Late-life depression is one of the most common psychiatric disorders in older adults. Reference Byers, Yaffe, Covinsky, Friedman and Bruce1 It is associated with significant functional impairment, variable treatment response, chronicity, high recurrence rates, and high rates of medical comorbidity and mortality. Older adults with late-life depression, including those with early-onset depression and late-onset depression, exhibit significant cognitive impairment that may not fully recover after successful antidepressant treatment and there is a relationship between history of depression and increased risk of dementia. Reference Butters, Becker, Nebes, Zmuda, Mulsant and Pollock2,Reference Butters, Young, Lopez, Aizenstein, Mulsant and Reynolds3 A previous meta-analysis of case-control and cohort studies found that depression was associated with an increased risk for incident Alzheimer's disease (pooled odds ratio (OR) = 1.90), but it did not include studies of other dementia syndromes such as vascular dementia or mixed dementias as possible outcomes. Reference Ownby, Crocco, Acevedo, John and Loewenstein4 However, recent studies have reported a significant association between these conditions. Reference Lenoir, Dufouil, Auriacombe, Lacombe, Dartigues and Ritchie5
To date there has been no systematic evaluation of whether a depressive episode increases the risk of vascular dementia and other dementia syndromes in addition to Alzheimer's disease. The understanding of whether late-life depression increases the risk of dementia syndromes in addition to Alzheimer's disease could lead to better long-term predictive models and more rational preventive interventions. This is of utmost importance given that the estimated number of cases of Alzheimer's disease attributable to depression is 781 000 (95% CI 506 000-1 078 000) and the prevention of 10 to 25% of depression cases may reduce the prevalence of Alzheimer's disease by 68 000 to 173 000 respectively. Reference Barnes and Yaffe6 Therefore, our aim was to carry out a systematic review and meta-analysis of population-based, prospective cohort studies to evaluate the pooled risk of incident all-cause dementia, and more specifically of Alzheimer's disease and vascular dementia in individuals experiencing a late-life depression episode. We hypothesised that depression increases the risk of all-cause dementia, including both Alzheimer's disease and vascular dementia in older adults. Secondarily, we aimed to compare the magnitudes of the relative risk posed by late-life depression separately for Alzheimer's disease and vascular dementia.
Method
This meta-analysis followed the PRISMA guidelines for conducting and reporting systematic reviews.
Search strategy
We conducted a comprehensive search for potentially relevant studies of late-life depression and dementia risk in the electronic bibliographic databases PubMed and Scopus, which provide broad coverage of biomedical journals published worldwide. There were no time or language limits for the searches. We also searched references of selected publications, in particular of previous systematic reviews and meta-analyses, for additional potentially relevant studies. The literature search was conducted in February 2012. Searches included the following terms: incidence* (as a Medical Subject Heading (MeSH) term) OR cohort studies (MeSH term). Results of these searches were combined with sets created with depression OR depressive; Alzheimer disease; Vascular Dementia; dementia OR dementing. This search retrieved a total of 1839 references (Fig. 1).
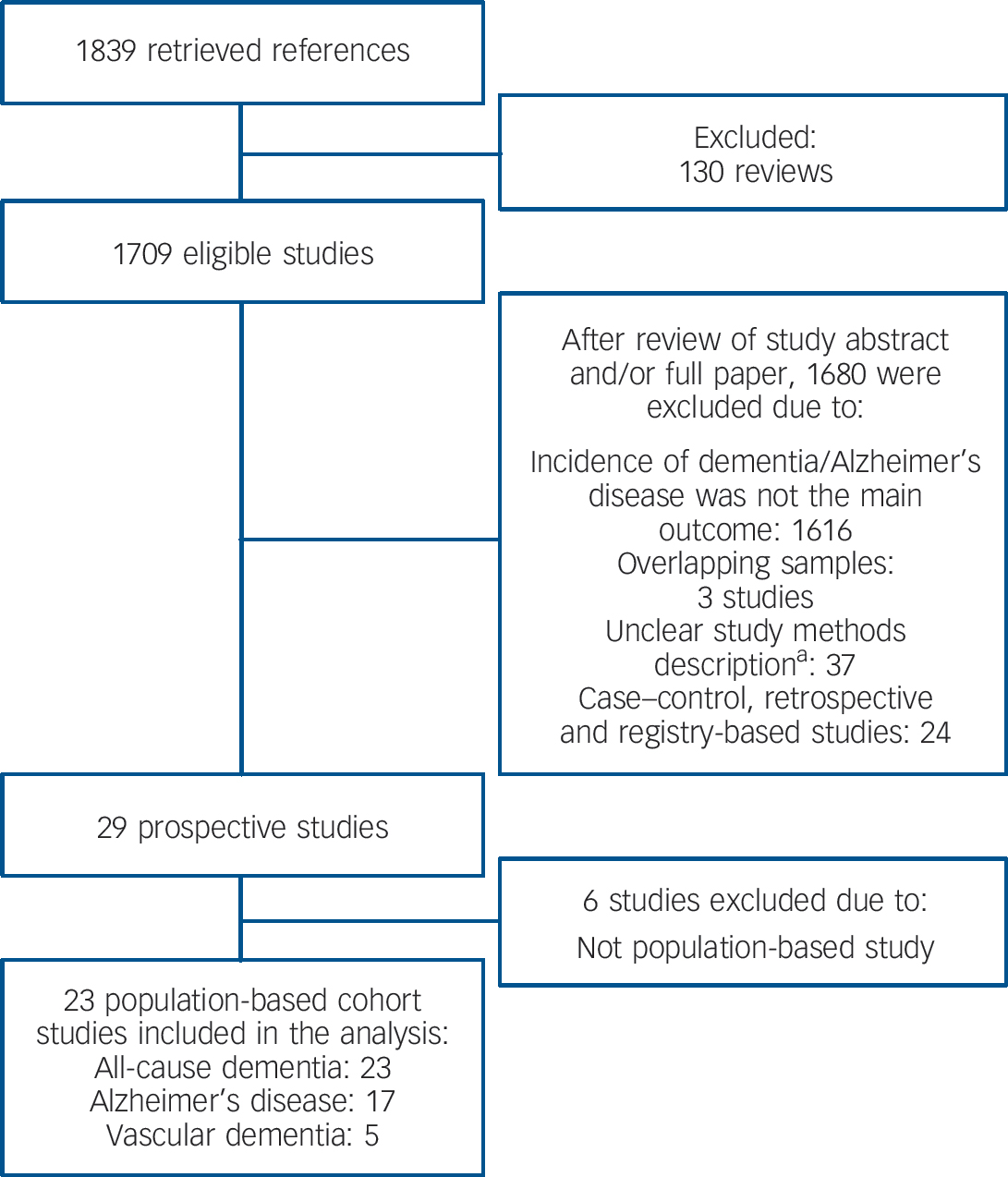
Fig. 1 Flow chart of the study search and selection for inclusion in the meta-analysis.
a. No description of sample setting, of baseline diagnosis of depression or the outcome diagnosis of dementia/Alzheimer's disease, risk measure not calculated or not reported.
Study selection and quality assessment
After reviewing the references, we selected the studies for data extraction and analysis based on the following criteria: (a) community-based prospective cohort studies; (b) identification of baseline depression ‘caseness’ (based on predefined cut-off on depression assessment scales); (c) age over 50 years at baseline assessment for participants with late-life depression and controls; (d) absence of dementia in the baseline assessment; (e) information on incidence of dementia (all cause) and/or Alzheimer's disease and/or vascular dementia; and (f) risk measure information (odds ratio or hazard ratio (HR)) and its 95% CI for the risk of dementia (all cause) and/or Alzheimer's disease and/or vascular dementia in participants with late-life depression as compared with participants without depression. As most of the studies selected in the systematic review and meta-analysis did not clearly differentiate between those with early-onset and late-onset depression, we broadly defined late-life depression to include individuals in either clinical classification.
We used the Newcastle-Ottawa Scale (NOS) Reference Wells, Shea, O'Connell, Peterson, Welch and Losos7 to assess the method quality of each study selected for inclusion in the meta-analysis. This measure assesses methodological aspects of observational studies such as selection of cases and controls, comparability of population ascertainment of exposure to risk, quality of case ascertainment and outcome assessment (online Table DS1).
Data extraction and statistical analysis
For each study included in the meta-analysis, we extracted the reported risk measure and 95% confidence interval for all-cause dementia, and, when available, for Alzheimer's disease and/or vascular dementia. Then, we log-transformed the risk measure and calculated the standard error from the 95% confidence interval for each study. We calculated separate pooled hazard ratios and odds ratios for the risk of all-cause dementia, Alzheimer's disease and vascular dementia. Then, we grouped all studies to calculate a pooled risk effect for these categories. We used the generic inverse variance method with a random-effects model for all analyses. Random-effects models are more appropriate than fixed-effect models for dealing with studies characterised by heterogeneous methodological approaches, such as those included in this meta-analysis. We assessed heterogeneity in the meta-analysis by means of the Q-test and I 2 index. If the P-value was below 0.05 in the Q-test and/or the I 2 index was higher than 50%, the pooled analysis was considered significantly heterogeneous.
We performed sensitivity analyses by excluding one study at a time and recalculating the risk effect, in order to evaluate whether the summary risk effect was biased by the effect of any individual study. Publication bias was ascertained by visual inspection of a funnel plot. All analyses were carried with the statistical software RevMan 5.1 on Windows 7 (The Nordic Cochrane Centre, Copenhagen, Denmark, http://ims.cochrane.org/revman/download). The authors B.S.D. and M.A.B. were involved in the study selection, data extraction, quality assessment and statistical analysis processes. Regular meetings between authors were held to minimise the risk of errors for each of the review processes. If there were inconsistencies between the authors (M.A.B. and B.S.D.) regarding any of these processes, the other authors were asked to provide a final decision.
Results
A total of 23 community-based prospective cohort studies met criteria for inclusion in the meta-analysis (Fig. 1). Two studies reported independent risk measures for two or more distinct stratifications (i.e. gender and country) Reference Dal Forno, Palermo, Donohue, Karagiozis, Zonderman and Kawas8,Reference Chen, Hu, Wei, Qin, McCracken and Copeland9 and data were extracted independently for each stratification. When a study reported data on more than one follow-up period, the longest follow-up point was chosen for data extraction. The median follow-up interval for all-cause dementia studies was 5 years; 5 years for Alzheimer's disease studies and 6.1 years for vascular dementia studies. Online Table DS2 shows the characteristics of all included studies.
The total sample included in the pooled analysis for all-cause dementia was 49 612 participants (5116 with late-life depression and 44 496 non-depressed controls). Analyses for Alzheimer's disease were based on data from 28 746 participants (3437 with late-life depression and 25 309 non-depressed controls); and for vascular dementia, 14 901 participants (1801 with late-life depression and 13 100 non-depressed controls).
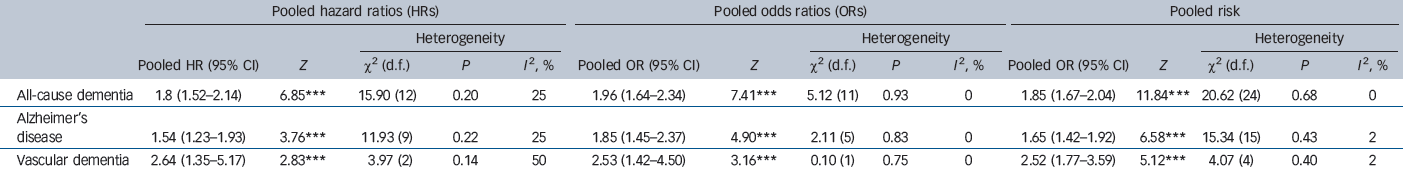
Table 1 and Figs 2, 3, 4 show the pooled hazard ratios, pooled odds ratios and pooled risk effect for all-cause dementia, Alzheimer's disease and vascular dementia. We found that individuals with late-life depression had a significantly higher risk for incident all-cause dementia, Alzheimer's disease and vascular dementia compared with the elderly controls. Results for the heterogeneity analysis are also shown in Table 1. We found low heterogeneity for vascular dementia and all-cause dementia, but moderate heterogeneity for Alzheimer's disease studies. A comparison of the pooled risk measure for Alzheimer's disease and vascular dementia showed that the risk of incident vascular dementia was significantly higher than Alzheimer's disease in individuals with late-life depression (χ2 = 4.65, d.f. = 1, P = 0.03) (online Fig. DS1).
Sensitivity analysis revealed no significant influence of any individual studies in the pooled hazard ratios, pooled odds ratios and pooled risk measure for all-cause dementia, Alzheimer's disease or vascular dementia. Visual inspection of the funnel plot did not reveal significant publication bias (online Fig. DS2). The quality assessment of the studies included in the meta-analysis was moderate to high and would not have biased the present results (online Table DS1).
Table 1 Meta-analysis results of risk measures for all-cause dementia, Alzheimer's disease and vascular dementia
| Pooled hazard ratios (HRs) | Pooled odds ratios (ORs) | Pooled risk | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Heterogeneity | Heterogeneity | Heterogeneity | |||||||||||||
| Pooled HR (95% CI) | Z | χ2 (d.f.) | P | I 2, % | Pooled OR (95% CI) | Z | χ2 (d.f.) | P | I 2, % | Pooled OR (95% CI) | Z | χ2 (d.f.) | P | I 2, % | |
| All-cause dementia | 1.8 (1.52–2.14) | 6.85Footnote *** | 15.90 (12) | 0.20 | 25 | 1.96 (1.64–2.34) | 7.41Footnote *** | 5.12 (11) | 0.93 | 0 | 1.85 (1.67–2.04) | 11.84Footnote *** | 20.62 (24) | 0.68 | 0 |
| Alzheimer's disease | 1.54 (1.23–1.93) | 3.76Footnote *** | 11.93 (9) | 0.22 | 25 | 1.85 (1.45–2.37) | 4.90Footnote *** | 2.11 (5) | 0.83 | 0 | 1.65 (1.42–1.92) | 6.58Footnote *** | 15.34 (15) | 0.43 | 2 |
| Vascular dementia | 2.64 (1.35–5.17) | 2.83Footnote *** | 3.97 (2) | 0.14 | 50 | 2.53 (1.42–4.50) | 3.16Footnote *** | 0.10 (1) | 0.75 | 0 | 2.52 (1.77–3.59) | 5.12Footnote *** | 4.07 (4) | 0.40 | 2 |
*** P<0.0001.
Because depressive symptoms were assessed with different depression scales (most commonly the Center for Epidemiologic Studies Depression Scale (CES-D) and its variants), we conducted an additional subgroup analysis to evaluate whether the observed effects were related to the different scales used in the studies (CES-D v. other scales). We observed no significant difference in the risk of incident dementia as a function of the assessment of depressive symptoms (dementia, CES-D: 1.81 (95% CI 1.60-2.04) v. other scales: 1.84 (95% CI 1.39-2.42); P = 0.9).
The relationship between depression and risk of dementia can be confounded by several factors, such as sociodemographics (e.g. age, education, gender), cognitive and functional performance, and biological status (e.g. health status, vascular risk factors, ApoE genotype, hippocampal volume, white matter lesions). To address these potential confounders on the risk of incident all-cause dementia, Alzheimer's disease and vascular dementia, we carried out a subgroup analysis including only studies that reported risk measures adjusted for confounders (online Table DS3). The strength of the association between late-life depression and the risk of all-cause dementia, Alzheimer's disease and vascular dementia was reduced, but was still significant: all-cause dementia (17 studies), pooled risk, 1.59 (95% CI 1.41-1.80), Z = 7.51, d.f. = 16, P<0.001; Alzheimer's disease (8 studies), pooled risk, 1.55 (95% CI 1.29-1.87), Z = 4.65, d.f. = 7, P<0.001; vascular dementia (3 studies), pooled risk 2.02 (95% CI 1.27-3.21), d.f. = 2, P = 0.003.
Discussion
Main findings
To the best of our knowledge, this is the first meta-analysis of population-based prospective cohort studies that specifically addressed the risk of all-cause dementia, Alzheimer's disease and vascular dementia in older adults with late-life depression. We found that late-life depression increases the risk of incident all-cause dementia and, more specifically, of both vascular dementia and Alzheimer's disease. After including only those studies that reported risk measures adjusted for multiple confounders, the strength of the association between late-life depression and the risk of all-cause dementia, Alzheimer's disease and vascular dementia was reduced, but still statistically significant. Additionally, we demonstrated, for the first time, that the risk of vascular dementia is significantly higher than the risk of Alzheimer's disease in older adults with late-life depression in population-based prospective cohort studies.
Findings from other studies
Our results are in line with a recent report that showed an increased risk of all-cause dementia, vascular dementia and Alzheimer's disease in participants with mid- and late-life depression in a retrospective analysis of 13 535 elder participants followed on the Kaiser Permanente Medical Care Program of Northern California. Reference Barnes, Yaffe, Byers, McCormick, Schaefer and Whitmer30
We found a less strong association between late-life depression and Alzheimer's disease compared with Ownby and colleagues Reference Ownby, Crocco, Acevedo, John and Loewenstein4 for cohort prospective studies (Ownby study: OR = 1.90, 95% CI 1.55-2.33 v. present study: OR = 1.65, 95% CI 1.42-1.92), although this difference is not statistically significant (χ2 = 1.81, d.f. = 1, P = 0.17). Methodological differences between the two studies may help to explain such differences. First, the present meta-analysis focuses on the risk of dementia among individuals with late-life depression in contrast to the Ownby meta-analysis, which included studies of mid- and late-life depression. We included a larger number of population-based prospective cohort studies (23 v. 8 studies), yielding a homogeneous pool of studies and a much larger sample of older adults. In addition, the combination of retrospective, case-control and prospective studies and the inclusion of mid- and late-life depression cases in Ownby's study led to high heterogeneity in the analysis, possibly inflating the association between depression and Alzheimer's disease. Finally, we evaluated the risk of all-cause dementia and, more specifically, of vascular dementia in addition to and separately from Alzheimer's disease. Thus, the current results go beyond and extend the findings from this previous meta-analysis, being specific to older adults and permitting broader generalisation to the population of older adults with major depression.
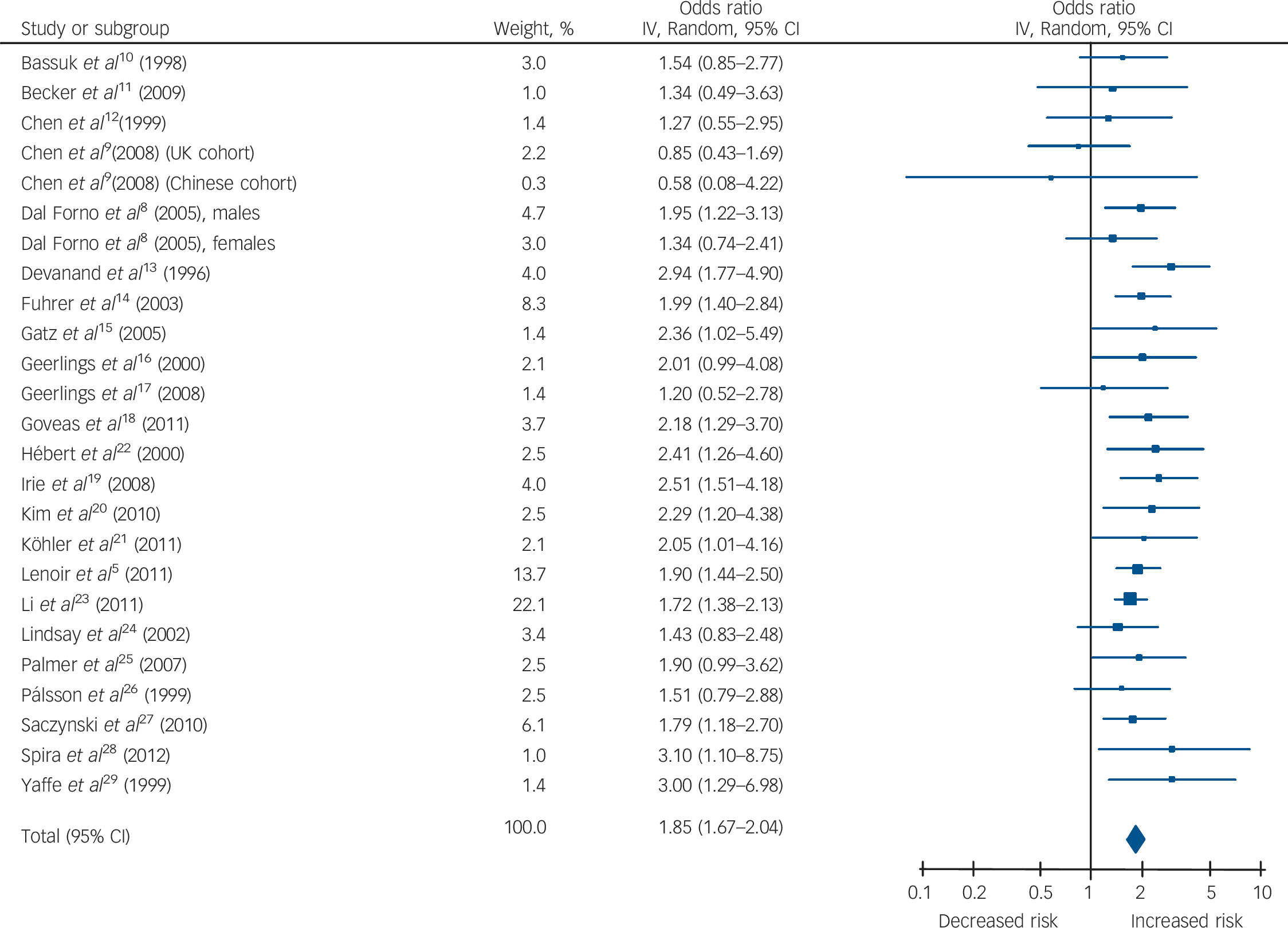
Fig. 2 Forest plot for the risk of all-cause dementia in participants with late-life depression.
Vascular dementia
This is the first meta-analysis to show that late-life depression increases the risk of vascular dementia and that the risk of vascular dementia is greater than the risk of Alzheimer's disease in these individuals. In general, late-life depression is associated with poorer general health and, in particular, has a strong association with higher burden of cardiovascular and cerebrovascular disease. Reference Charlson and Peterson31,Reference Sheline, Pieper, Barch, Welsh-Bohmer, McKinstry and MacFall32 Thus, our finding of an increased risk of vascular dementia in individuals with late-life depression provides additional evidence of a negative interaction between vascular pathology and late-life depression. Moreover, vascular disease co-occurs with Alzheimer's disease at a very high rate and may moderate when clinical Alzheimer's disease symptoms appear, raising the possibility that in the original studies some cases of diagnosed Alzheimer's disease are indeed cases of mixed dementia (Alzheimer's disease + vascular dementia), Reference Laukka, Fratiglioni and Backman33,Reference Gold, Giannakopoulos, Herrmann, Bouras and Kovari34 stressing the importance of vascular pathology and poor outcomes in late-life depression. Nonetheless, the finding of increased risk of vascular dementia, and of significantly higher risk as compared with Alzheimer's disease, should be viewed with caution, as it is based on only five studies. Also, most studies did not directly compare the risk of Alzheimer's disease v. vascular dementia in participants with late-life depression, except Lenoir et al, Reference Lenoir, Dufouil, Auriacombe, Lacombe, Dartigues and Ritchie5 who found that the risk for vascular dementia was significantly higher than for Alzheimer's disease (P = 0.002).
Implications
Recent evidence suggest that depression is a potentially preventable medical condition across the lifespan (including in older adults) and may be a modifiable risk factor for preventing or delaying dementia in later life. Reference Cuijpers, Beekman and Reynolds35,Reference Reynolds, Cuijpers, Patel, Cohen, Dias and Chowdhary36 Thus, strategies to promote the prevention, early diagnosis and adequate treatment of depression may have a major public health impact as they may potentially prevent or delay dementia in older adults. Also, the prevention and treatment of cardiovascular risk factors and an improvement of general health in people with late-life depression may have a significant impact not only in a reduction of late-life depression cases but also a reduction of dementia cases (vascular dementia and Alzheimer's disease) associated with this disorder. Given the present results in this meta-analysis, the estimated number of cases of dementia attributable to depression, including lifetime and late-life depression, is probably higher than those reported by Barnes & Yaffe, Reference Barnes and Yaffe6 and the prevention of lifetime and late-life depression would possibly have an even greater impact on the reduction of Alzheimer's disease and vascular dementia cases than previously hypothesised. Reference Barnes and Yaffe6 Therefore, it is necessary to conduct novel clinical trials to investigate the potential impact of prevention of depression on risk of cognitive impairment and dementia in elderly people.
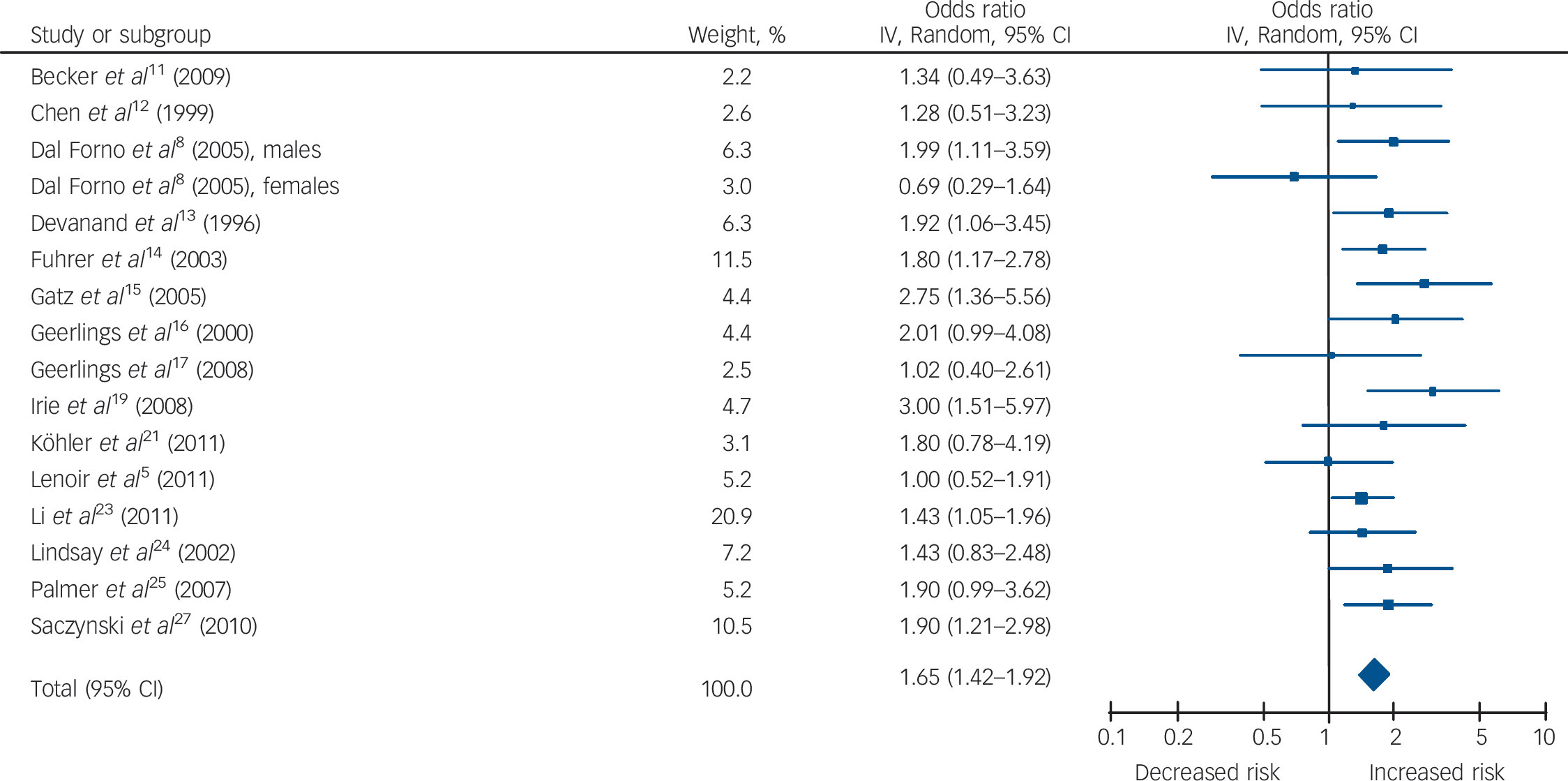
Fig. 3 Forest plot for the risk of incident Alzheimer's disease in participants with late-life depression.
Limitations
The studies included in the meta-analysis reported either the odds ratio or the hazard ratio for the association between late-life depression and dementia. Thus, we calculated separately the pooled OR and the HR for the association between late-life depression and dementia. Although both measure the association between two conditions, they are not interchangeable. Odds ratio is a measure of association between two conditions (such as in logistic regression models); whereas the hazard ratio is a measure of the strength of association between two conditions in time-to-event statistical analysis (for example in Cox proportional hazard models). Given this, we should interpret the results from the pooled risk analysis with caution as we included studies that reported hazard ratios and odds ratios together. Nonetheless, the results are very consistent across all analyses for all-cause dementia, Alzheimer's disease and vascular dementia.
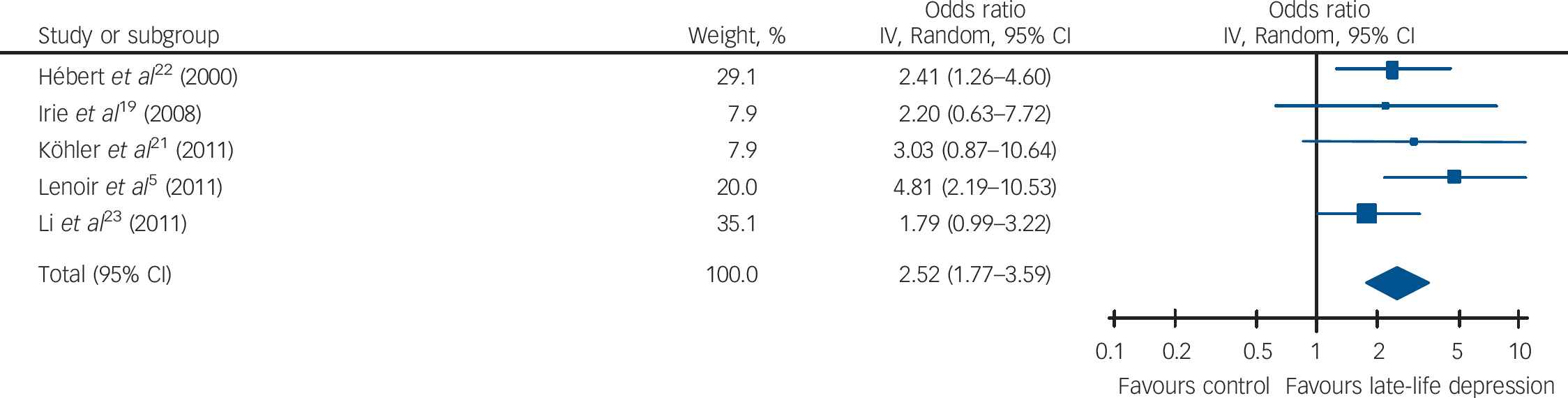
Fig. 4 Forest plot for the risk of incident vascular dementia in participants with late-life depression.
The present results should also be considered in light of the limitations of the studies included. First, in the included studies depressive symptoms were measured with rating scales, using pre-established cut-off scores. As no structured interviews were conducted for diagnostic assessment of depressive disorders, this may have introduced significant heterogeneity in the classification of cases and non-cases, in particular in individuals with mild depression. In addition, the included studies had widely varying follow-up intervals, ranging from 1 to 17 years, which might have introduced heterogeneity into the present results. Also, some studies were not representative of the entire population (such as studies including only men or women) or the study samples were not randomly selected (for example including all participants from a catchment area). These biases might have led to an overestimation of the association between late-life depression and dementia, which could have led to the higher and significant association between late-life depression and dementia found in this meta-analysis. Nonetheless, the current results were consistent across analyses and robust to sensitivity and subgroup analyses and we found low heterogeneity for all-cause dementia and vascular dementia, but moderate heterogeneity for Alzheimer's disease studies, which strengthens the present results.
The adjustments of pooled odds ratios to account for potential confounders on the relationship between late-life depression and dementia, Alzheimer's disease and vascular dementia were based on the adjusted risk measures reported by individual studies. As expected, the strength of the relationship between late-life depression and these disorders was reduced, although still significant, after adjustments. Nonetheless, each study adjusted by its own set of variables (mostly baseline sociodemographic and cognitive function, but also for cardiovascular risk factor and medication use) may have introduced significant heterogeneity in the statistical analysis and biased the results. However, Q-tests and I 2 index did not show evidence for significant heterogeneity in these subanalyses that might have biased the results. We did not carry out a separate analysis of the effect of recurrent depressive episodes on the risk of dementia. However, there is evidence that individuals with a history of recurrent depressive episodes have a greater risk of all-cause dementia and Alzheimer's disease than individuals with a history of a single depressive episode. Reference Dotson, Beydoun and Zonderman37,Reference Kessing and Andersen38 We did not include case-control studies that evaluated history of lifetime depression and the risk of dementia. Thus, our results are specific to older adults with depressive symptoms and do not address the moderating effect of lifetime depressive episodes on the risk of incident dementia.
Another potential limitation of this meta-analysis is that our search was limited to PubMed and Scopus databases (the largest biomedical databases available with broad coverage of publications from different countries). We also did a careful review of all references of potentially relevant publications, previous meta-analyses and systematic reviews published on this topic. Although the search of other international databases (such as EMBASE and PsycINFO) might have led to the identification of additional studies that could have been included in this meta-analysis, we believe that the literature search was comprehensive and identified all potentially relevant articles for this meta-analysis.
In conclusion, the present study provides robust evidence that depression is independently associated with an independent increased risk of Alzheimer's disease and vascular dementia in older adults. In addition, the risk of vascular dementia may be significantly higher than for Alzheimer's disease in these individuals. The prevention of depression along with stimulating general healthy behaviours and lifestyles, reduction of cardiovascular burden and other mental health-related problems should be considered in public health policies aiming at the prevention and/or delaying of the presentation of dementia syndromes in the older adult population. In view of the strong evidence provided by this meta-analysis that late-life depression is an independent risk factor for distinct dementia syndromes, namely Alzheimer's disease and vascular dementia, it is necessary to conduct novel clinical trials to investigate the potential impact of prevention of depression on the risk of cognitive impairment and dementia in older adults.
Funding
This work was supported in part by John Hartford Foundation and the UPMC Endowment in Geriatric Psychiatry, National Institute of Health grants R01 MH072947, R01 MH080240, P30 MH090333, UL1 RR024153 and UL1TR000005. B.S.D. receives research support from the John A. Hartford Foundation. M.A.B. receives support from the National Institute of Mental Health (NIMH) and National Institute on Aging (NIA). C.F.R. receives support from the NIMH, NIA, National Center for Minority Health Disparities (NIMHD), National Heart Lung and Blood Institute (NHLBI), Center for Medicare and Medicaid Services, John A. Hartford Foundation, American Foundation for Suicide Prevention, Commonwealth of Pennsylvania, American Association for Geriatric Psychiatry for services as associate editor, and the UPMC Endowment in Geriatric Psychiatry which supports his endowed professorship.








eLetters
No eLetters have been published for this article.