Many patients with bipolar disorder experience benefit from mood-stabilising treatments, but do not achieve full remission of affective symptoms. Low-grade depression, mood cycling and/or anxiety are common, present for a large proportion of time and are associated with a higher risk of relapse and ongoing disability. Reference Judd, Schettler, Akiskal, Coryell, Leon and Maser1 Another factor contributing to disability in bipolar disorder are cognitive changes that usually do not respond well to standard long-term treatments. Bipolar disorder is viewed by many as a progressive condition leading to impairment in cognitive functioning and to grey matter loss in certain brain regions. Reference Scott, Leboyer, Hickie, Berk, Kapczinski and Frank2 A number of studies have reported impaired neuropsychological performance in a number of domains. Reference Bourne, Aydemir, Balanza-Martinez, Bora, Brissos and Cavanagh3 Impairments have been demonstrated in global cognitive functioning, Reference Kessing4 visuospatial recognition memory, Reference Rubinsztein, Michael, Paykel and Sahakian5 verbal memory, Reference van Gorp, Altshuler, Theberge, Wilkins and Dixon6 general intelligence, reasoning, working memory and executive functions. Reference Tham, Engelbrektson, Mathe, Johnson, Olsson and Aberg-Wistedt7 Importantly, these deficits seem to persist into the euthymic state. Reference Bourne, Aydemir, Balanza-Martinez, Bora, Brissos and Cavanagh3 Notably, the past number of depressions was predictive of cognitive functioning in several studies. Reference Kessing4,Reference van Gorp, Altshuler, Theberge, Wilkins and Dixon6 There is therefore, a need for treatments that could better control residual and cognitive symptoms leading us to examine some of the older medications that could be re-purposed for these indications. Methylene blue was investigated in bipolar disorder more than 30 years ago and its effects have been described in case reports and case series Reference Naylor, Dick, Johnston, Hopwood, Dick and Smith8–Reference Naylor, Smith and Connelly11 as well as in a double-blind trial. Reference Naylor, Martin, Hopwood and Watson12 Their rationale was based on the assumption that high vanadium concentrations inhibited sodium-potassium adenosine triphosphatase and that methylene blue catalysed the conversion of vanadate to less active vanadyl. Reference Naylor, Dick, Johnston, Hopwood, Dick and Smith8,Reference Naylor, Smith and Trotter13 The first report included the cases of two individuals who were previously non-responsive to lithium and treated successfully with methylene blue for mood cycling. Reference Naylor, Dick, Johnston, Hopwood, Dick and Smith8 Next, the same group reported a case series of 24 patients treated in an open-label trial from 3 days to 19 months with 200 mg to 300 mg of methylene blue. Out of 19 patients in the sample who had bipolar disorder, 14 were judged improved. Reference Narsapur and Naylor9 In a double-blind crossover 2-year trial, patients on 300 mg of methylene blue had less severe symptoms of depression than on 15 mg. Reference Naylor, Martin, Hopwood and Watson12 Unfortunately, this important study was limited by a relatively small sample (17 patients completed the trial) and by simple symptom rating measures. Finally, Naylor et al found some support for benefits of short-term treatment with a low dose of methylene blue in patients with depressive disorder, Reference Naylor, Smith and Connelly14 and no positive effects on symptoms of mania. Reference Naylor, Smith and Connelly11
In addition to clinical studies in bipolar disorder, methylene blue has been recognised as potentially antidepressant and anxiolytic in animal models, Reference Eroglu and Caglayan15 possibly by increasing both serotonin and dopamine levels in the hippocampus through various mechanisms. Reference Wegener, Volke and Rosenberg16 In recent years there has been renewed interest in methylene blue and its possible neuroprotective effect in light of newly discovered mechanisms. These effects include non-specific inhibition of nitric oxide synthase and of guanylate cyclase. In addition, methylene blue has been reported to improve mitochondrial function, specifically increasing cytochrome oxidase (complex IV) activity, and to decrease oxidative damage. Reference Rojas, Bruchey and Gonzalez-Lima17 We hypothesised that methylene blue could be particularly effective in combination with lamotrigine. Lamotrigine is an anticonvulsant commonly used as a mood stabiliser. Reference Yatham, Kennedy, Parikh, Schaffer, Beaulieu and Alda18 It is assumed to act on several levels, including inhibition of glutamate release and inhibition of voltage dependent cation channels. Reference von Wegerer, Hesslinger, Berger and Walden19–Reference Wang, Sihra and Gean21 Furthermore, it may increase gamma-aminobutyric acid (GABA)-ergic transmission Reference Hassel, Tauboll and Gjerstad22 although higher doses may paradoxically inhibit GABA release. Reference Cunningham and Jones23 The inhibition of glutamate release in particular may be responsible for the observed neuroprotective effect of lamotrigine. Reference Crumrine, Bergstrand, Cooper, Faison and Cooper24 Based on the current knowledge of the mechanism of action of lamotrigine, its combination with methylene blue might be especially effective by reducing glutamatergic effect. This may be mediated by an inhibitory action at multiple steps of the signalling cascade: first, by inhibiting glutamate release and, second, by attenuating the production of nitric oxide. The role of nitric oxide signalling in cognition is complex. Nitric oxide may be important in long-term potentiation and synaptic plasticity, Reference Ingram, Spangler, Iijima, Kuo, Bresnahan and Greig25 but its effect may not be dependent on cyclic guanosine monophosphate, Reference Schuman, Meffert, Schulman and Madison26 and in fact, inhibition of nitric oxide synthase may enhance neurogenesis. Reference Park, Kang, Kwon, Chung, Ahn and Huh27 Based on the hypothesised mechanisms of action of methylene blue and on clinical case observations of several patients successfully treated in an open-label manner, we decided to study the drug in a randomised double-blind trial with the objective of investigating the efficacy of methylene blue as an add-on treatment of residual symptoms of bipolar disorder in participants treated with lamotrigine.
Method
Study design
This was a double-blind crossover study (Fig. 1). Patients with bipolar disorder received subtherapeutic (15 mg) and therapeutic (195 mg) doses of methylene blue, each given for 3 months in a random order. We chose this design because methylene blue discolours urine, and thus we could not use a traditional placebo. The study was conducted in two centres (The Mood Disorders Program at Dalhousie University, Halifax, Nova Scotia and The Mood Disorders Program, McMaster University, Hamilton, Ontario). The enrolment for the study took place from 2004 to 2008. The study protocol was approved by Health Canada and by the Research Ethics Boards at Capital District Health Authority in Halifax and St Joseph's Healthcare Centre in Hamilton. All participants signed written informed consent prior to their participation. The trial has been registered at ClinicalTrials.gov (registration number NCT00214877).
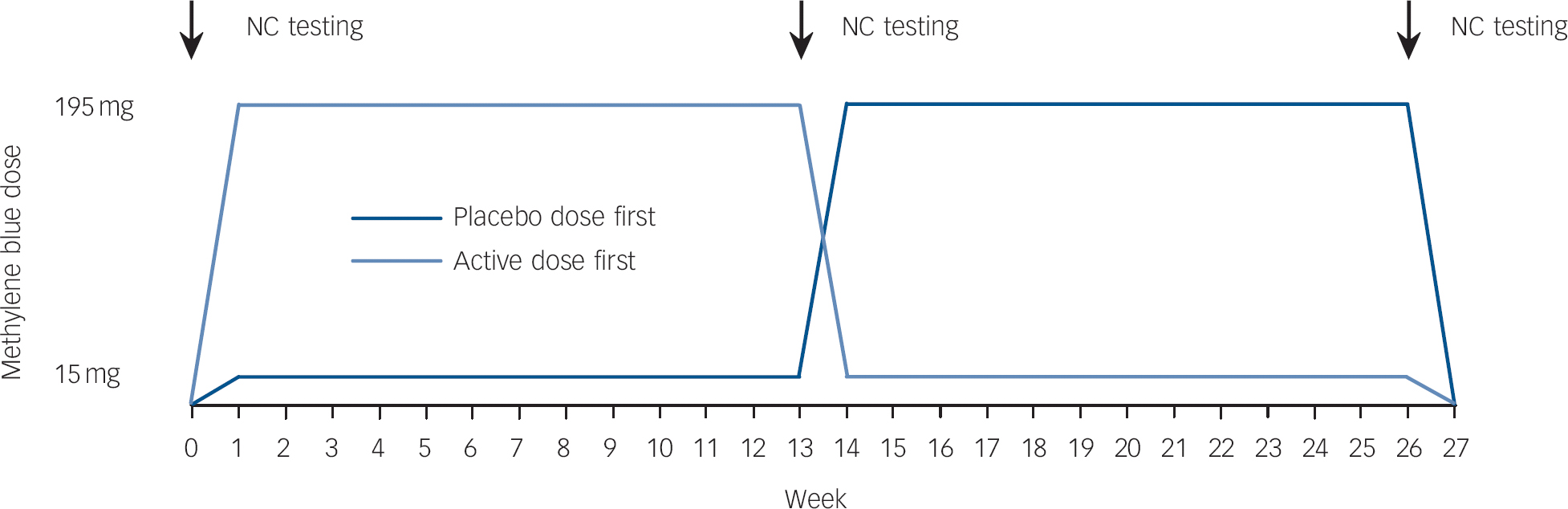
Fig. 1 Study design.
NC, neurocognitive.
Participants
Patients with bipolar disorder partially stabilised on lamotrigine were eligible to take part in the study if they met the following criteria.
Inclusion criteria
Men or women between the ages of 18 and 65 who meet both Research Diagnostic Criteria (RDC) Reference Spitzer, Endicott and Robins28 and DSM-IV 29 criteria for bipolar I or bipolar II disorder were recruited. All participants were interviewed using the Schedule for Affective Disorders and Schizophrenia (SADS-L) interview Reference Endicott and Spitzer30 with added questions so as to arrive at DSM-IV diagnoses as well. All patients had to be treated with lamotrigine as their main mood stabiliser. Patients recruited for the study were expected to show partial response to long-term treatment with respect to their mood symptoms. Hamilton Rating Scale For Depression (HRSD, 17 item) Reference Hamilton31 scores at study entry had to be 15 or lower and Young Mania Rating Scale (YMRS) Reference Young, Biggs, Ziegler and Meyer32 scores less than 15. The protocol did not specify the minimum scores, but in the opinion of the investigators the patients had shown incomplete response and required additional treatment.
Exclusion criteria
Patients meeting any of the following criteria were excluded from the trial: (a) unable to provide informed consent; (b) active substance misuse or dependence or a history of such within the past 2 years; (c) significant unstable physical illness, especially liver and kidney disorders and glucose-6-phosphate dehydrogenase (G-6-PD) deficiency; (d) previous treatment with methylene blue; (e) pregnant or breastfeeding women; (f) electroconvulsive therapy within the past 2 years; (g) known history of brain injury or loss of consciousness with a duration greater than 10 min; (h) use of concurrent medications known to have cognitive effects, for example beta-blockers.
Drug formulation and dosage schedule
Low and high doses of methylene blue were administered in identical-looking capsules containing either 5 mg or 65 mg of methylene blue. Both active drug and placebo were supplied by Star Pharmaceuticals (http://www.starpharm.com/). The study medication was dispensed by hospital pharmacies at each site and given as one capsule three times daily. The titration took place over a period of 1 week, followed by 12 weeks of treatment, 1 week of crossover and another 12 weeks of treatment. The order of treatments (active–subtherapeutic, subtherapeutic–active, each with 50% probability) was chosen at random. The randomisation was done in blocks of six participants, separately for each centre; the medication codes were kept at respective hospital pharmacies and were unavailable to the investigators and patients.
Study procedures
All patients were seen at baseline and then in 2-week intervals with additional visits at weeks 13 and 26; at each visit their symptoms were rated and side-effects assessed. The neurocognitive testing was done at baseline and at weeks 13 and 26.
Every effort was made to perform the cognitive testing and symptom ratings in patients who terminated the treatment trial prematurely. We did not specify any rescue medication as this was an add-on study. Furthermore, the target symptoms included residual symptoms and cognitive dysfunction, for which treatment options are currently limited. Patients continued receiving all their regular mood-stabilising drugs. In the event of worsening of symptoms, patients were treated using current best practice, and withdrawn from the study. There was no washout period at the beginning of the trial. The switch (crossover) from low to high (or high to low) dose of methylene blue was completed over a 1-week period.
Patients were enrolled in the trial if they were on a stabilised dose of lamotrigine for a minimum of 3 months. Participants treated with additional medications were not excluded as long as their doses remained stable over the course of the trial. Medications, such as beta-blockers or anticholinergic drugs known to cause memory difficulties were not allowed during the study.
Use of other additional medications was assessed on a case-by-case basis.
Outcome measures
Primary outcome measures – clinical status
For assessment of specific mood changes we used the following rating scales: the 17-item HRSD and Montgomery–Åsberg Depression Rating Scale (MADRS) Reference Montgomery and Åsberg33 for symptoms of depression and YMRS for assessment of symptoms of mania. The Hamilton Rating Scale for Anxiety (HRSA) Reference Hamilton34 was used to evaluate changes in severity of anxiety symptoms. For global ratings of psychiatric morbidity we used the Clinical Global Impression Scale modified for use in bipolar disorder (CGI-BP) Reference Spearing, Post, Leverich, Brandt and Nolen35 and the Affective Morbidity Index (AMI). Reference Coppen, Montgomery, Gupta and Bailey36
Secondary outcome measures – cognitive tests
We completed a targeted cognitive assessment based on documented abnormalities in adults with bipolar disorder. Two tasks examined recollection memory, the California Verbal Learning Task (CVLT) Reference Delis, Kramer, Kaplan and Ober37 and a process-dissociation task. Reference Jacoby38 The CVLT is a measure of episodic verbal learning and memory, retroactive and proactive interference and strategy employed to remember information that has been widely used in adults with mood disorders. Reference van Gorp, Altshuler, Theberge and Mintz39,Reference Martinez-Aran, Vieta, Colom, Reinares, Benabarre and Torrent40 The process-dissociation task is a sensitive, computerised task that has been validated as a method of examining recollection memory (hippocampus-dependent) processes independent of habit memory (hippocampus-independent) within a single paradigm. Three scores are generated reflecting recollective memory performance, habit memory and the propensity to guess at correct responses. We have extensive data demonstrating that patients with mood disorders Reference MacQueen, Galway, Hay, Young and Joffe41 are impaired on the recollection component of this task.
The Trails B task Reference Reitan42 was administered as a test of executive function, chosen because, in previous studies of patients with bipolar disorder, abnormalities on this measure have been reliably demonstrated, Reference Martinez-Aran, Vieta, Colom, Reinares, Benabarre and Torrent40 and may reflect underlying changes in frontal regions. It is also a well-studied task that is simple, reliable and easy to administer. Two tasks focused on selective attention, negative priming Reference Tipper43 and inhibition of return Reference Posner, Cohen, Bouma and Bouwhuis44 tasks. The negative priming paradigm and inhibition of return task examine inhibitory components of selective attention, examining both spatial- and feature-based stimulus properties. Finally, a visual backward masking task that has been well-studied in patients with bipolar disorder was also administered; the mechanism and underlying circuit deficits have been described in adult populations with bipolar disorder. Reference MacQueen, Grof, Alda, Marriott, Young and Duffy45 The cognitive measures were administered before treatment, before crossover and at the end of the trial.
Safety analysis
The patients were monitored regularly and any adverse events were documented at each visit in a standard clinical trial format.
Statistical analysis
The analyses were all specified prior to commencing the data collection. The clinical outcome and cognitive data were analysed by analysis of variance with two within-participant factors: (a) evaluation period (beginning v. end of each treatment), and (b) methylene blue dose (active and placebo dose), and one between-participant factor (treatment order). Thus, the null hypothesis is that there is no interaction between methylene blue dose and evaluation period at the point of medication crossover (week 13). The baseline value for the second treatment period was the last rating (week 13) of the first treatment period (i.e. this value was duplicated in the ANOVA model). Post hoc comparisons were done by t-tests and non-parametric tests as deemed appropriate. The analyses of all data were repeated twice – first for completers only and second, based on intent to treat using the last-observation-carried-forward (LOCF) method. Given the subtle nature of cognitive impairments in bipolar disorder, we chose not to correct the results of the cognitive tests for multiple comparisons in order to best illuminate what are often small differences in performance in this population. The planned sample size of 40 patients had 87% power to detect an effect size of 0.5 or larger.
Results
Characteristics
In total, 47 patients at the two centres met the inclusion criteria; of these, 37 agreed to participate in the study (Fig. 2). There were 11 men and 26 women, who ranged in age from 22 to 64 years. Further clinical characteristics of the participants are summarised in Table 1. Mood state at the beginning of the trial was characterised as mildly or moderately depressed in 34 participants and as cycling in 3 patients. The initial HRSD and MADRS scores at baseline were 7.6 (s.d. = 4.6) and 12.7 (s.d. = 7.8), respectively. Concomitant medication included lamotrigine in all participants, lithium (n = 17), sodium divalproex (n = 4), gabapentin (n = 3), antipsychotics (n = 20, in 19 participants atypical) and antidepressants (n = 20).
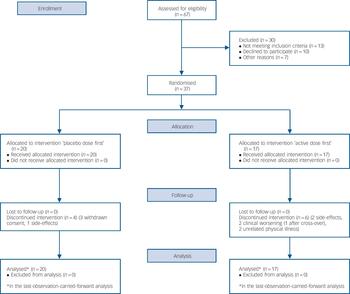
Fig. 2 Flow diagram.
Table 1 Participants
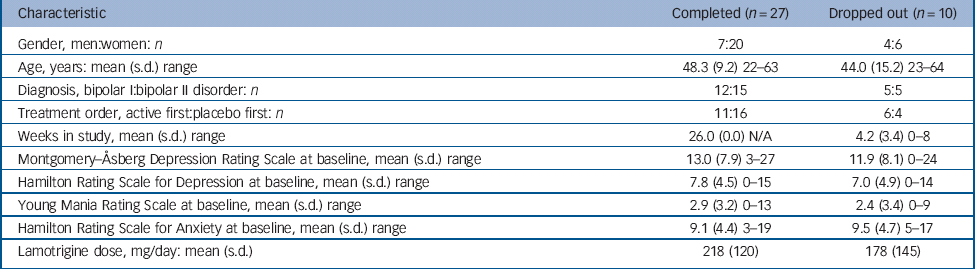
| Characteristic | Completed (n = 27) | Dropped out (n = 10) |
|---|---|---|
| Gender, men:women: n | 7:20 | 4:6 |
| Age, years: mean (s.d.) range | 48.3 (9.2) 22–63 | 44.0 (15.2) 23–64 |
| Diagnosis, bipolar I:bipolar II disorder: n | 12:15 | 5:5 |
| Treatment order, active first:placebo first: n | 11:16 | 6:4 |
| Weeks in study, mean (s.d.) range | 26.0 (0.0) N/A | 4.2 (3.4) 0–8 |
| Montgomery–Åsberg Depression Rating Scale at baseline, mean (s.d.) range | 13.0 (7.9) 3–27 | 11.9 (8.1) 0–24 |
| Hamilton Rating Scale for Depression at baseline, mean (s.d.) range | 7.8 (4.5) 0–15 | 7.0 (4.9) 0–14 |
| Young Mania Rating Scale at baseline, mean (s.d.) range | 2.9 (3.2) 0–13 | 2.4 (3.4) 0–9 |
| Hamilton Rating Scale for Anxiety at baseline, mean (s.d.) range | 9.1 (4.4) 3–19 | 9.5 (4.7) 5–17 |
| Lamotrigine dose, mg/day: mean (s.d.) | 218 (120) | 178 (145) |
Clinical effects
Depression
On both the HRSD and MADRS, we found a significant effect of treatment. The group starting on an active dose had lower depression ratings at midpoint in comparison with the placebo-first group. These differences were statistically significant among study completers showing as an interaction between medication (active dose v. placebo dose) and assessment point (start v. end of each of the two treatments). The effect size (ES) for the HRSD was 0.71 (95% CI 0.15–1.25), F = 5.01, d.f. = 1,25, P = 0.034) and for the MADRS it was 0.80 (95% CI 0.27–1.30), F = 6.16, d.f. = 1,25, P = 0.020, Fig. 3. When we repeated these analyses for all participants who received at least one dose of study medication (LOCF analysis), the results remained similar (HRSD: F = 4.15, d.f. = 1,35, P = 0.049, ES = 0.56, 95% CI 0.09–1.02; MADRS: F = 5.99, d.f. = 1,35, P = 0.020, ES = 0.68, 95% CI 0.20–1.14). None of the analyses showed effects of treatment order, medication or assessment point alone.

Fig. 3 Symptom ratings on (a) Hamilton Rating Scale for Depression (HRSD), (b) Montgomery–Åsberg Depression Rating Scale (MADRS), (c) Hamilton Rating Scale for Anxiety (HRSA) and (c) Young Mania Rating Scale (YMRS).
The graph shows all ratings to better visualise the time course of the symptoms. The shaded symbols at weeks 0, 13 and 26 indicate values on which the analysis of variance was based.
Mania
Neither the active dose nor the placebo dose of methylene blue had any effect on mania both in study completers and in the LOCF analysis, with both simple effect of dose, treatment order and point of evaluation as well as their interactions showing no significance (Fig. 3). All patients had very low scores on the YMRS throughout the trial.
Anxiety
Symptoms of anxiety were evaluated using the HRSA. The active dose of methylene blue was superior to the placebo dose as indicated by the significant interaction between medication and the assessment point (for study completers: F = 5.48, d.f. = 1,25, P = 0.028, ES = 0.79 (95% CI 0.22–1.33; and for LOCF: F = 6.55, d.f. = 1,35, P = 0.015, ES = 0.72 (95% CI 0.25–1.19)). At the same time, in both analyses, we noticed a significant simple effect of the point of assessment, indicating an overall decrease of anxiety ratings in the course of the trial (completers: F = 9.87, d.f. = 1,25, P = 0.0043; LOCF: F = 10.02, d.f. = 1,35, P = 0.0032), Fig. 3. Inspection of the data and post hoc analysis indicate that this effect appears to be primarily as a result of the lasting improvement of anxiety in the active-dose-first group after switching to a placebo dose.
The changes in anxiety were noticeable both on the psychic and somatic dimensions of the HRSA, except for a non-significant effect on somatic anxiety in study completers (psychic anxiety – completers: F = 7.78, d.f. = 1,25, P = 0.010; LOCF: F = 6.47, d.f. = 1,35, P = 0.016; somatic anxiety – completers: F = 2.56, d.f. = 1,25, P = 0.123; LOCF: F = 4.56, d.f. = 1,35, P = 0.040).
CGI-BP and AMI scores
The CGI-BP includes a total of nine measures (severity of mania, depression and overall bipolar disorder and for each of these it also measures their changes since the previous assessment and from the worst phase of illness). The analyses of the individual items confirmed the positive effects of methylene blue on depression severity, with no effect on mania, and non-significant trends for improvement of severity of bipolar disorder as a whole (online Table DS1). The effects of methylene blue on the AMI were not significant, most likely because of the overall low absolute AMI values.
Cognitive measures
There were practically no differences between the low and standard doses of methylene blue on any of the cognitive measures (CVLT, trails B, process-dissociation task, location negative priming, inhibition of return, and visual backward masking tasks). The results are summarised in online Table DS2.
Subjective effects of treatment, drug tolerability and side-effects
Most patients felt subjectively improved over the course of the study and six participants decided to continue their treatment after completing the trial. A total of 27 patients out of 37 (73%) completed the 6-month long trial. The reasons for dropping out were worsening of bipolar disorder (n = 1), blue urine colour (n = 1), increase in anxiety (n = 1), fluid retention (n = 1), treatment of pre-existing physical condition precluding continuation of the trial in two cases; four participants withdrew their consent.
Methylene blue was generally well tolerated with no severe side-effects. The proportion of participants who reported any side-effects in the placebo-first group changed from 13% at the trial onset to 20% at crossover and to 0% at the end of treatment with the active dose. In the active-dose-first group the proportion of those with side-effects changed from 57% at the beginning of the trial to 29% at crossover and to 0% at the termination of the study. The most commonly reported side-effects were mild and transient. They included urinary tract burning, diarrhoea and nausea, and headaches; these side-effects are similar to those reported by Naylor et al. Reference Naylor, Martin, Hopwood and Watson12 We did not observe any changes in the routine laboratory tests performed at baseline, crossover and termination visits. Vital signs (blood pressure, heart rate measured at each visit) remained stable throughout the trial.
Discussion
Main findings
Our results indicate that methylene blue used as an adjunctive medication significantly improved residual symptoms of depression in patients with bipolar disorder. We did not observe any changes in the level of manic symptoms, but there was a significant improvement in symptoms of anxiety. These findings are consistent with previous reports Reference Naylor, Martin, Hopwood and Watson12 and confirm that methylene blue could be considered a safe and effective add-on treatment for patients with persisting and residual symptoms.
Limitations
In contrast to the effects of methylene blue on mood, the cognitive effects were all non-significant. However, there was also no evidence of any negative cognitive effects of methylene blue. It is notable that cognitive performance at baseline was not included as one of the selection criteria of this study. It is therefore possible that our patients had relatively preserved cognitive functioning with little room for further improvement. This is one limitation of this study. Furthermore, the cognitive functioning could be influenced by severity of mood symptoms, which was relatively low in this study. We also have to consider the possibility that even the placebo dose (in the order of 0.15 to 0.3 mg/kg) may have had some beneficial effect on residual or cognitive symptoms, however unlikely. The study by Naylor et al Reference Naylor, Smith and Connelly14 would suggest that this remains a possibility, although the same group found a significantly better effect of a higher dose in their long-term trial. Reference Naylor, Martin, Hopwood and Watson12 Another limitation is the requirement of lamotrigine as the main mood stabiliser. Although this made the patient group more homogeneous, we cannot necessarily generalise our findings to patients treated with other mood stabilisers. As this was an add-on study and all participants continued their regular medication, we did not require any washout period.
Safety issues
Some authors have raised concerns about the risk of serotonin syndrome in individuals treated with methylene blue, Reference Gillman46 possibly as a result of its inhibitory effect on monoamino-oxidase. In 2011 the US Food and Drug Administration (FDA) issued a safety alert and warned against the use of methylene blue in patients treated with serotonergic agents 47 updated several months later suggesting that the risk was possibly higher only for some antidepressants and in conjunction with intravenous methylene blue administration. 48 In our study, none of the patients showed any signs of serotonergic toxicity. Methylene blue was well tolerated with only transient and mild side-effects present both during placebo-dose and active-dose phases of treatment.
In summary, the results from this study provide further support for the use of methylene blue in the treatment of bipolar disorder. Given its intriguing mechanism of action, this drug deserves further attention in both preclinical and clinical applications.
Funding
The study was funded by a grant from the Stanley Foundation (now Stanley Medical Research Institute).
Acknowledgements
We wish to thank Star Pharmaceuticals for supplying both active medication and the placebo.
Our sincere thanks are to Deborah Snow, CCRP, pharmacy technician at Capital District Health Authority who prepared, masked and dispensed the medication. Gilbert Matte, PhD, helped greatly with preparation of the study protocol submission to Health Canada.







eLetters
No eLetters have been published for this article.