Minor distress and emotional disorders are the commonest mental health problems encountered in primary care. Reference Bijl, de Graaf, Hiripi, Kessler, Kohn and Offord1,2 Mixed anxiety and depressive symptoms that do not reach thresholds for formal psychiatric diagnosis make up nearly half of all psychological problems in the UK and are nearly four times more common than depressive disorder alone. Reference Singleton, Bumpstead, O'Brien, Lee and Meltzer3 Nevertheless, there has been much debate about the inclusion of mixed anxiety and depression as a new category in DSM–5, Reference Frances4 particularly since it is unclear when symptoms of milder distress that may be transient, understandable and part of normal human emotional responses should be seen as a disorder. There is little consensus about the impact of milder distress on mental health outcomes, quality of life, functional impairment and economic costs. Minor, ‘subthreshold’ or ‘subclinical’ disorders are commonly defined as having above threshold scores for significant distress on a validated measure, but without fulfilling the criteria for any other diagnosis according to the ICD–10 5 or the DSM–IV. 6 In the proposed criteria for DSM-5 7 mixed anxiety and depression patients must have three to four symptoms of major depression, accompanied by anxious distress; whereas in ICD–10 mixed anxiety and depressive disorder (MADD) symptoms of anxiety and depression should be present (with no specified number) but neither should be clearly predominant or severe enough to justify an individual diagnosis, and some autonomic symptoms must be present. Since these criteria differ, studies cannot easily be compared across both systems. There are few studies reporting outcomes for minor distress with mixed anxiety and depression symptoms, in particular the group defined by ICD–10 as MADD. Those that exist are mostly small, methodologically heterogeneous and inconsistent in their findings. Some evidence has suggested that MADD may not be a stable diagnosis, with most either remitting or shifting to other diagnoses over time. Reference Barkow, Heun, Wittchen, Ustun, Gansicke and Maier8 A small number of studies have demonstrated an increased risk of major depression in those with either minor depression or ‘subsyndromal’ depressive symptoms Reference Cuijpers and Smit9,Reference Forsell10 as defined by DSM–IV. However, in absolute terms only a minority (around 10–20%) appear to develop more severe disorders, with the majority improving over time. Reference Forsell10–Reference Jackson, Passamonti and Kroenke12 There is currently insufficient evidence to accurately estimate recovery rates for those with ICD–10 MADD. Our objectives in this study were to: describe the characteristics of people with MADD in primary care; determine their short- to medium-term outcomes in terms of persistent/recurrent psychological distress, associated quality of life, health services use and general practitioner (GP) diagnosis; and identify potential predictive factors for persistent/recurrent distress in people with MADD.
Method
This was a cohort study of consecutive people aged 18 years and over attending seven general practices in suburban and urban London, UK. Those unable to complete a written questionnaire in English, with significant cognitive impairment or incapacitating physical illness were excluded. Participants were selected in a two-stage sampling design from respondents to a waiting room screening questionnaire including the General Health Questionnaire 12-item version (GHQ–12). Reference Goldberg13 The methods/results of this initial screening are published in detail elsewhere. Reference Walters, Buszewicz, Weich and King14 Participants who scored as having mild–moderate distress (in the range 2–6 on the GHQ–12) in the initial questionnaire survey were selected for a full psychiatric diagnostic interview using the Revised Clinical Interview Schedule (CIS–R). Reference Lewis, Pelosi, Glover, Wilkinson, Stansfeld and Williams15,Reference Lewis, Pelosi, Araya and Dunn16 The CIS–R was used to categorise participants into three groups:
-
(a) MADD with significant symptoms and scoring above the CIS–R threshold (11/12) for a ‘case’ of psychological disorder but not reaching eligibility for any other formal ICD–10 diagnosis;
-
(b) other ICD–10 psychiatric diagnosis; and
-
(c) no psychiatric disorder.
The computerised version of the CIS–R was chosen as a widely used and validated diagnostic instrument for common mental disorders in primary care and community settings. Reference Singleton, Bumpstead, O'Brien, Lee and Meltzer3,Reference Lewis, Pelosi, Glover, Wilkinson, Stansfeld and Williams15,Reference Lewis, Pelosi, Araya and Dunn16 We used the ICD–10 definition of MADD as the DSM–IV/DSM–5 definition is currently still in proposed form.
Measurements
Baseline
All participants had a baseline interview including the CIS–R conducted either face to face or by telephone. Reference Evans, Kessler, Lewis, Peters and Sharp17 They also completed several self-complete measures, including baseline psychological distress (GHQ–28) Reference Goldberg13,Reference Ormel, Koeter, Van den Brink and Giel18,Reference Goldberg, Gater, Sartorius, Ustun, Piccineli and Gureje19 quality of life (version 2 of the 12-item Short Form Health Survey, SF–12), Reference Gandek, Ware, Aaronson, Apolone, Bjorner and Brazier20,Reference Ware, Turner-Bowker, Kosinski and Gandek21 disability (World Health Organization Disability Assessment Schedule, WHO–DAS) 22 somatic dysfunction (15-item somatic subscale of the Patient Health Questionnaire, PHQ). Reference Kroenke, Spitzer and Williams23 Information on sociodemographic characteristics (age, gender, ethnicity, employment, housing tenure), social factors (marital status, who they lived with, number of children, carer status), past psychiatric history, physical ill health (self-reported major physical illness, number of prescriptions categorised into 0–2 or 3 or more), lifestyle (alcohol and drugs), life events in past year, treatments received for emotional problems (self-report and from GP records), help-seeking behaviour and help-seeking preferences were also collected.
Outcomes
Participants were followed up by postal questionnaire at 3 months and 1 year and case-note review of GP records at 1-year follow-up. The primary outcome was psychological distress, measured using the GHQ–28, a widely used and validated measure containing four subscales of anxiety, depression, somatic symptoms and social dysfunction. Reference Goldberg13,Reference Ormel, Koeter, Van den Brink and Giel18,Reference Goldberg, Gater, Sartorius, Ustun, Piccineli and Gureje19 Secondary outcomes were quality of life (SF–12), GP diagnosis and GP consultation rate.
Sample size
We chose to sample people who scored in the low/moderate range at screening on the GHQ–12 in order to identify a sufficiently large sample of people with MADD to potentially identify predictors of outcome in this group. Using conservative estimates that 30% of our MADD group and 8% of our no diagnosis (control) group would be GHQ–28 ‘cases’ at 1 year follow-up, Reference King, Nazareth, Levy, Walker, Morris and Weich24 at 90% power and 0.05 level of significance this would require 74 participants in each of these arms.
Data analysis
Analysis was performed using Stata release 10.0 software for Windows. Unadjusted associations between outcomes and main explanatory variables and each potential confounder were tested using two-tailed chi-squared, t- and equality of medians tests. Colinearity was assessed for conceptually similar variables by cross-tabulation and scatter plots/correlation as appropriate. Given there was a marked right skewed distribution on the GHQ–28 at both 3 months and 1 year, with a large proportion of respondents scoring zero, it was necessary to dichotomise the outcome measure and choose an acceptable and valid case threshold to indicate ‘caseness’. We calculated a receiver operating characteristics (ROC) curve against the baseline CIS–R scores for our sample. We chose a relatively high case threshold of 8/9 for further analysis, as the threshold that achieved an adequate level of specificity (79.6%), while maintaining a reasonable, albeit lower level of sensitivity (68.6%).
Modified Poisson modelling was used for our main outcome the GHQ–28, using a dichotomous case/non-case definition of outcome, and for the secondary outcomes of GP diagnosis and GP consultation rate. Quality of life (SF–12) was sufficiently normally distributed to fit population-averaged panel-data models using generalised estimating equations (GEE). The variables from each group of potentially colinear variables that had the strongest association with outcomes and exposures on crude analysis and on theoretical grounds from the literature were selected for the modelling process. To avoid overloading models and potential colinearity a number of the variables were omitted. Interactions were assessed between the main explanatory variables and each of the other key variables in the model and also between age and gender. Robust standard errors were used to account for clustering by GP practice. We used a complete case analysis for responders to the two follow-up postal questionnaires. Where a single item was missing from our main outcome measure (GHQ–28) we imputed the missing data in Stata based on the scores of the other items in that subscale. We conducted a sensitivity analysis of worst and best case scenarios for the imputations to determine the potential impact on our findings.
Results
Sample characteristics
Response rates and attrition
In total 1383/1528 (91%) of consecutive adult GP attendees from a range of GP surgery sessions spread throughout the week completed a waiting room screening questionnaire. Of these, 304/447 (68%) individuals who were eligible for inclusion opted to participate in the main cohort study. Interviews were completed for 250/304 (82%) participants (Fig. 1). Overall 193/250 (77%) returned postal follow-up questionnaires at 3 months and 172/250 (69%) at 1 year. In total 215/250 (86%) returned questionnaires at either 3 months or 1 year and 153/250 (61%) returned questionnaires at both 3 months and 1 year. Case-note follow-up data were obtained for 238/250 (95%) participants.
Non-responders and missing data
The cohort study participants were similar to non-responders at baseline in terms of gender and GHQ–12 screening score (mean score 3.6 for both groups), but were slightly older (mean age 46 v. 42 years for non-responders). Non-responders to the postal questionnaire at 3-month follow-up were slightly younger (mean age 41 v. 48 years), from Black and minority ethnic groups (44% v. 25%) and non-home owners (68% v. 55%). Results were similar for non-responders at 1 year. There was no difference in response rates for those in the MADD group compared with the no psychiatric diagnosis group, but more non-responders were in the other ICD–10 diagnosis group (21% v. 11%). Non-responders had slightly higher levels of psychological distress at baseline (GHQ–28 score 9.8, 95% CI 8.0–11.6) than participants (GHQ–28 score 8.0, 95% CI 7.3–8.8), but confidence intervals were overlapping. There were no meaningful differences in other sociodemographic characteristics. The sensitivity analysis of the imputation of single missing items of data for our main outcome, the GHQ–28, demonstrated no appreciable difference in our findings using best or worse case scenarios for the imputations.
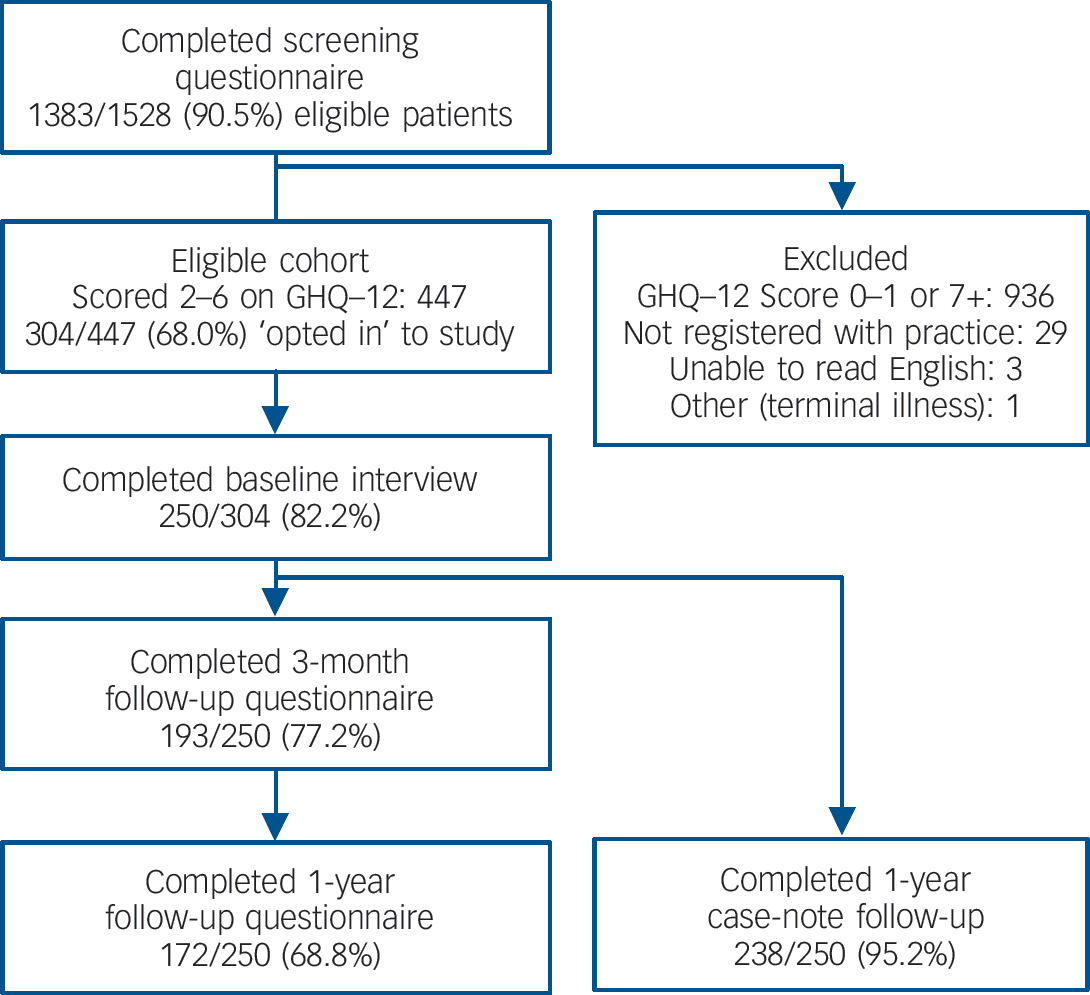
Fig. 1 Study response rates and attrition
Sociodemographic characteristics
The mean age for the whole sample was 46 years (95% CI 44–48 years) and 71% were women (online Table DS1). The sample was ethnically diverse, with only 137/250 (55%) identifying themselves as ‘White British’ and a further 25/250 (10%) as ‘White European’. The largest Black and minority ethnic groups were ‘Black African or Caribbean’, and ‘Asian’ (Indian, Pakistani and Bangladeshi). The sample was socially diverse, with just under half (120/250, 48%) in paid employment and less than half (107/250, 43%) were owner-occupiers. More than half reported a significant life event in the past year (143/250, 57%), the most common of which were major illness in themselves or someone close to them, changing or losing their job, relationship breakdown and bereavement.
Baseline psychological symptoms, diagnoses and functioning
The mean total scores were 8.4 (95% CI 7.7–9.1) for the GHQ–28 and 13.5 (95% CI 12.4–14.5) for the CIS–R within the sample as a whole at baseline (Table 1). Using the CIS–R 103/250 (41%) had MADD, 34/250 (14%) had another ICD–10 psychiatric diagnosis and 113/250 (45%) had no diagnosis and scored lower than the threshold of 11 symptoms on the CIS–R. For those with other ICD–10 diagnoses the majority had mild, moderate or severe depressive disorder (21/34, 62%) and the remainder had panic disorder/agoraphobia (4/34), other specific phobias (6/34) or obsessive–compulsive disorder (2/34). The quality of life measured using the SF–12 in the overall sample was lower for the mental health subscale (mean 42.4, 95% CI 40.9–43.8) than for the physical health subscale (mean 45.2, 95% CI 43.7–46.7). Both of these scores were below general population norms. Reference Gandek, Ware, Aaronson, Apolone, Bjorner and Brazier20 There was a wide variation in degree of disability, with a median score of 15 and an interquartile range (IQR) of 5–30 on the WHO–DAS 12-item screen.
Table 1 Baseline psychological distress, quality of life, disability and somatic dysfunction, by diagnostic group

| Variable | Overall (n = 250) | MADD group (n = 103) | Other ICD–10 diagnosis group (n = 34) | No diagnosis group (n = 113) | MADD v. no diagnosis group, P |
|---|---|---|---|---|---|
| CIS–R: total score, mean (95% CI) (n = 250) | 13.5 (12.4–14.5) | 17.9 (16.9–18.9) | 24.1 (21.3–26.8) | 6.2 (5.6–6.8) | 0.001 a |
| GHQ–28 (n = 246) | |||||
| Overall, mean score (95% CI) | 8.4 (7.7–9.1) | 10.3(9.4–11.2) | 12.6 (10.5–14.8) | 5.4 (4.6–6.2) | 0.001 a |
| Subscales, median (IQR) | |||||
| Anxiety | 3 (1–5) | 4 (2–5) | 4 (2–6) | 1 (0–3) | <0.001 b |
| Somatic | 3 (1–4) | 4 (2–5) | 4.5 (2–6) | 2 (1–3) | <0.001 b |
| Social dysfunction | 1 (0–3) | 2 (1–4) | 2 (1–5) | 1 (0–2) | <0.001 b |
| Depression | 0 (0–0) | 0 (0–1) | 1 (0–2) | 0 (0–0) | 0.007 b |
| Quality of life (SF–12 score), mean (95% CI) (n = 250) | |||||
| Physical health functioning | 45.2 (43.7–46.7) | 44.9 (42.5–47.4) | 41.0 (36.1–45.9) | 46.7 (44.7–48.7) | 0.27a |
| Mental health functioning | 42.4 (40.9–43.8) | 38.3 (36.3–40.3) | 35.0 (31.8–38.2) | 48.1 (46.3–50.0) | <0.001 a |
| Somatic dysfunction (PHQ–15 score), median (IQR) | 1 (0–3) | 1 (0–3) | 3 (1–4) | 1 (0–2) | 0.003 b |
| Disability (WHO–DAS score), median (IQR) (n = 241) | 15 (5–30) | 19 (10–30) | 26 (14.5–30) | 10 (2–20) | <0.001 b |
| Disability days,c median (IQR) | 5 (0–14) | 6.5 (2–15) | 15 (4.5–30) | 2 (0–8) | 0.001 b |
Univariable associations
Associations with MADD at baseline
There were significant associations between some sociodemographic variables and MADD compared with those with no psychiatric diagnosis. These included female gender (odds ratio (OR) = 2.40, 95% CI 1.28–4.48), having children under 16 years of age (OR = 1.83, 95% CI 1.00–3.33), somatic dysfunction (OR = 2.49, 95% CI 1.28–4.84) and a self-reported past psychiatric history (OR = 2.56, 95% CI 1.44–4.54) (online Table DS1). Those in the MADD group at baseline had increased psychological distress, including CIS–R overall score (P<0.001), GHQ–28 overall score (P<0.001) and all four subscales of the GHQ–28: anxiety (P<0.001), depression (P = 0.007), somatic symptoms (P<0.001) and social dysfunction (P<0.001) compared with those in the no psychiatric diagnosis group. They also had higher associated somatic dysfunction (‘bothered a lot’ by three or more somatic symptoms) using the PHQ somatic subscale (P = 0.003), disability WHO–DAS 12-item screen (P<0.001), number of disability days (P<0.001) and lower quality of life on the mental health subscale of the SF–12 (P<0.001) (Table 1). There was no significant association with age or the physical health subscale of the SF–12.
Primary outcome
The median GHQ–28 score was significantly higher in both the MADD and other ICD–10 diagnosis groups when compared with the no psychiatric diagnosis group at all three time points (Table 2). The median GHQ–28 scores improved substantially in the MADD group at 3 months and 1 year, but remained higher in the other ICD–10 diagnosis group. In the overall sample the only significant associations of sociodemographic variables with being a GHQ–28 ‘case’ for psychological distress (using the 8/9 threshold) were with self-reported past psychiatric history at both 3 months (crude OR = 2.03, 95% CI 1.04–3.96) and 1 year (crude OR = 4.05, 95% CI 1.84–8.90) and with somatic dysfunction at both 3 months (crude OR = 3.15, 95% CI 1.49–6.69) and 1 year (crude OR = 2.74, 96% CI 2.74–6.10). In the MADD group 47/77 (61%) were below the 8/9 threshold for psychological distress at 3 months and 48/70 (69%) at 1 year follow-up.
Table 2 Median General Health Questionnaire (GHQ–28) scores at baseline, 3 months and 1 year by diagnostic group

| GHQ–28 score | ||||||
|---|---|---|---|---|---|---|
| Baseline | 3 months | 1 year | ||||
| Group | Median (IQR) | P a | Median (IQR) | P a | Median (IQR) | P a |
| No diagnosis | 4 (2–8) | 1 (0–4) | 1 (0–4) | |||
| Mixed anxiety and depressive disorder | 10 (7–13) | <0.001 | 5 (2–10) | <0.001 | 4.5 (0–10) | 0.003 |
| Other ICD–10 disorder | 13 (9–15) | <0.001 | 9.5 (2–13) | 0.022 | 14 (4–19) | <0.001 |
Multivariable analysis
Psychological distress
Those in the MADD group were more than twice as likely as the no psychiatric diagnosis group to be a GHQ–28 case at both 3 months and 1 year (Table 3). When this was adjusted for age, gender, past psychiatric history, somatic dysfunction and clustering by practice the risk was reduced, but remained significant at 3 months, with an adjusted incidence rate ratio (IRR) of 2.39 (95% CI 1.29–4.42). By 1-year follow-up, the point estimates for being a GHQ–28 case were still raised in the MADD group, but the disparity with the no psychiatric diagnosis group was no longer significant (IRR = 1.73, 95% CI 0.87–3.47). For the other ICD–10 disorders group the risk of being a GHQ case of distress at follow-up compared with risk in the no psychiatric diagnosis group was increased still further, with a nearly fourfold increase in risk at 3 months and a nearly fivefold increased risk at 1 year. After adjustment for confounding factors the point estimates were reduced slightly, but remained significant at both 3 months (IRR = 2.87, 95% CI 1.41–5.88) and 1 year (IRR = 3.18, 95% CI 1.56–6.51).
Table 3 Multivariable analysis: crude and adjusted risk of General Health Questionnaire (GHQ-28) caseness at 3 months and 1-year follow-up in the mixed anxiety and depressive disorder (MADD) and other ICD–10 diagnoses groupsa

| 3-month follow-up (n = 179) | 1-year follow-up (n = 160) | |||||
|---|---|---|---|---|---|---|
| Model | GHQ–28 case, n (%) | Crude IRR (95% CI)b | Adjustedc IRR (95% CI)b | GHQ–28 case, n (%) | Crude IRR (95% CI)b | Adjustedc IRR, (95% CI)b |
| No diagnosis (3 month n = 84, 1 year n = 72) | 12 (14.3) | 1.0 | 1.0 | 10 (13.9) | 1.0 | 1.0 |
| Mixed anxiety depressive disorder (3 month n = 77, 1 year n = 70) | 30 (39.0) | 2.73 (1.50–4.95) | 2.39 (1.29–4.42) | 22 (31.4) | 2.26 (1.15–4.44) | 1.73 (0.87–3.47) |
| Other ICD–10 diagnosis (3 month n = 18, 1 year n = 18) | 10 (55.6) | 3.89 (1.99–7.59) | 2.87 (1.41–5.88) | 12 (66.7) | 4.80 (2.47–9.32) | 3.18 (1.56–6.51) |
Quality of life
There was a significantly lower quality of life (mental functioning) for both the MADD (–5.36, 95% CI –8.40 to –2.31) and other ICD–10 diagnosis (–4.92, 95% CI –8.80 to –1.64) groups in comparison to the no diagnosis group at 3 months, and this was of a similar degree for both groups (Table 4). At 1-year follow-up the lowered mental functioning quality of life persisted at a similar level in the MADD group (–5.04, 95% CI – 8.43 to –1.64), but had markedly deteriorated in the other ICD–10 diagnoses group (–12.09, 95% CI –17.07 to – 7.11). There was no significant difference in physical functioning quality of life at either 3 months or 1 year for either the MADD or other ICD–10 diagnostic groups.
Table 4 Secondary outcomes: quality of life, general practitioner (GP) consultation rate and GP diagnosis over 1-year follow-up in mixed anxiety and depressive disorder and other ICD–10 disorders compared with the no diagnosis groupa

| 3-month follow-up | 1-year follow-up | |
|---|---|---|
| 12-item Short Form, difference in comparison with no diagnosis,b mean (95% CI) | ||
| Mental functioning | ||
| Mixed anxiety and depressive disorder | –5.36 (-8.40 to –2.31) | –5.04 (– 8.43 to –1.64) |
| Other ICD–10 disorders | 4.92 (–8.80 to –1.64) | –12.09 (–17.07 to –7.11) |
| Physical functioning | ||
| Mixed anxiety and depressive disorder | – 1.66 (–4.67 to 1.34) | – 1.48 (–4.59 to 1.62) |
| Other ICD–10 disorders | – 3.58 (–8.07 to 0.92) | – 3.40 (–7.98 to 1.19) |
| GP consultations, IRR (95% CI)c | ||
| Mixed anxiety and depressive disorder | - | 0.98 (0.79 to 1.20) |
| Other ICD–10 disorders | - | 1.46 (1.08 to 1.99) |
| GP diagnosis, IRR (95% CI)d | ||
| Mixed anxiety and depressive disorder | - | 1.27 (0.72 to 2.31) |
| Other ICD–10 disorders | - | 2.55 (1.36 to 4.75) |
Consultation rate
There was no significant difference in GP consultation rates between those in the MADD group and the no diagnosis group (Table 4). The consultation rate was, however, significantly higher in the other ICD–10 diagnoses group, even after adjustment for comorbid physical illness and other demographic factors (IRR = 1.46, 95% CI 1.08–1.99).
GP diagnosis
There was no significant difference in a GP-reported diagnosis of a mental health problem between the MADD and no diagnosis groups over the year's follow-up (Table 4). Those with other ICD–10 disorders were, however, two and a half times more likely to have a mental health problem recorded in their case notes over the whole year follow-up period than those in the no diagnosis group (IRR = 2.55, 95% CI 1.36–4.75).
Discussion
Main findings
People with MADD were more likely to be women, have multiple somatic symptoms at baseline and a history of mental health difficulties when compared with those with no psychiatric diagnosis. Around two-thirds of those with MADD did not have levels of distress high enough to reach the threshold to be considered distressed on the GHQ–28 at 3 months (61%) or 1 year (69%). However, those with MADD were more than twice as likely to be psychologically distressed 3 months from baseline as those with no diagnosis. Those with other ICD–10 diagnoses remained the most affected of the three groups at both 3 and 12 months. No variables were found to be significant predictors of persistent or recurrent distress in the MADD group at either 3 months or 1 year. The pattern of findings for the quality of life mental health functioning subscale was similar to those for psychological distress across the three groups, but there were no significant differences in physical health functioning. Participants with MADD attended their GP practice no more often than those without a diagnosis, and were no more likely to have a mental health problem recorded by their GP during the year's follow-up. Participants with other ICD–10 diagnoses were the most frequent users of GP services and were more likely to have a GP-recorded mental health problem.
Strengths and limitations
This is one of very few prospective studies specifically designed to measure the outcomes of people attending their general practice with MADD. The sample was recruited from those attending seven UK GP practices in urban/suburban settings, with excellent response rates to the initial screening questionnaire. There were lower rates of participation in the main cohort, but participants were similar in terms of age, gender and mean level of distress experienced when compared with those declining to take part. There was good follow-up of participants, with most (86%) completing at least one of the two follow-up questionnaires and nearly complete (95%) case-notes follow-up. Non-responders to follow-up questionnaires had slightly higher baseline levels of distress, although confidence intervals were overlapping. Attrition rates were similar for the MADD and no diagnosis groups, but slightly higher in the other ICD–10 diagnosis group, and the findings from this study may over-represent those with less severe symptoms.
All participants had between two and six symptoms of psychological distress on the baseline GHQ–12 screening questionnaire 2 weeks earlier. This means that the sample under-represented both those with no symptoms of distress and those with severe distress, which would have the effect of biasing our study towards finding no difference between the groups. As the distribution of GHQ–28 scores at follow-up was so skewed towards complete recovery we were not able to analyse these as a continuous measure in our multivariable analysis. A higher case threshold was chosen for the GHQ–28 than is commonly used in other studies. Reference Goldberg13 This high threshold was chosen as it represented the best balance of sensitivity and specificity against the CIS–R within the sample. There was insufficient power to fully determine potential predictors of persistent distress/recovery within the MADD group. In common with any observational study there may have been unmeasured confounding.
Interpretation in relation to existing literature
The lack of statistically significant associations with sociodemographic factors in our study is consistent with the little existing work in the area, which has also shown a lack of strong associations with MADD. Reference Ormel, Oldehinkel, Brilman and Van den Brink25–Reference Das-Munshi, Goldberg, Bebbington, Bhugra, Brugha and Dewey27 Those with MADD had higher disability and lower quality of life than the no diagnosis group at baseline, consistent with the literature. Reference Das-Munshi, Goldberg, Bebbington, Bhugra, Brugha and Dewey27,Reference Da Silva Lima and de Almeida Fleck28 Using the 8/9 threshold on the GHQ–28, around two-thirds of those in the MADD group were non-cases at 3 months (61%) and 1 year (69%). These rates are broadly similar to the majority of studies evaluating MADD Reference Ormel, Oldehinkel, Brilman and Van den Brink25,Reference Pini, Perkonnig, Tansella, Wittchen and Psich26,Reference Simon, Ormel, von Korff and Barlow29 and somewhat higher than the WHO Collaborative study using a different instrument for case definition where only 49% recovered. Reference Barkow, Heun, Wittchen, Ustun, Gansicke and Maier8 Recovery rates for MADD are lower than for adjustment disorder, a disorder with similar symptoms but differences in the nature of stressors, where studies suggest most symptoms resolve rapidly. Reference Snyder, Strain and Wolf30,Reference Despland, Monod and Ferrero31 The findings for MADD also contrast with the literature on comorbid threshold disorders where comorbid depression and anxiety or ‘cothymia’ has a worse prognosis with more associated disability and more persistent symptoms than either depression or anxiety disorders alone. Reference Tyrer, Seivewright and Johnson32,Reference Beard, Heathcote, Brooks, Earnest and Kelly33
It appears that, for the majority of those with MADD this can be considered a self-limiting condition, with improvements expected for most in the short and longer term. However, one-third of those with MADD had significant psychological distress at follow-up and those with MADD were more than twice as likely to be significantly distressed at 3 months follow-up when compared with the no diagnosis group, although this lessened to non-significant levels by 1 year. There was no other study in the literature of reasonable quality that reported the relative risk of psychiatric caseness at follow-up in comparison with a no psychiatric diagnosis group for those with MADD, with which we could directly compare our figures. Past studies on MADD have reported simple proportions who have either recovered or who are ICD-/DSM-defined psychiatric cases at follow-up. Reference Barkow, Heun, Wittchen, Ustun, Gansicke and Maier8,Reference Ormel, Oldehinkel, Brilman and Van den Brink25,Reference Pini, Perkonnig, Tansella, Wittchen and Psich26,Reference Simon, Ormel, von Korff and Barlow29 A number of studies have reported the risk of major depression in those with either minor depression or subsyndromal depression. These found increased odds of around fivefold of developing major depression for those with minor depression defined by DSM–IV or ICD–10 Reference Forsell10,Reference Horwath, Johnson, Klerman and Weissman34 and fourfold for those with subsyndromal depressive symptoms. Reference Cuijpers and Smit9,Reference Forsell10 These studies were heterogeneous, using a variety of case definitions and outcome measures.
In this study, those with MADD had poorer mental health related quality of life than those with no psychiatric diagnosis, but a better quality of life than those with other ICD–10 diagnoses. This is consistent with previous published research. Reference Das-Munshi, Goldberg, Bebbington, Bhugra, Brugha and Dewey27,Reference Da Silva Lima and de Almeida Fleck28,Reference Kessler, Merikangas, Berglund, Eaton, Koretz and Walters35 There was no evidence from this study that those with MADD consumed more health resources than those with no psychiatric diagnosis, since they did not consult their GP more frequently and did not receive more diagnoses of mental health problems. These findings contrast with past work in other countries, which has found higher health services use in those with minor distress and subthreshold disorders in comparison with those with no psychiatric symptoms. Reference Kessler, Merikangas, Berglund, Eaton, Koretz and Walters35 In our initial survey of 1357 people attending their GP less than one-half of those with mild–moderate distress reported wanting help if they were ‘feeling stressed, worried or low and it was affecting their daily life’. Reference Walters, Buszewicz, Weich and King14
In our study no factors included in the models were identified as significant predictors of persistent distress within the MADD group at follow-up. There has been limited past research on this and no robust evidence for any one factor in predicting recovery. It is therefore currently difficult to predict which people with MADD have a worse or better prognosis, and further research is needed.
Implications
Our results suggest that for many people with MADD their symptoms will improve over time and around two-thirds will not be significantly distressed 3 months or 1 year later. However, those with MADD are more than twice as likely as those with no psychiatric diagnosis to have significant distress at 3 months, and also have persistently lower mental health related quality of life. This supports a recommendation of ‘active monitoring’ for those with MADD (discussion of problem/concerns, psychoeducation and active follow-up within 2 weeks), which is the current advice given to GPs for those with mild or subthreshold depressive symptoms. 36
Although those with MADD were at higher risk of psychological distress at follow-up, unlike those with other ICD–10 diagnoses, they did not present to their GP more frequently or have more diagnoses of mental health problems than the no diagnosis group. This situation could conceivably change if a policy of ‘active monitoring’ were followed in this group. We identified no strong predictors in our cohort to assist with identifying those at higher risk of persistent symptoms, but this was limited by our sample size. The MADD group appears heterogeneous, and it is currently unclear which subgroups are likely to have a poorer prognosis and reduced quality of life. Further research is needed to address this. Including MADD as a diagnostic category in DSM–5 could lead to the medicalisation of many people who have minor, self-limiting symptoms of distress.
To conclude, many of those with MADD have a self-limiting course, but they are at short-term increased risk of significant distress, have a persistently lower quality of life in terms of mental health functioning and around one-third have significant symptoms of psychological distress at follow-up. Despite this, they are no more likely to seek help from their GP or be diagnosed with mental health problems over the following year than those with no psychiatric diagnosis. We cannot currently predict which people in this heterogeneous group have a better or worse prognosis.
Funding
This study was supported by a Medical Research Council Special Training Fellowship in Health Services Research () for the main author (K.W.).
Acknowledgements
We thank Susannah Payne for her help with collecting the data, and all study practices and participants.








eLetters
No eLetters have been published for this article.