The most likely time for a woman to become depressed is after childbirth. Reference Kendler, Neale, Kessler, Heath and Eaves1 Post-partum depression affects approximately 13% of women. Reference O'Hara and Swain2,Reference García-Esteve, Ascaso, Ojuel and Navarro3 Post-partum depression has a great impact on the family and economy, and is considered a major public health problem. Reference Petrou, Cooper, Murray and Davidson4,Reference Wisner, Chambers and Sit5 There is general agreement that the dramatic physiological changes that occur post-partum increase a woman's vulnerability to depressive symptoms, including post-partum depression. Reference Bloch, Daly and Rubinow6,Reference Serreti, Olgiati and Colombo7
Pregnancy and delivery are accompanied by hormonal changes as well as lower plasma tryptophan levels, both of which are thought to be aetiologically relevant to the mood changes that follow childbirth. Reference Bloch, Daly and Rubinow6,Reference Maes, Ombelet, Verkerk, Bosmans and Scharpé8,Reference Payne9 Although plasma tryptophan availability is not directly related to mood changes, Reference Maes, Ombelet, Verkerk, Bosmans and Scharpé8 the brain tryptophan availability index is decreased after delivery and is related to depressive symptoms. Reference Baïlara, Henry, Lestage, Launay, Parrot, Swendsen, Sutter, Roux, Dallay and Demotes-Mainard10 The mood-lowering effects of experimental tryptophan depletion are controversial, Reference Van der Does11 perhaps because of differences in 5-HTT genotype–tryptophan interaction. In women with previous depressive episodes, 5-HTT genotype may moderate the risk for depressive symptoms after tryptophan depletion. Reference Moreno, Rowe, Kaiser, Chase, Michaels, Gelernter and Delgado12,Reference Neumeister, Hu, Luckenbaugh, Sachwrz, Nugent, Bonne, Herscovitch, Goldman, Drevets and Charney13 If childbirth is considered an environmental factor, there may be a strong pathophysiological link between post-partum mood changes and the genes that moderate 5-hydroxy-tryptamine (5-HT) signalling.
Jans et al Reference Jans, Riedel, Markus and Blokland14 have proposed the concept of ‘serotonergic vulnerability’. Disruption of the serotonergic system may occur at several levels, including tryptophan availability, 5-HT synthesis, release, reuptake or metabolism, and/or pre- or post-synaptic 5-HT receptors. 5-HTT has received special attention because it plays a crucial role in the regulation of serotonergic function. Reference Murphy, Li, Engel, Wichems, Andrews, Lesch and Uhl15,Reference Lesch and Gutknecht16
In the light of these findings, we designed a prospective longitudinal multicentre study to evaluate interactions between 5-HTT genotype and post-partum mood changes. We hypothesised that 5-HTT genotype would shape the risk for depressive symptoms in post-partum women. We considered three genotype combinations of 5-HTTLPR and Stin2 VNTR polymorphisms that predict differential 5-HTT expression. Reference Hranilovic, Stefulj, Schwab, Borrmann-Hassenbach, Albus, Jernej and Wildenauer17 Given that the acute decline in tryptophan availability that follows childbirth resembles experimental tryptophan depletion, we hypothesised that high-expression 5-HTT genotypes would be associated with depressive symptoms in the weeks following childbirth.
Method
Participants
Between December 2003 and October 2004 women (2–3 days post-partum) were recruited in seven acute care teaching hospitals in Spain and invited to participate in a 32-week follow-up study. All participants were Spanish, not under psychiatric care during pregnancy, and able to understand and answer the clinical questionnaire. Women whose children died after birth were excluded. This study was approved by the institutional review boards of the participating hospitals. All women gave written informed consent.
Measures
All participants completed a semi-structured interview that included socio-demographic data: age, education level, marital status, number of children and employment status during pregnancy. Personal and family history of psychiatric illness was also recorded.
Depression
Depressive symptoms were assessed using the total score of the Edinburgh Postnatal Depression Scale (EPDS) Reference Cox, Holden and Sagovsky18 with a Spanish validated version. Reference Navarro, Ascaso, García-Esteve, Aguado, Torres and Martín-Santos19 The EPDS Reference Cox, Holden and Sagovsky18 is a 10-item self-report scale with four possible responses and a total score from 0 to 30. The EPDS was administered at baseline (2–3 days post-partum), 8 weeks and 32 weeks post-partum. All women who scored 9/10 in the EPDS were defined as probable cases of major depression. The cut-off of 9/10 for major depression maximises the sensitivity to 100% and reaches a specificity of 89%. Reference Navarro, Ascaso, García-Esteve, Aguado, Torres and Martín-Santos19 All probable cases of major depression were evaluated using the Spanish version of the Diagnostic Interview for Genetics Studies (DIGS) Reference Nurnberger, Blehar and Kaufmann20,Reference Roca, Martín-Santos, Sainz, Obiols, Serrano, Torrens, Subirà, Gili, Navinés, Ibañez, Nadal, Barrantes and Cañellas21 adapted for post-partum depression. All interviews were conducted by clinical psychologists who were previously trained in DIGS using the same video case records. A high level of reliability (kappa>0.8) was obtained among interviewers.
Genotyping
The Puregene DNA purification kit (Gentra Systems) was used to extract genomic DNA from peripheral blood samples. Two polymorphisms of 5-HTT were analysed, both of which affect 5-HTT expression: 5-HTTLPR, a 44-base pair insertion/deletion in the promoter region, and STin2, a multi-allelic 17-base pair variable number of tandem repeats (VNTR) within intron 2. Alleles of the 5-HTTLPR polymorphism were termed S (short allele with the deletion) and L (long allele with the insertion); the L allele shows higher basal transcription than the S allele. Reference Heils, Teufel, Petri, Stöber, Riederer, Bengel and Lesch22 Two main alleles of the STin2 VNTR polymorphism have been described: STin2.10 and STin2.12, with 10 or 12 repeats respectively. STin2.12 displays higher transcriptional activity than STin2.10. Reference Fiskerstrand, Lovejoy and Quinn23,Reference Mackenzie and Quinn24 STin2 alleles with seven and nine repeats occur at very low frequencies, and thus they were eliminated from the statistical analysis. Linked to 5-HTTLPR deletion insertion of a single nucleotide polymorphism (A/G) has been described. Reference Nakamura, Ueno, Sano and Tanabe25 This single nucleotide polymorphism somehow modulates the functional effect on 5-HTTLPR promoter polymorphisms on gene expression. However, the Stin2 VNTR polymorphism acts as an enhancer having a dramatic effect on gene expression. Moreover, it has been experimentally showed that the combination of both polymorphisms (5-HTTLPR and Stin2 VNTR) strongly affect the transcriptional level of the 5-HTT gene. Reference Hranilovic, Stefulj, Schwab, Borrmann-Hassenbach, Albus, Jernej and Wildenauer17 So, it seems more relevant to take into consideration the effect of both polymorphisms as a whole.
Taking into account the combined effect of both polymorphisms, significant differences in expression levels could be established based on high expression at one, both or neither of the loci. Reference Hranilovic, Stefulj, Schwab, Borrmann-Hassenbach, Albus, Jernej and Wildenauer17 Three types of 5-HTT expression genotype combinations were used for the statistical analysis: no low-expressing genotype at either of the loci (LL/12.12); low-expressing genotype at one of the loci (LL/12.10, LL/10.10, LS/12.12, SS/12.12); and low-expressing genotypes at both loci (LS/12.10, LS/10.10, SS/12.10, SS/10.10).
Statistical methods
Outcomes were the EPDS score and major depression diagnosis at 8 weeks and 32 weeks post-partum. The independent variable was the expression level genotype.
Multiple regression analysis was used to determine whether expression level genotype was associated with EPDS scores. The linear trends were assessed in relation to the degree of genotype loading. The regression model of EPDS score at 8 weeks was corrected using the EPDS score at baseline, and the regression model of EPDS score at 32 weeks was corrected with the EPDS score at 8 weeks. The chi-squared test was used to estimate the significance of association between expression level genotype and major depression at 8 and 32 weeks post-partum. A likelihood ratio test for interaction was used to test whether the association between expression level genotype and depression at 8 weeks differed from expression level genotype at 32 weeks. Given the low power of these tests, a P-value of 0.1 was considered suggestive of differences in effect size at 8 and 32 weeks. STATA version 9.2 was used for the statistical analyses. Two-sided statistical significance was set at P<0.05.
Results
Participants
There were 1974 women who fitted the inclusion criteria for the study, 94 (5%) women refused to participate and 76 (3.8%) women were excluded because their EPDS questionnaires were incomplete. Thus, the final sample comprised 1804 women. At the 8-week follow-up, 1407 (78%) women remained in the study. At 32 weeks, 1337 (74.1%) women were evaluated (Fig. 1). Those women who dropped out during the follow-up period were compared with the final sample, revealing that women from lower social classes were the most likely to drop out (P=0.005). The mean age of the participants was 31.7 years (s.d.=4.6), range 18–46. Of the participants, 32% had attended primary school, 41% finished secondary school and 27% had a college degree. Most participants (68%) were employed, 9% were homemakers or students, 12% were unemployed and 11% were on sick leave or maternity leave. Forty-six per cent of the women were primiparous. Thirty-one per cent of the total sample has a family history of previous psychiatric treatment. Sixteen per cent had a previous personal history of psychiatric treatment.
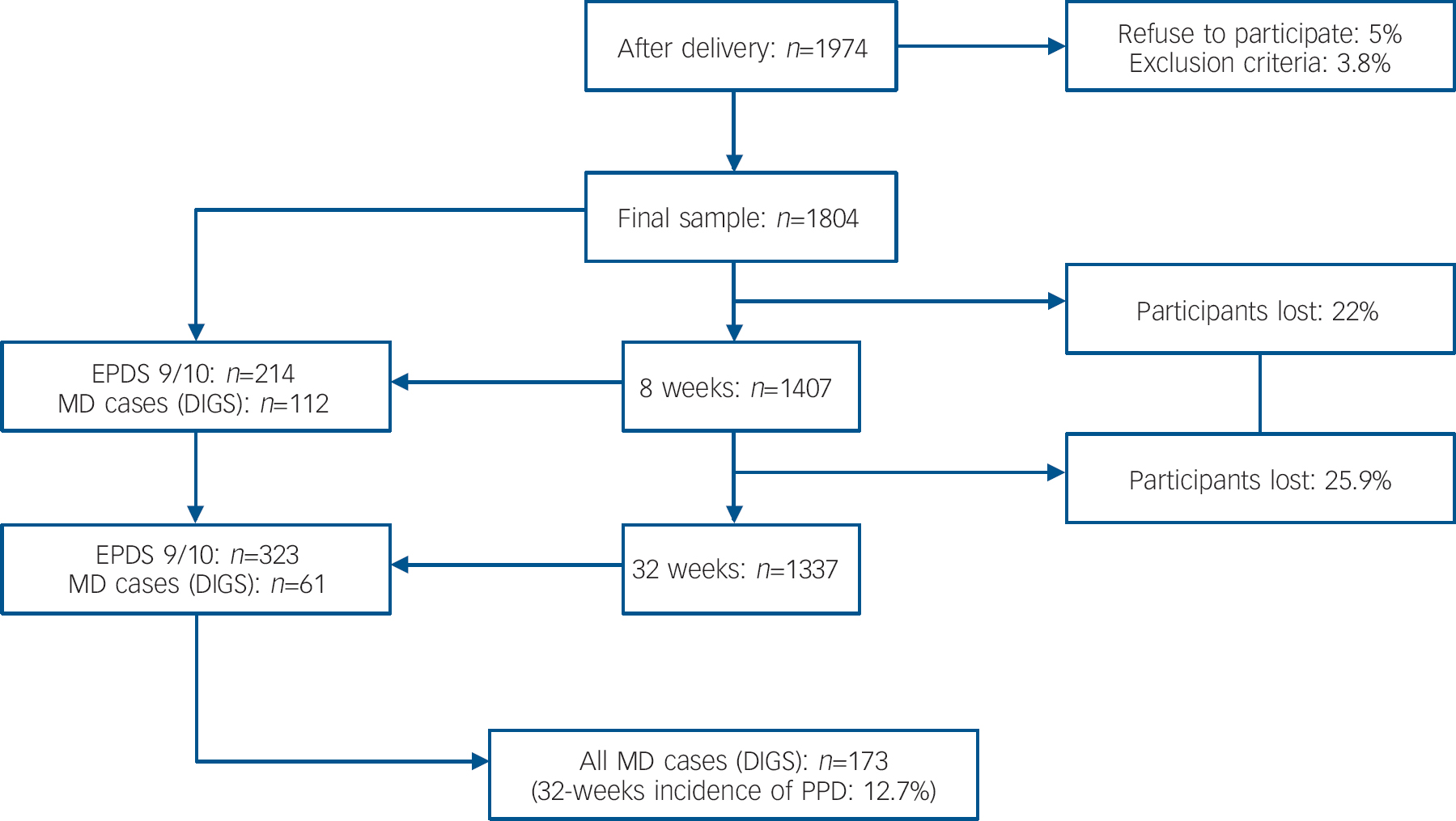
Fig. 1 Follow-up study of post-partum depression; EPDS, Edinburgh Postpartum Depression Scale; MD, major depression: DSM–IV major depression episode; DIGS, Diagnostic Interview for Genetics Studies; PPD, post-partum depression.
Clinical variables
Of the 1407 women studied at 8 weeks post-partum, 214 scored 9/10 on the EPDS scale. A diagnosis of major depression was confirmed by DIGS in 112 (7.9%) women. At 32 weeks post-partum, 323 of 1337 women scored 9/10 on the EPDS, but only 61 (4.5%) new cases of major depression were confirmed by DIGS. Overall, 173 women (12.7%) had a major depression episode during the first 32 post-partum weeks (Fig. 1). There were no differences in socio-demographic variables (i.e. age, educational level, employment) between women diagnosed with major depression at 8 weeks post-partum and women diagnosed at 32 weeks post-partum.
Edinburgh Postnatal Depression Scale scores declined over the post-partum period. The mean EPDS score was 6.1 (s.d.=4.5) at baseline, 5.3 (s.d.=4.6) at 8 weeks post-partum and 4.4 (s.d.=4.7) at 32 weeks post-partum (Fig. 2).

Fig. 2 Edinburgh Postnatal Depression Scale (EPDS) score over time (2–3 days, 8 weeks and 32-weeks post-partum) in relation to the different 5-HTT genotype combinations. HE, no low-expressing genotype at either of the loci; ME, low-expressing genotype at one of the loci; LE, low-expressing genotypes at both loci.
Frequency of polymorphisms
Genotype analysis of 5-HTTLPR and Stin2 VNTR polymorphisms in the 1804 women revealed the following frequencies: 0.28 LL, 0.48 SL, and 0.24 SS for 5-HTTLPR, and 0.46 STin2.12/STin2.12, 0.42 STin2.12/STin2.10, 0.10 STin2.10/STin2.10, 0.1 STin2.12/STin2.9, and 0.1 STin2.10/STin2.9 for STin2 VNTR. Allele frequencies for the 5-HTTLPR polymorphism were 0.52 for L and 0.48 for S. For the STin2 VNTR polymorphism, STin2.12, STin2.10, and STin2.9 alleles occurred at a frequency of 0.67, 0.32 and 0.01 respectively. Both polymorphisms were in Hardy–Weinberg equilibrium (5-HTTLPR P=0.19; STin2 VNTR P=0.62). No significant differences in the two polymorphisms were observed between samples from the seven different centres, and frequencies were similar to those reported for other Caucasian populations. Reference Fan and Sklar26 The frequencies of the expression genotypes were: no low-expressing genotype at either of the loci=0.088; low-expressing genotype at one of the loci=0.572; and low-expressing genotypes at both loci=0.34.
Genotype frequencies according to serotonin transporter expression
No significant difference in the distribution of major depression according to genotype combination was observed at 8 weeks (P=0.089) or 32 weeks (P=0.125) post-partum.
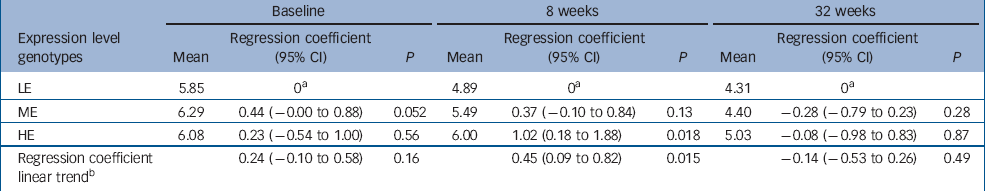
At baseline, genotype was not a predicator of EPDS score. At 8 weeks after childbirth, EPDS scores were related to 5-HTT expression levels in a dose–response fashion (regression coefficient B=0.45, 95% CI 0.09 to 0.82, P=0.015). At 32 weeks post-partum, EPDS was not related to genotype (B=–0.14, 95% CI −0.53 to 0.26, P=0.49) (Table 1, Fig. 2). The test for interaction between time and expression level genotype suggested the difference between 8 and 32 weeks was not due to chance (χ2=2.7, d.f.=1, P=0.1).
Table 1 Frequency of different 5-HTT genotype combinations (5-HTT-GC) and Edinburgh Postnatal Depression Scale (EPDS) score at baseline (2–3 days post-partum), 8 weeks post-partum, and 32 weeks post-partum
| Baseline | 8 weeks | 32 weeks | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Expression level genotypes | Mean | Regression coefficient (95% CI) | P | Mean | Regression coefficient (95% CI) | P | Mean | Regression coefficient (95% CI) | P | ||||||
| LE | 5.85 | 0 a | 4.89 | 0 a | 4.31 | 0 a | |||||||||
| ME | 6.29 | 0.44 (–0.00 to 0.88) | 0.052 | 5.49 | 0.37 (–0.10 to 0.84) | 0.13 | 4.40 | –0.28 (–0.79 to 0.23) | 0.28 | ||||||
| HE | 6.08 | 0.23 (–0.54 to 1.00) | 0.56 | 6.00 | 1.02 (0.18 to 1.88) | 0.018 | 5.03 | –0.08 (–0.98 to 0.83) | 0.87 | ||||||
| Regression coefficient linear trend b | 0.24 (–0.10 to 0.58) | 0.16 | 0.45 (0.09 to 0.82) | 0.015 | –0.14 (–0.53 to 0.26) | 0.49 | |||||||||
HE, no low-expressing genotype at either of the loci; ME, low-expressing genotype at one of the loci; LE, low-expressing genotypes at both loci
a. Reference category
b. Summary increase in EPDS score with one unit change in genotypic loading. P-values refer to the differences of ME and HE genotype with LE genotype as reference group
The differences remains significant after correcting for multiple comparison using Bonferroni correction between high expression (no low-expressing genotype at either of the loci) and low expression groups (low-expressing genotypes at both loci and low-expressing genotype at one of the loci) (P=0.045). We also introduce age in our linear regression model with not significant effect on the results (P=0.069).
Discussion
The incidence of post-partum depression in this study (12.7%) is in concordance with previous reports. Reference O'Hara and Swain2,Reference García-Esteve, Ascaso, Ojuel and Navarro3 Although there was a trend (P=0.089), no significant interaction between the expression level genotype and major depression was found. The EPDS score at 8 weeks post-partum was associated with the high-expression genotype. These results are in agreement with our hypothesis that 5-HTT genotype may modulate the mood changes, mainly depressive symptoms, that women experience just after delivery.
A number of factors may explain why the 5-HTT genotype was significantly associated with the EPDS score at 8 weeks, but not with major depression. First, it could be that the lack of significance (P=0.089) may be a statistical problem related to the relatively small sample size of women with major depression (n=173) and/or a weak effect of the 5-HTT genotype. High-expression 5-HTT polymorphisms may promote tryptophan depletion and induce major depression post-partum, but only when other genetic and/or environmental factors are present (e.g. lack of social support or life events). It seems unlikely that EPDS score at 8 weeks may reflect the ‘blues’, a transient emotional liability that affects about 50% of post-partum women. Reference Seyfried and Marcus27 The ‘blues’ are quantitatively and qualitatively different from depression; Reference Miller, Rukstalis and Miller28 they emerge during the first post-partum week and disappear by the second week. Moreover, there is some evidence that the DSM–IV 29 or ICD–10 30 definition of major depression is an arbitrary diagnostic convention imposed upon a continuum of depressive symptoms. Reference Goldberg31–Reference Aggen, Neale and Kendler33
Reduced availability of brain tryptophan during the post-partum period may explain the high (>9) EPDS scores. Experimental tryptophan depletion frequently leads to transient symptoms of depression in vulnerable individuals. Reference Van der Does11 The brain tryptophan availability index decreases by 15% after delivery and is associated with depressive symptoms. Reference Baïlara, Henry, Lestage, Launay, Parrot, Swendsen, Sutter, Roux, Dallay and Demotes-Mainard10 This reduction in the early post-partum period is not associated with plasma tryptophan levels Reference Maes, Ombelet, Verkerk, Bosmans and Scharpé8 but rather with a dramatic increase in circulating levels of free amino acids that compete with tryptophan, such as leucine, isoleucine, valine and tyrosine, resulting in a significantly impaired transport of tryptophan across the blood barrier. The brain tryptophan availability index is calculated according to the Michaelis model for substrate competition on enzymes or transporters taking into account the total plasma tryptophan concentration and the blood concentration of competitor amino acids. So, the post-partum period is a ‘natural model’ of tryptophan depletion, and individuals with the LL 5-HTTLPR genotype indeed present with more depressive symptoms after tryptophan depletion. In a study of 43 individuals currently in remission from major depression, those with the LL genotype had significantly higher scores on the Hamilton Rating Scale for Depression (HRSD) than LS or SS carriers. Reference Moreno, Rowe, Kaiser, Chase, Michaels, Gelernter and Delgado12 Moreno et al suggested that rapid uptake of 5-HT in people with LL, combined with decreased brain 5-HT availability during tryptophan depletion, produces a substantial decrease in serotonergic transmission, thereby enhancing depressive symptoms. Reference Moreno, Rowe, Kaiser, Chase, Michaels, Gelernter and Delgado12 A recent study Reference Neumeister, Hu, Luckenbaugh, Sachwrz, Nugent, Bonne, Herscovitch, Goldman, Drevets and Charney13 confirms that people with previous depressive episodes and the LL genotype have the greatest increase in HRDS scores after tryptophan depletion. The gene–tryptophan interaction is further supported by a double-blind placebo study of 15 healthy SS and 15 healthy LL volunteers. The SS genotype group outperformed the LL genotype group in tests of episodic memory and attention. Reference Roiser, Müller, Clark and Sahakian34 Thus, it may be hypothesised that women with high-expression 5-HTT genotypes have an increased risk of depressive symptoms post-partum because serotonergic transmission is reduced. In line with the above observation, the genotype effect disappears by 32 weeks post-partum.
The decline of EPDS scores over the post-partum period is also consistent with previous observations. Reference Evans, Heron, Francomb, Oke and Golding35,Reference Dennis36 Depressive symptoms in women with a high-expression 5-HTT genotype show the least change over time, possibly reflecting differences in the rate of brain tryptophan availability index normalisation during the post-partum period. Other aetiological mechanisms of post-partum depression, such as familiality, are in agreement with the time of onset observed in our study. Reference Forty, Jones, Macgregor, Caesar, Cooper, Hough, Dean, Dave, Farmer, McGuffin, Brewster, Craddock and Jones37 In tryptophan depletion studies, high-expression 5-HTT genotypes are at risk, whereas low-expression variants have been implicated in life event interactions. Reference Caspi, Sugden, Moffitt, Taylor, Craig, Harrington, McClay, Mill, Martin, Braithwaite and Poulton38–Reference Cervilla, Molina, Rivera, Torres-González, Bellón, Moreno, Luna, Lorente, Mayoral, King and Nazareth44 This suggests that gene effects on acute tryptophan depletion and stress response are distinct. According to the ‘serotonergic vulnerability’ model, Reference Jans, Riedel, Markus and Blokland14 mood changes after delivery are the result of complex interactions between multiple variables, including acute hormonal changes and tryptophan depletion, which is mediated by 5-HTT genotype. In support of this hypothesis the risks of post-partum depression have been associated with a tryptophan hydroxylase gene polymorphism. Reference Sun, Tsai, Ko, Chang and Yeh45
To the best of our knowledge, there are two studies of 5-HTT expression during pregnancy and delivery. Coyle et al 48 found a significant (P<0.003) over-representation of STin2.12 allele (the high expression allele) in 127 women with bipolar disorder, who later developed puerperal psychosis within a few days of delivery, compared with 380 controls. Scheid et al Reference Scheid, Holzman, Jones, Friderici, Nummy, Symonds, Sikorskii, Regier and Fisher47 performed a cross-sectional study of 568 pregnant women to examine possible interactions between 5-HTTLPR polymorphisms and different types of life stressors relative to depressive symptoms. The only significant interaction identified was the sub-construct of ‘childhood abuse’. Their study, however, was conducted during pregnancy, not during the post-partum period, and therefore it is unlikely it tapped into specific mechanisms associated with tryptophan depletion.
The present study has several limitations. First, the brain tryptophan availability index was not measured. Second, the results pertain to White Spanish women and may or may not be applicable to other ethnic groups. Lastly, it is impossible to exclude the possibility that depressive symptoms account for the 27% attrition rate at 32 week post-partum. However, it is unlikely that genotype has a differential effect on attrition, making this an unlikely cause of bias. It is also worth mentioning several strengths of our study. First, our sample is relatively large. Second, the study was longitudinal and depression was evaluated categorically and dimensionally to increase sensitivity of the measure. Third, we analysed the combination of two (5-HTTLPR and Stin2 VNTR) polymorphisms related to functional expression. Finally, the homogeneity of our sample may be one of the principal methodological strengths. All participants were White Spanish women, all were young and, perhaps most importantly, all were evaluated during the very well-defined post-partum risk period.
In summary, our results suggest that there are no ‘bad’ or ‘good’ 5-HTT genotypes in relation to depression. High-expression 5-HTT genotypes might be a risk factor under certain environmental conditions such as tryptophan depletion after childbirth. This study supports a new hypothesis for understanding the biological mechanisms underlying depressive symptoms after delivery and encourages further study of gene–tryptophan interactions in mood disorders.
Acknowledgements
This work was supported by the Instituto Carlos III (Spanish Ministry of Health) grant numbers: PI041635, PI041783, PI041779, PI041758, PI041761, PI041791, PI041766 and PI041782, as well as The Spanish Psychiatric Genetics and Genotyping network GO3/184 and the Spanish Mental Health Network: CIBER Enfermedades Mentales. We also thank M. Pulido for editing the manuscript.






eLetters
No eLetters have been published for this article.