the NART restandardisation sample (10% of healthy controls showed an IQ ‘decline’, whereas 13% showed an IQ ‘gain’, of this magnitude), Reference Nelson16 represents the average deterioration between pre- and post-schizophrenia, Reference Seidman, Buka, Goldstein and Tsuang2,Reference Goldberg, Torrey, Gold, Bigelow, Ragland and Taylor21 and differentiates effectively between neuropsychological subtypes of the disorder. Reference Weickert, Goldberg, Gold, Bigelow, Egan and Weinberger3–Reference Joyce, Hutton, Mutsatsa and Barnes5 In addition, in order to classify a participant as ‘stable’, both premorbid and current IQ had to fall on the same side of the cut-off for ‘good’ intellectual functioning. Thus, participants were allocated to one of six a priori defined IQ categories: ‘improved good’ (WAIS–R IQ≥90, gain≥10 points), ‘improved poor’ (WAIS–R IQ<90, gain≥10 points), ‘stable good’ (NART–R/WAIS–R IQs≥90, discrepancy <10 points), ‘stable poor’ (NART–R minus WAIS–R IQ<90, discrepancy<10 points), ‘deteriorated good’ (WAIS–R IQ≥90, decline≥10 points) and ‘deteriorated poor’ (WAIS–R IQ<90, decline≥10 points).
The number of individuals in the schizophrenia group falling within each of the above categories was 6 (6%), 3 (3%), 22 (22%), 19 (19%), 6 (6%), and 37 (37%), respectively, but 8 people (8%) did not fall in any a priori defined category (e.g. discrepancy<10 points, but NART–R IQ and WAIS–R IQ did not fall on the same side of the cut-off for good functioning). The corresponding numbers of controls were 50 (16%), 3 (1%), 137 (43%), 14 (4%), 44 (14%) and 26 (8%), but 43 (14%) did not fall in any a priori defined category.
Participants with schizophrenia and controls differed significantly in their distribution in the ‘improved’, ‘stable’ and ‘deteriorated’ categories (Pearson χ2 = 18.00, P = 0.001) (controls were over-represented in the ‘improved’ categories compared with participants with schizophrenia: 17% v. 9%; individuals with schizophrenia were over-represented in the ‘deteriorated’ categories compared with controls: 43% v. 22%). As a result of the small number of individuals with schizophrenia falling in the ‘improved’ (n = 9) and ‘deteriorated good’ (n = 6) subgroups, the latter IQ categories were not included in the analysis.
Neuropsychological assessment
Verbal learning, short-term verbal recall and delayed verbal recall were assessed using trials 1–5, 6 and 7, respectively, of the Rey Auditory Verbal Learning Test (RAVLT). Reference Spreen and Strauss22 Immediate visual memory was examined using Visual Reproduction I of the Wechsler Memory Scale – Revised (WMS–R). Reference Wechsler23 Working memory and executive function were evaluated using Trail Making – part B, Reference Reitan24 Letter–number span, Reference Gold, Carpenter, Randolph, Goldberg and Weinberger25 Raven's coloured progressive matrices (CPM) – Set B, Reference Raven26 Category fluency (categories ‘body parts’, ‘fruits’ and ‘animals’), and Letter fluency (letters F, A, S). Processing speed was measured using Trail Making – part A Reference Reitan24 and the WAIS–R Digit symbol subtest. Reference Wechsler17 Raven's coloured progressive matrices – Set A Reference Raven26 and WAIS–R Block design Reference Wechsler17 were employed to assess visual and visuospatial perception and organisation, whereas WAIS–R Vocabulary Reference Wechsler17 and Comprehension Reference Wechsler17 were used to reflect verbal ability.
Statistical analysis
The statistical analysis was performed using STATA 8.0 for Windows. Differences in sociodemographic/IQ characteristics among the six participating groups (stable good schizophrenia group, stable good control group, deteriorated poor schizophrenia group, deteriorated poor control group, stable poor schizophrenia group, stable poor control group) were assessed using analysis of variance (ANOVA) models, Pearson chi-square and Fisher's exact tests. Differences in neuropsychological characteristics among the six groups were assessed using analysis of covariance (ANCOVA) models, controlling for age, gender, ethnicity and years of education (the latter variables showed significant/suggestive differences across groups; see Results). All AN(C)OVAs were performed using robust standard errors to safeguard against potential violations of the standard ANOVA assumptions. The AN(C)OVAs that gave rise to significant results (P<0.0027 using a Bonferroni correction: 0.05/18 neuropsychological and intellectual variables – including WAIS–R, NART–R and NART–R minus WAIS–R) were followed by planned post hoc contrasts between intellectually matched cases and controls (α was set at 0.05). As significance tests are dependent on sample size (which ranged from small to large across groups), for each neuropsychological variable we further estimated the standardised difference (effect size) between the means of intellectually matched participants with schizophrenia and controls using Cohen's d (d = Mcases – Mcontrols/σcontrols). Cohen Reference Cohen27 classified effect sizes ranging from 0.2 to 0.49 as small, from 0.5 to 0.79 as medium, and from 0.8 and above as large. As a deficit of half a standard deviation (equivalent to an effect size of 0.5) compared with established norms or healthy controls is regarded as the threshold of clinical or practical importance, we used this convention in addition to (or regardless of) statistical significance to characterise neurocognitive variables with a likely deficit in the people with schizophrenia. Negative effect sizes indicated better performance in the people with schizophrenia compared with their control counterparts.
Results
Sociodemographic and intellectual characteristics
Table 1 shows the demographic and intellectual characteristics of the six participating groups, which differed significantly in age, years of education, ethnicity, NART–R IQ, WAIS–R IQ, NART–R IQ minus WAIS–R IQ, and, at a trend level, in gender. The mean IQs of the schizophrenia (n = 78) and control (n = 177) samples were 95.7 (s.d. = 14.1) and 106.3 (s.d. = 12.3), respectively, on the NART–R (t 253 = −6.08, P<0.0001), and 86.0 (s.d. = 15.1) and 102.9 (s.d. = 15.2), respectively, on the WAIS–R (t 253 = −8.24, P<0.0001).
Table 1 Demographic and intellectual characteristics of individuals in the stable good, deteriorated poor and stable poor schizophrenia and control groups

| Stable good | Deteriorated poor | Stable poor | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Group differences, statistical test | Schizophrenia (n = 22) | Control (n = 137) | Schizophrenia (n = 37) | Control (n = 26) | Schizophrenia (n = 19) | Control (n = 14) | |||
| Age, years: a mean (s.d.) | F 5,249 = 10.94, P < 0.0001 | 27.0 (7.7) | 38.2 (13.4) | 27.2 (8.9) | 33.9 (8.1) | 26.7 (11.8) | 34.1 (13.0) | |||
| Gender, b n | χ2 = 10.01, P < 0.10 | |||||||||
| Males | 12 | 60 | 23 | 12 | 14 | 5 | ||||
| Females | 10 | 77 | 14 | 14 | 5 | 9 | ||||
| Ethnicity, c n | χ2 = 53.58, P < 0.0001 | |||||||||
| White British | 17 | 95 | 17 | 12 | 9 | 8 | ||||
| African—Caribbean | 2 | 7 | 10 | 7 | 8 | 5 | ||||
| Black African | 0 | 1 | 2 | 1 | 0 | 0 | ||||
| Asian | 0 | 1 | 3 | 1 | 0 | 0 | ||||
| Other | 3 | 33 | 5 | 5 | 2 | 1 | ||||
| Education, years: d mean (s.d.) | F 5,249 = 13.14, P < 0.0001 | 13.7 (2.4) | 13.6 (2.4) | 12.3 (2.1) | 12.5 (2.3) | 11.3 (1.0) | 11.9 (1.7) | |||
| Highest educational level attained, d n | χ2 = 75.29, P < 0.001 | |||||||||
| No qualifications | 2 | 19 | 8 | 5 | 10 | 8 | ||||
| GCSE/CSE | 3 | 20 | 13 | 9 | 5 | 2 | ||||
| ‘O’ Levels | 2 | 9 | 5 | 4 | 2 | 1 | ||||
| ‘A’ Levels | 5 | 34 | 2 | 0 | 1 | 0 | ||||
| Vocational/college | 5 | 11 | 4 | 5 | 1 | 3 | ||||
| Teaching/HND/nursing | 0 | 12 | 2 | 0 | 0 | 0 | ||||
| University/professional | 5 | 32 | 3 | 3 | 0 | 0 | ||||
| NART—R IQ, e mean (s.d.) | F 12,242 = 61.10, P < 0.0001 | 107.2 (8.5) | 109.6 (10.3) | 98.1 (10.3) | 101.3 (8.9) | 77.5 (5.7) | 83.3 (5.5) | |||
| WAIS—R IQ, f mean (s.d.) | F 12,242 = 81.81, P < 0.0001 | 106.4 (10.8) | 109.0 (11.0) | 78.9 (6.7) | 82.7 (5.8) | 76.2 (5.5) | 80.7 (5.3) | |||
| NART—R IQ — WAIS—R IQ, g mean (s.d.) | F 12,242 = 37.29, P < 0.0001 | 0.8 (6.0) | 0.6 (5.1) | 19.2 (8.3) | 18.6 (7.0) | 1.3 (5.0) | 2.6 (4.8) | |||
NART—R, National Adult Reading Test — Revised; WAIS—R, Wechsler Adult Intelligence Scale — Revised.
a. Planned post hoc contrasts between intellectually matched participants with schizophrenia and controls indicated that those with schizophrenia were statistically significantly younger than controls in the stable good and deteriorated poor intellectual categories (P<0.05).
b. Planned comparisons between intellectually matched participants with schizophrenia and controls indicated that there were more males than females in the stable poor group with schizophrenia compared with the stable poor controls (Fisher's exact P=0.04).
c. Planned comparisons between intellectually matched participants with schizophrenia and controls showed no statistically significant differences or trends in ethnicity (all P>0.10).
d. Planned comparisons between intellectually matched participants with schizophrenia and controls showed no statistically significant differences or trends in years of education or highest level of education attained (all P>0.10).
e. Planned post hoc contrasts between intellectually matched participants with schizophrenia and controls showed no statistically significant differences or trends in NART—R IQ (all P>0.10).
f. Planned post hoc contrasts between intellectually matched participants with schizophrenia and controls showed no statistically significant differences or trends in WAIS—R IQ (all P>0.10).
g. Planned post hoc contrasts between intellectually matched participants with schizophrenia and controls showed no statistically significant differences or trends in NART—R IQ minus WAIS—R IQ (all P>0.10).
Within each IQ category (stable good, deteriorated poor, stable poor), there were no statistically significant differences/trends in NART–R IQ, WAIS–R IQ or NART–R IQ minus WAIS–R IQ between participants with schizophrenia and controls (Table 1).
Neuropsychological characteristics
Table 2 shows the means and standard deviations of the six groups in the 15 neurocognitive variables (adjusted for age, gender, ethnicity and years of education), the number of participants in each statistical comparison, the results of the ANCOVAS and post hoc contrasts, and the standardised mean differences (Cohen's d) between intellectually matched individuals with schizophrenia and controls. A visual display of the average effect sizes across all component processes in each neuropsychological domain in the three groups with schizophrenia is presented in Fig. 1.
Table 2 Adjusted mean (s.d.) neurocognitive scores, a number of participants included in the statistical analysis, results of ANCOVAs and post hoc contrastsb and effect sizes (Cohen's d) b

| Stable good | Deteriorated poor | Stable poor | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Results of ANCOVAs c | Schizophrenia (N = 22) Mean (s.d.) n | Control (N = 137) Mean (s.d.) n | d | Schizophrenia (N = 37) Mean (s.d.) n | Control (N = 26) Mean (s.d.) n | d | Schizophrenia (N = 19) Mean (s.d.) n | Control (N = 14) Mean (s.d.) n | d | ||||||
| Verbal memory and learning (Rey Auditory Verbal Learning Test) | ||||||||||||||||
| Trials 1-5 | F 12,229 = 14.09 | 51.1 (9.1) 21 | 53.8 (9.6) 133 | 0.3 | 41.0 (9.6) 32 | 45.6 (8.9) 25 | 0.5 | 38.7 (9.5) 17 | 43.0 (9.0) 14 | 0.5 | ||||||
| Trial 6 | F 12,228 = 10.89 | 11.1 (2.7) 21 | 11.3 (2.9) 132 | 0.1 | 7.7 (2.9) 32 | 8.8 (2.7) 25 | 0.4 | 7.1 (2.8) 17 | 8.6 (2.7) 14 | 0.6 | ||||||
| Trial 7 | F 12,223 = 10.03 | 10.0 (3.1) 21 | 11.1 (3.3) 130 | 0.3 (*) | 7.1 (3.3) 30 | 8.8 (3.1) 25 | 0.6(*) | 7.2 (3.2) 16 | 8.7 (3.1) 14 | 0.5 | ||||||
| Visual memory (Wechsler Memory Scale - Revised) | ||||||||||||||||
| Visual reproduction | F 12,230 = 10.43 | 11.1 (2.6) 21 | 11.6 (2.8) 134 | 0.2 | 8.7 (2.8) 32 | 8.4 (2.6) 24 | -0.1 | 8.8 (2.7) 18 | 8.0 (2.6) 14 | -0.3 | ||||||
| Working memory and executive function | ||||||||||||||||
| Trail Making B | F 12,221 = 7.99 | 66.7 (42.1) 22 | 65.3 (44.4) 133 | 0.0 | 109.9 (44.7) 31 | 94.4 (41.2) 20 | 0.4 | 114.6 (44.1) 17 | 130.7 (41.5) 11 | -0.4 | ||||||
| Letter-number span | F 12,231 = 11.58 | 14.8 (3.6) 22 | 16.1 (3.8) 133 | 0.3* | 11.0 (3.7) 32 | 13.5 (3.5) 25 | 0.7* | 9.8 (3.7) 18 | 11.0 (2.9) 14 | 0.4 | ||||||
| Raven's coloured progressive matrices - B | F 12,238 = 8.41 | 10.2 (1.8) 22 | 10.9 (1.9) 137 | 0.4* | 8.6 (1.9) 35 | 9.3 (1.8) 25 | 0.4 | 8.5 (1.9) 18 | 8.4 (1.8) 14 | -0.1 | ||||||
| Category fluency | F 12,219 = 13.99 | 42.3 (7.5) 19 | 43.9 (7.8) 131 | 0.2 | 33.7 (7.8) 31 | 39.3 (7.4) 24 | 0.8** | 30.6 (7.6) 14 | 40.4 (7.4) 13 | 1.3** | ||||||
| Letter fluency | F 12,220 = 10.47 | 28.3 (8.0) 18 | 27.0 (8.3) 133 | -0.2 | 20.1 (8.3) 31 | 24.2 (7.8) 24 | 0.5 (*) | 16.5 (8.1) 14 | 28.3 (7.9) 13 | 1.5*** | ||||||
| Processing speed | ||||||||||||||||
| Trail Making A | F 12,230 = 8.11 | 31.2 (18.4) 22 | 33.0 (19.4) 134 | -0.1 | 53.0 (19.5) 33 | 36.8 (18.1) 22 | 0.9* | 65.4 (19.1) 18 | 43.3 (18.3) 14 | 1.2* | ||||||
| Digit symbol | F 12,239 = 22.83 | 8.6 (2.2) 22 | 9.8 (2.3) 136 | 0.5** | 6.0 (2.3) 35 | 6.5 (2.1) 26 | 0.2 | 4.9 (2.3) 19 | 6.6 (2.2) 14 | 0.8** | ||||||
| Visuospatial perception and organisation | ||||||||||||||||
| Raven's coloured progressive matrices - A | F 12,238 = 5.00 | 11.1 (1.2) 22 | 11.3 (1.3) 137 | 0.2 | 10.4 (1.3) 35 | 10.3 (1.2) 25 | -0.1 | 10.1 (1.3) 18 | 9.7 (1.2) 14 | -0.3 | ||||||
| Block design | F 12,236 = 25.31 | 11.9 (2.4) 20 | 11.5 (2.5) 136 | -0.2 | 6.9 (2.5) 35 | 7.9 (2.4) 25 | 0.4 | 6.9 (2.5) 19 | 7.8 (2.4) 14 | 0.4 | ||||||
| Verbal IQ | ||||||||||||||||
| Vocabulary | F 12,242 = 32.44 | 10.4 (1.9) 22 | 10.1 (2.0) 137 | -0.2 | 7.7 (2.0) 37 | 7.7 (1.9) 26 | 0.0 | 6.6 (2.0) 19 | 6.9 (1.9) 14 | 0.2 | ||||||
| Comprehension | F 12,240 = 37.87 | 11.2 (2.2) 22 | 10.7 (2.4) 136 | -0.2 | 6.8 (2.3) 36 | 7.1 (2.2) 26 | 0.1 | 6.5 (2.4) 19 | 6.7 (2.2) 14 | 0.1 | ||||||
a. Scaled scores were used for Digit symbol, Block design, Vocabulary and Comprehension; raw scores were used for the remaining cognitive domains; all scores have been adjusted for age, gender, ethnicity and years of education.
b. Between intellectually matched participants with schizophrenia and controls (negative d indicates better performance in the participants with schizophrenia). Effect sizes: small from 0.2 to 0.49; medium from 0.5 to 0.79; and large from 0.8 and above.
c. ANCOVAS were performed on unadjusted raw or scaled scores covarying for age, gender, ethnicity and years of education. All P ≤0.0001.
(*) P <0.10, *P ≤0.05, **P ≤0.01, ***P ≤0.001.
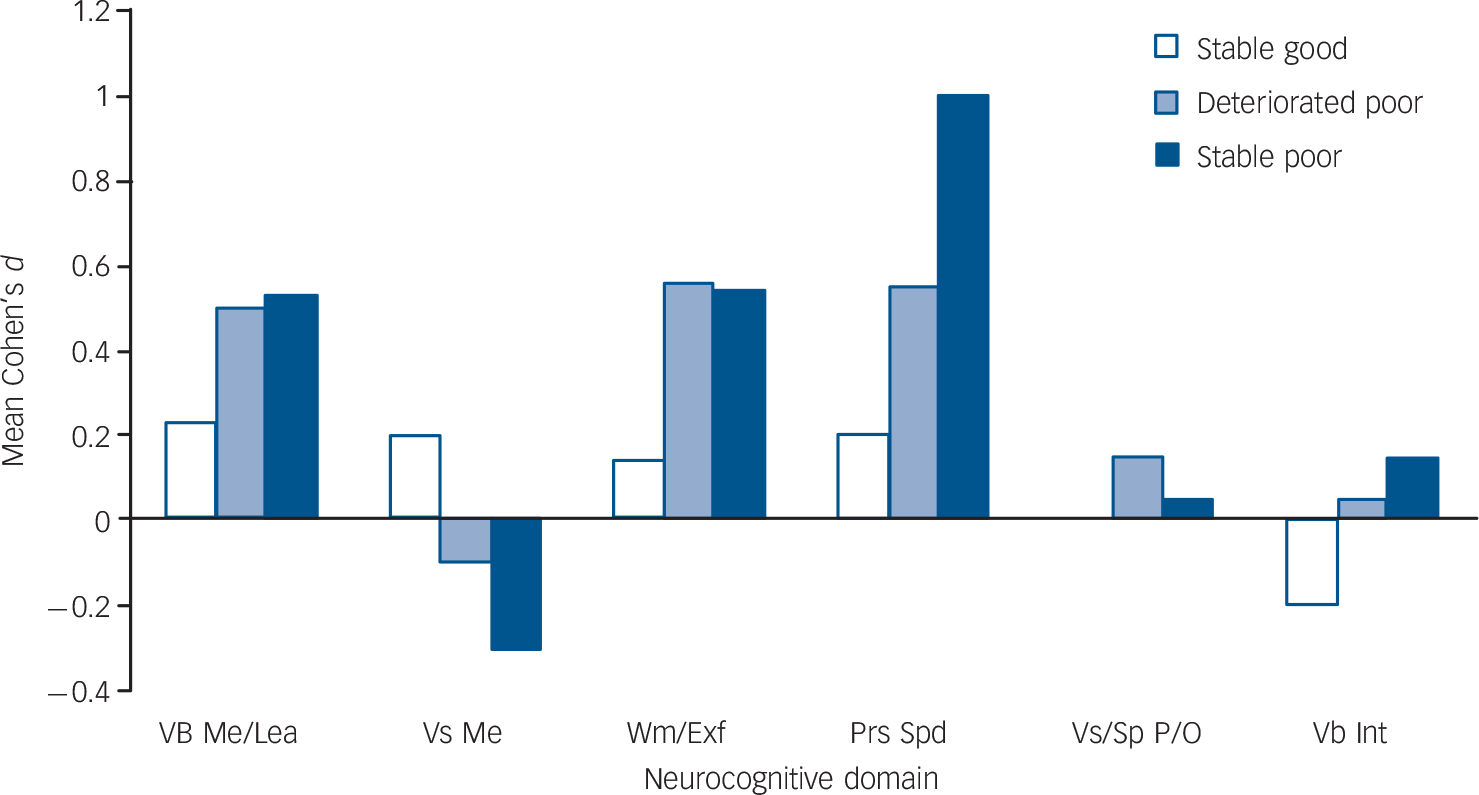
Fig. 1 Mean Cohen's d per neurocognitive domain in the IQ subgroups for participants with schizophrenia.
Vb Me/Lea, Verbal memory and learning; Vs Me, Visual memory; Wm/Exf, Working memory and executive function; Prs Spd, Processing speed; Vs/Sp P/O, Visuospatial perception and organisation; Vb Int, Verbal intelligence.
The ANCOVAs revealed significant differences across groups in all the neuropsychological variables (Table 2). Compared with intellectually matched controls, all groups with schizophrenia showed significant (P≤0.05–0.001) or suggestive (P<0.10) deficits, and/or moderate to large effect sizes in verbal memory and learning, working memory, executive function, and processing-speed variables (Table 2). The mean effect size across the variables that showed suggestive or significant deficits, and/or moderate to large effect sizes (Table 2), ranged from 0.4 (small) in the stable good group, to 0.7 (medium) in the deteriorated poor group, to 0.9 (large) in the stable poor group.
Role of working memory and processing speed
It has been argued that ‘core’ deficits in working memory and processing speed Reference Badcock, Dragovic, Waters and Jablensky4,Reference Silver, Feldman, Bilker and Gur28–Reference Dickinson, Ramsey and Gold30 may underlie impairments in several other cognitive domains in schizophrenia. To test this hypothesis, the ANCOVAS and post hoc contrasts between intellectually matched cases and controls were repeated for those neurocognitive variables that gave rise to significant/suggestive deficits and/or medium/large effect sizes (Table 2) while covarying separately for working memory (Letter–number span) (Table 3) and processing speed (Trail Making – part A and Digit symbol) (Table 4). Addition of either covariate to the analysis only slightly modified the pattern of findings (effect sizes in verbal memory and learning decreased slightly) (Tables 3 and 4). The same pattern was observed after controlling for Category and Letter fluency, the executive components that elicited the largest deficit in the ‘poor’ groups (results available on request).
Table 3 Adjusted mean (s.d.) raw scores on verbal memory and learning, working memory and executive function, a number of participants included in the statistical analysis, results of ANCOVAs and post hoc contrasts b and effect sizes (Cohen's d) b after covarying for processing speed (Trail Making A and Digit symbol)

| Stable good | Deteriorated poor | Stable poor | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Results of ANCOVAs c | Schizophrenia (N = 22) Mean (s.d.) n | Control (N = 137) Mean (s.d.) n | d | Schizophrenia (N = 37) Mean (s.d.) n | Control (N = 26) Mean (s.d.) n | d | Schizophrenia (N = 19) Mean (s.d.) n | Control (N = 14) Mean (s.d.) n | d | ||||||
| Verbal memory and learning (Rey Auditory Verbal Learning Test) | ||||||||||||||||
| Trials 1-5 | F 14,221 = 13.60 | 51.1 (9.0) 21 | 52.7 (10.6) 130 | 0.2 | 42.9 (10.4) 32 | 46.7 (9.1) 22 | 0.4 | 41.1 (10.4) 17 | 44.5 (9.1) 14 | 0.4 | ||||||
| Trial 6 | F 14,220 = 11.21 | 11.1 (2.7) 21 | 11.0 (3.1) 129 | -0.0 | 8.2 (3.1) 32 | 9.0 (2.7) 22 | 0.3 | 7.7 (3.1) 17 | 9.2 (2.7) 14 | 0.6 | ||||||
| Trial 7 | F 14,215 = 9.85 | 10.0 (3.1) 21 | 11.0 (3.6) 127 | 0.3 | 7.4 (3.6) 30 | 9.0 (3.1) 22 | 0.5 | 7.6 (3.6) 16 | 9.2 (3.1) 14 | 0.5 | ||||||
| Working memory and executive function | ||||||||||||||||
| Letter-number span | F 14,223 = 10.82 | 14.7 (3.5) 22 | 15.7 (4.0) 131 | 0.3 | 11.5 (3.9) 31 | 14.2 (3.5) 22 | 0.8** | 10.7 (4.0) 18 | 11.4 (3.5) 14 | 0.2 | ||||||
| Raven's coloured progressive matrices - B | F 14,226 = 7.45 | 10.0 (1.8) 22 | 10.6 (2.1) 133 | 0.3 (*) | 9.0 (2.1) 33 | 9.3 (1.8) 22 | 0.2 | 9.4 (2.1) 17 | 8.7 (1.8) 14 | -0.4 | ||||||
| Category fluency | F 14,209 = 19.13 | 41.5 (7.2) 19 | 42.3 (8.2) 128 | 0.1 | 36.6 (8.2) 29 | 40.3 (7.3) 21 | 0.5 (*) | 35.3 (8.1) 14 | 42.1 (7.2) 13 | 0.9* | ||||||
| Letter fluency | F 14,210 = 14.35 | 27.6 (7.6) 18 | 25.6 (8.7) 130 | -0.2 | 23.1 (8.7) 29 | 24.9 (7.7) 21 | 0.2 | 21.1 (8.5) 14 | 30.2 (7.6) 13 | 1.2*** | ||||||
a. All scores have been adjusted for age, gender, ethnicity, years of education and processing speed (Trail Making A and Digit symbol).
b. Between intellectually matched participants with schizophrenia and controls (negative d indicates better performance in the participants with schizophrenia). Effect sizes: small from 0.2 to 0.49; medium from 0.5 to 0.79; and large from 0.8 and above.
c. ANCOVAS were performed on unadjusted raw scores covarying for age, gender, ethnicity, years of education and processing speed (Trail Making A and Digit symbol). All P ≤ 0.0001.
(*) P < 0.10, *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
Table 4 Adjusted mean (s.d.) scores on verbal memory/learning, executive function and processing speed, a number of participants included in the statistical analysis, results of ANCOVAs and post hoc contrasts, b and effect sizes (Cohen's d) b after covarying for working memory (Letter-number span)

| Stable good | Deteriorated poor | Stable poor | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Results of ANCOVAs c | Schizophrenia (N = 22) Mean (s.d.) n | Control (N = 137) Mean (s.d.) n | d | Schizophrenia (N = 37) Mean (s.d.) n | Control (N = 26) Mean (s.d.) n | d | Schizophrenia (N = 19) Mean (s.d.) n | Control (N = 14) Mean (s.d.) n | d | ||||||
| Verbal memory and learning (Rey Auditory Verbal Learning Test) | ||||||||||||||||
| Trials 1-5 | F 13,223 = 17.31 | 51.0 (8.8) 21 | 52.6 (10.0) 130 | 0.2 | 43.1 (9.8) 30 | 46.2 (8.7) 25 | 0.4 | 41.7 (9.6) 17 | 45.4 (9.0) 14 | 0.4 | ||||||
| Trial 6 | F 13,222 = 11.89 | 11.1 (2.6) 21 | 11.0 (3.0) 129 | -0.0 | 8.4 (3.0) 30 | 9.0 (2.6) 25 | 0.2 | 7.9 (2.9) 17 | 9.4 (2.7) 14 | 0.6 | ||||||
| Trial 7 | F 13,217 = 10.07 | 10.0 (3.0) 21 | 10.8 (3.4) 127 | 0.2 | 7.8 (3.4) 28 | 9.0 (3.0) 25 | 0.4 | 8.1 (3.3) 16 | 9.6 (3.1) 14 | 0.5 | ||||||
| Executive function | ||||||||||||||||
| Raven's coloured progressive matrices - B | F 13,229 = 7.65 | 10.1 (1.8) 22 | 10.8 (2.0) 133 | 0.4 (*) | 8.9 (2.0) 32 | 8.7 (1.9) 25 | -0.1 | 8.7 (2.0) 17 | 9.3 (1.8) 14 | 0.3 | ||||||
| Category fluency | F 13,212 = 12.34 | 42.2 (7.4) 19 | 43.1 (8.2) 128 | 0.1 | 35.1 (8.1) 28 | 39.7 (7.3) 24 | 0.6* | 32.5 (7.9) 14 | 41.7 (7.5) 13 | 1.2** | ||||||
| Letter fluency | F 13,213 = 10.14 | 28.3 (7.9) 18 | 26.4 (8.7) 130 | -0.2 | 21.2 (8.6) 28 | 24.6 (7.8) 24 | 0.4 | 18.3 (8.4) 14 | 29.6 (8.0) 13 | 1.4*** | ||||||
| Processing speed | ||||||||||||||||
| Trail Making A | F 13,224 = 7.29 | 31.2 (18.4) 22 | 33.1 (20.8) 131 | -0.1 | 51.2 (20.5) 31 | 36.8 (18.1) 22 | 0.8* | 64.6 (20.3) 18 | 42.9 (18.8) 14 | 1.2* | ||||||
| Digit symbol | F 13,230 = 20.42 | 8.6 (2.1) 22 | 9.6 (2.4) 133 | 0.4* | 6.2 (2.3) 32 | 6.8 (2.1) 25 | 0.3 | 5.5 (2.3) 18 | 7.0 (2.2) 14 | 0.7** | ||||||
a. Scaled scores were used for Digit symbol; raw scores were used for the remaining cognitive domains; all scores have been adjusted for age, gender, ethnicity, years of education and working memory (Letter-number span).
b. Between intellectually matched participants with schizophrenia and controls (negative d indicates better performance in the participants with schizophrenia). Effect sizes: small from 0.2 to 0.49; medium from 0.5 to 0.79; and large from 0.8 and above.
c. ANCOVAS were performed on unadjusted raw or scaled scores covarying for age, gender, ethnicity, years of education and working memory (Letter-number span). All P ≤ 0.0001.
(*) P < 0.10, *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
Role of disease-related variables
No significant associations emerged between cognitive impairment and medication dose, positive symptoms or depressive symptoms in the combined sample of individuals with schizophrenia (stable good, deteriorated poor, stable poor combined). Higher levels of negative symptoms correlated with poorer verbal learning (Pearson r = −0.50, P = 0.0001), short-term verbal recall (Pearson r = −0.29, P = 0.04), delayed verbal recall (Pearson r = −0.29, P = 0.04) and working memory (Pearson r = −0.31, P = 0.02). Longer duration of untreated psychosis was associated with poorer short-term (Pearson r = −0.30, P = 0.02) and delayed (Pearson r = −0.28, P = 0.04) verbal recall.
To examine whether negative symptoms could account for the deficits in verbal learning, verbal recall and working memory in the groups with schizophrenia, we compared these domains between individuals with subclinical negative symptoms (18 stable good, 24 deteriorated poor, 10 stable poor) and intellectually matched controls. The pattern of findings as outlined in Table 2 remained the same (results available on request).
Examining potential biases in intellectual classification
The formation of intellectual subgroups in the present study was based on a priori defined, previously validated, criteria for a meaningful discrepancy between premorbid and current IQ. Reference Seidman, Buka, Goldstein and Tsuang2–Reference Joyce, Hutton, Mutsatsa and Barnes5,Reference Nelson16,Reference Goldberg, Torrey, Gold, Bigelow, Ragland and Taylor21 However, the percentage of controls showing a decline of at least 10 IQ points from premorbid levels (22%) was higher than expected. Reference Nelson16 We therefore repeated the classification and analysis using a considerably more conservative cut-off for premorbid/current IQ discrepancy (17 points), as less than 10% of our controls showed a NART–R minus WAIS–R discrepancy of at least this magnitude. The pattern of findings as outlined in Table 2 remained the same (results available on request).
To address the possibility that current IQ was overestimated in the individuals with schizophrenia (WAIS–R Vocabulary is considered to be relatively resistant to brain damage), Reference Bilder, Lipschutzbroch, Reiter, Geisler, Mayerhoff and Lieberman31 participants were reclassified to intellectual subgroups using an estimate of WAIS–R IQ based on Comprehension and Digit symbol (the most sensitive subtest to brain damage). Reference Bilder, Lipschutzbroch, Reiter, Geisler, Mayerhoff and Lieberman31 This led to an average decrease of 3.0 points in the current IQ of the participants with schizophrenia, but, again, did not affect the pattern of findings (results available on request).
Using continuous IQ predictors of neuropsychological function
As mentioned earlier, 8% of the 101 people with schizophrenia and 14% of the 317 controls did not fall in any a priori defined IQ category, and 15% of the individuals with schizophrenia (and their intellectually matched controls) were excluded from the analysis because of the small sizes of their representative intellectual categories. To address this methodological downside, we carried out an alternative analysis: the effects of WAIS–R IQ and premorbid/current IQ discrepancy (NART–R IQ minus WAIS–R IQ) on neurocognitive function were simultaneously addressed in multiple regression models in each of the total schizophrenia (n = 101) and control (n = 317) samples, covarying for age, gender, ethnicity and years of education. The independent effect of WAIS–R IQ was statistically significant for all neurocognitive variables (α was set at 0.05, P<0.05–0.001), contributing 6–39% of unique variance in the participants with schizophrenia, and 2–22% of unique variance in the controls. The independent effect of NART–R IQ minus WAIS–R IQ was statistically significant for a smaller set of functions (P<0.05–0.01), contributing 0–10% of unique variance in the participants with schizophrenia, and 0–4% of unique variance in the controls (results available on request). These findings suggest that current IQ and IQ trajectory may have independent roles in neuropsychological function. They further suggest a relatively greater contribution of current IQ compared with IQ trajectory, and a somewhat greater explanatory power in relation to the performance of the participants with schizophrenia than that of the controls' performance.
Discussion
Main findings
This is the first population-based study to investigate neuropsychological function in people with first-episode schizophrenia and control subgroups that mirrored each other in both intellectual trajectory and current IQ. The epidemiological framework, large and well-characterised sample, novel matching strategy and use of standard cognitive tests in an original and informative way, give our study a powerful foundation and fills a gap in the literature, while supporting previous findings. Our strategy enabled us to isolate neuropsychological deficits in the cohorts of individuals with schizophrenia that were unlikely to have simply resulted from a generic effect of low/lowered IQ on neurocognitive function, sampling biases, long-standing clinical illness, medication or state-dependent factors. We were further able to investigate the utility of intellectual factors in defining meaningful schizophrenia subgroups, whose IQ trajectory and neuropsychological function can inform current theoretical accounts of the developmental and pathophysiological heterogeneity of schizophrenia.
A number of tentative conclusions can be drawn from the present findings. First, teasing out the effects of different aspects of intellectual performance, our study corroborated findings from earlier studies of schizophrenia, which variably identified ‘core’ deficits in executive function, Reference Weickert, Goldberg, Gold, Bigelow, Egan and Weinberger3–Reference Kremen, Seidman, Faraone and Tsuang6 processing speed Reference Badcock, Dragovic, Waters and Jablensky4,Reference Rodríguez-Sánchez, Crespo-Facorro, González-Blanch, Perez-Iglesias and Vázquez-Barquero32 or verbal declarative memory. Reference Kremen, Seidman, Faraone and Tsuang6,Reference Saykin, Gur, Gur, Mozley, Mozley and Resnick33,Reference Hoff and Kremen34 Combining statistical tests and effect sizes, our approach demonstrated that deficits in all the above domains are likely to be prototypical of schizophrenia. Second, impairments in the respective domains may reflect independent pathophysiological processes. Although deficits in working memory and processing speed have been suggested to cause cascading effects on other neurocognitive functions, Reference Mitropoulou, Harvey, Zegarelli, New, Silverman and Siever29,Reference Rodríguez-Sánchez, Crespo-Facorro, González-Blanch, Perez-Iglesias and Vázquez-Barquero32,Reference Brebion, David, Bressan and Pilowsky35,Reference Elvevag and Goldberg36 controlling for either process or for verbal fluency (the most impaired executive component in the ‘poor’ groups) did not abolish deficits in the remaining domains. Third, visual memory, visuospatial organisation and verbal intellectual ability are relatively spared in schizophrenia, as the respective effect sizes were small (or even negative) and non-significant across the schizophrenia groups. Finally, the graded pattern of average effect sizes across the affected domains in the stable good, deteriorated poor and stable poor groups (ranging from a small 0.4 to a medium 0.7 to a large 0.9) suggests that the ‘normal’ relationship between IQ and neuropsychological function breaks down incrementally in schizophrenia as one moves from high to low current and premorbid IQ.
Our analysis suggested that current IQ and IQ trajectory may have independent roles in neuropsychological function. This result parallels a finding by Kremen and colleagues, Reference Kremen, Buka, Seidman, Goldstein, Koren and Tsuang37 who reported that a static intellectual deficit at age 7, and a large IQ decline between ages 4 and 7 against a background of normal IQ, were both associated with adult risk for psychosis. The above evidence suggests that IQ tests are indexing something fundamental about schizophrenia, and that the neurodevelopmental substrates of the disorder involve both static and dynamic elements, which may dissociate with different subtypes of the disorder. This issue is further discussed below.
Intellectual and neurocognitive subtypes in schizophrenia
Our findings corroborate earlier attempts at subtyping schizophrenia using intellectual criteria. Reference Weickert, Goldberg, Gold, Bigelow, Egan and Weinberger3–Reference Kremen, Seidman, Faraone and Tsuang6 In particular, the proportions of individuals falling in the stable good, deteriorated good/poor (collapsed in previous studies) and stable poor categories are comparable with those reported in earlier investigations. Reference Weickert, Goldberg, Gold, Bigelow, Egan and Weinberger3–Reference Kremen, Seidman, Faraone and Tsuang6
About a quarter of people with schizophrenia are reported to obtain normal scores on global measures of intellectual or neuropsychological functioning. Reference Weickert, Goldberg, Gold, Bigelow, Egan and Weinberger3,Reference Allen, Goldstein and Warnick9,Reference Palmer, Heaton, Paulsen, Kuck, Braff and Harris10 This sizeable minority (22% in our study) showed small isolated deficits in verbal memory, working memory and executive function and a moderate deficit in processing speed. These findings lend support to the argument that the corresponding group might be more accurately referred to as ‘high-functioning’ rather than neuropsychologically intact, as these individuals show deficits in a restricted range of cognitive functions in several studies. Reference Allen, Goldstein and Warnick9
A substantial proportion of participants with schizophrenia (37%) showed below average intellectual functioning and a meaningful estimated decline from premorbid levels. Their neuropsychological impairment encompassed the same broad neurocognitive domains as those showing deficits in the stable good group, but involved more subcomponent processes and larger effect sizes. Our findings concur with those of a population-based study in Israel, which demonstrated that 40% of 17-year-olds with a later admission to hospital for schizophrenia had experienced IQ decline from childhood to adolescence. Reference Reichenberg, Weiser, Rapp, Rabinowitz, Caspi and Schmeidler8 It is tempting to view this group as representing a ‘late neurodevelopmental’ subtype of schizophrenia. Reference Reichenberg, Weiser, Rapp, Rabinowitz, Caspi and Schmeidler8 The latter has been hypothesised to involve a genetic regulatory defect in brain development, exerting active effects in adolescence and early adulthood. Reference Kremen, Buka, Seidman, Goldstein, Koren and Tsuang37,Reference Feinberg38 The plausibility of this form is supported by evidence suggesting that some brain maturational events of adolescence (e.g. electrophysiological changes) are abnormal in about 40% of people with schizophrenia; Reference Feinberg38,Reference Woods39 and the distinctiveness of this form is supported by findings that individuals with deteriorating intellectual functions have more chronic symptoms and are less responsive to treatment. Reference Rabinowitz, Haim, Reichenberg, Harvey, Weiser and Kaplan40,Reference Harvey, Silverman, Mohs, Parrella, White and Powchik41 Importantly, both our study and the Israeli series suggest that the late neurodevelopmental subtype might be the predominant form of schizophrenia.
A sizeable minority of the cohort of individuals with schizophrenia (19%) showed below-average IQ and a stable IQ trajectory. These people demonstrated deficits in the same broad neurocognitive domains as those eliciting impairments in the stable good and deteriorated poor groups. Their deficit, however, involved more subcomponent functions and larger effect sizes compared with the former groups. These individuals may be the prototypical ‘early neurodevelopmental’ group, in which a deviation in early development as a result of genetic and/or environmental factors establishes a neuronal phenotype (static encephalopathy) that predisposes to, or determines, the onset of schizophrenia. Reference Weinberger42–Reference Murray, O'Callaghan, Castle and Lewis44 According to this model, the period during which the causative agent actively damages the central nervous system is short (the pathogenetic process is virtually static) and any behavioural consequences remain relatively latent until the onset of psychosis.
In the participants with schizophrenia in this study the likelihood of falling in the stable poor category was about half that of falling in the deteriorated poor group. In line with this finding, Kremen and colleagues reported that the rate of future psychosis among 7-year-olds with low but stable IQ was about half that among peers with normal but deteriorated IQ. Reference Kremen, Buka, Seidman, Goldstein, Koren and Tsuang37 Both studies suggest that a declining IQ trajectory during development is a stronger risk factor for adult schizophrenia than a static intellectual deficiency.
Using the NART–R and the WAIS–R to measure intellectual change
The NART–R has been validated against the WAIS–R, Reference Nelson16 and, in healthy control samples, NART–R IQs and WAIS–R IQs differ by an average of 1.0–3.8 points. Reference Joyce, Hutton, Mutsatsa and Barnes5,Reference Bright, Jaldow and Kopelman45,Reference Berry, Carpenter, Campbell, Schmitt, Helton and Lipke-Molby48 This range is comparable to the NART–R minus WAIS–R difference of 3.4 points found in our extended control sample. However, several drawbacks need to be acknowledged. In particular, the NART–R should be used with caution where IQ deviates from general population means, as it may over- or underestimate premorbid IQ. Reference Russell, Munro, Jones, Hayward, Hemsley and Murray46,Reference Graves47 Importantly, the latter possibility is more salient in people with first-episode schizophrenia compared with controls, as education is likely to be interrupted and adult vocabulary incompletely attained in the former group. In contrast to earlier studies, which compared IQ subgroups of participants with schizophrenia with a single, intellectually normal, healthy control group, the present study compared subgroups of people with schizophrenia and controls that did not differ in current IQ, level of education or vocabulary.
The percentage of controls showing cognitive instability in our study was relatively high: about twice as many ÆSOP controls (22%) showed a ‘deficit’ of at least 10 points relative to their predicted IQ as participants in the NART restandardisation study (10%). Reference Nelson16 Differences in the characteristics of the NART–R sample and the ÆSOP controls may partly explain this finding. In particular, individuals with high IQ were over-represented in the NART–R sample compared with the general population, Reference Nelson16 whereas ÆSOP controls were epidemiologically ascertained. The presence of non-psychotic psychiatric disorders in the ÆSOP controls may have increased rates of cognitive decline compared with the NART–R sample. However, an alternative explanation is that score differences between the NART–R and the WAIS–R reflect differences between verbal and non-verbal, or acquired and innate, cognitive abilities, or simply measurement error. In particular, the NART–R measures only acquired verbal function, whereas the WAIS–R measures both verbal and non-verbal IQ, and aspects that are more and less acquired. Given the different psychometric properties of the two instruments, and the lack of prospective studies which support their combined use in the study of intellectual change, the validity and reliability of this approach remain to be established.
Other methodological considerations
Comparing symptoms, disease course, genetic indices or brain anatomical and functional measures between intellectual subtypes was beyond the scope of the present investigation. However, such strategy would have enhanced the interpretability of our findings in relation to the neurobiological or nosological ‘signature’ of the subtypes investigated. For example, Turetsky et al Reference Turetsky, Moberg, Mozley, Moelter, Agrin and Gur11 have reported ‘memory’ subtypes of schizophrenia to have distinct clinical, neuroanatomical and neurophysiological correlates. Albeit not cross-referenced with neurobiological investigations, the meaningfulness of subtyping individuals with schizophrenia based on IQ level and trajectory was supported by our neuropsychological findings, as well as results of regression analysis of continuous intellectual predictors. The latter approach further enabled us to address the ‘loss’ of some participants during subtyping.
To increase the distinctiveness and face validity of the IQ categories, the present study used more refined criteria for intellectual classification than those used in previous studies. Reference Weickert, Goldberg, Gold, Bigelow, Egan and Weinberger3–Reference Joyce, Hutton, Mutsatsa and Barnes5 The ‘deteriorated good’ participants were not lumped together with the ‘deteriorated poor’ individuals as in previous studies, Reference Weickert, Goldberg, Gold, Bigelow, Egan and Weinberger3,Reference Badcock, Dragovic, Waters and Jablensky4 since the differential ratio of deteriorated good/poor participants in the schizophrenia (0.2) and control (1.7) samples would have confounded the statistical comparison.
Performance on the WAIS–R was used to define both our matching criteria (full-scale IQ and IQ decline) and four outcome variables (Digit symbol, Block design, Vocabulary and Comprehension). As matching for WAIS–R IQ does not ensure equivalence on the cognitive components of general ability, Reference Leeson, Barnes, Harrison, Matheson, Harrison and Mutsatsa49 examining the neurocognitive processes measured by the four subtests was central to our discussion of relative deficits in schizophrenia. The findings of the present study closely parallel those of a recent investigation. Reference Leeson, Barnes, Harrison, Matheson, Harrison and Mutsatsa49 Leeson and colleagues compared individuals with first-episode psychosis and healthy controls, one-to-one matched for full-scale current IQ, on WAIS subtests of perceptual organisation, verbal comprehension, processing speed and working memory, as well as other tests of executive function and episodic memory. Reference Leeson, Barnes, Harrison, Matheson, Harrison and Mutsatsa49 In line with the present findings, the groups showed equivalent performance on all WAIS subtests except Digit symbol processing speed, on which the individuals with schizophrenia performed significantly worse. Reference Leeson, Barnes, Harrison, Matheson, Harrison and Mutsatsa49 In addition, covarying for processing speed did not abolish the deficit in working memory and verbal learning of the participants with schizophrenia. Reference Leeson, Barnes, Harrison, Matheson, Harrison and Mutsatsa49
Although the deficit in processing speed of the participants with schizophrenia was a robust finding, and our analysis corrected for age, we cannot exclude the possibility that the age ‘advantage’ of the stable good and the deteriorated poor schizophrenia subgroups relative to intellectually matched controls moderated the effect detected for processing speed, which is age-dependent (both the stable good and the deteriorated poor schizophrenia subgroups showed deficits in one test of processing speed, as opposed to the stable poor schizophrenia subgroup, who showed deficits in two tests).
Identifying ‘specific’ cognitive deficits in schizophrenia is a long-standing and controversial issue, which requires the use of theoretically relevant measures that do not ‘just correlate with IQ’. Adding to the complexity of the problem is the attempt to measure the same neurocognitive domain using more than one test, as this requires psychometrically matched instruments with comparable discriminating power. Failing to satisfy this criterion (or natural variations across samples) may explain why the same domain elicits deficits in some tests but not others. The use of psychometric tests with different reliabilities and discriminating powers poses a particular problem in studies that attempt to identify specific cognitive deficits in a disorder by trying to establish whether participants with schizophrenia and controls differ more on some theoretically relevant measures (focal tasks) than on control tasks measuring other constructs (reference tasks). Alternative methods proposed for overcoming this problem include matching participants with schizophrenia and controls on a highly reliable variable such as current IQ (averting confounds resulting from general performance deficiencies in schizophrenia), analysis of covariance (do statistical differences on the focal task remain after controlling for performance on control tasks?), process-oriented solutions (using well-established models from cognitive psychology to predict specific theory-driven patterns of performance within and across tasks) and psychometric matching. The current study adopted both IQ-matching and analysis of covariance. However, both methods have been criticised as much as process-oriented solutions and psychometric matching, and, currently, there is no unequivocally accepted method for establishing specific cognitive deficits in a disorder. For a further discussion of this important topic, the reader is referred to a special section of the Journal of Abnormal Psychology. Reference Strauss50–Reference Miller and Chapman54
The interpretation of effect sizes generally depends on the assumptions that the schizophrenia group and control group values are normally distributed and have the same standard deviations. Both assumptions were tenable for the majority, but not all, of our tasks, reducing the comparability of deficits across neuropsychological components.
In summary, subtyping schizophrenia on the basis of current IQ and IQ trajectory offers a potential means for resolving the phenotypic complexity of the disorder, and is in line with current theoretical models of developmental heterogeneity in schizophrenia. Our findings are in agreement with those of previous studies Reference Weickert, Goldberg, Gold, Bigelow, Egan and Weinberger3–Reference Joyce, Hutton, Mutsatsa and Barnes5,Reference Allen, Goldstein and Warnick9,Reference van Winkel, Myin-Germeys, Delespaul, Peuskens, De and van13 in suggesting that IQ decline in schizophrenia is not universal. Deficits in executive function, processing speed and verbal memory appear to be prototypical of the disorder, and may coexist with preserved IQ. Our findings further suggest that these key deficits may reflect independent pathophysiological processes.
Funding
The study was funded by grants from the Medical Research Council, London, England, and the Stanley Medical Research Institute, Bethesda, Maryland, USA. Both funding organisations provided financial support for the conduct of study, collection, management and analysis of data.
Acknowledgements
We would like to thank the staff in the mental health services who helped in the case ascertainment and the research participants. We gratefully acknowledge advice from the late R. E. Kendell, FRCPsych, regarding the design of the study. We wish to acknowledge the contributions of the entire ÆSOP study team, listed online at http://www.psychiatry.cam.ac.uk/aesop.








eLetters
No eLetters have been published for this article.