Intellectual disabilities are common and lifelong. For the USA 2000 birth cohort, it is estimated that the lifetime costs of intellectual disabilities will be $44.1 billion in excess of costs for people without intellectual disabilities (Reference Honeycutt, Grisse, Dunlap, Altman, Barnett and HendersonHoneycutt et al, 2003). The point prevalence of mental ill-health has been reported as 40.9% (Reference Cooper, Smiley and MorrisonCooper et al, 2007), which is higher than that of the general population, and is a significant contributor to both costs and quality of life. The factors associated with mental ill-health differ from findings in the general population (Reference Cooper, Smiley and MorrisonCooper et al, 2007). Prevalence of mental ill-health is determined by the incidence of episodes of mental ill-health and by the duration of episodes, and the factors predicting incidence and duration may differ. Associations found in cross-sectional studies cannot distinguish between factors predicting incidence, predicting duration, or both. Some associations may be the consequences of mental ill-health. The incidence rate and predictors of incident mental ill-health in this population are unknown (Reference SmileySmiley, 2005). Previous longitudinal studies in the general population did not include people with moderate to profound intellectual disabilities, but did demonstrate the higher prevalence of symptoms of depression and anxiety in adults with mild intellectual disabilities compared with the general population (Reference Maughan, Collishaw and PicklesMaughan et al, 1999; Reference Richards, Maughan and HardyRichards et al, 2001). They did not determine the factors predicting depression/anxiety scale scores, in view of the small numbers with mild intellectual disabilities (n=100 and n=41 in the respective studies) and low rates of (and bias in) cohort retention. The prevalence of problem behaviours was assessed in an institutional cohort of 67 adults with severe to profound intellectual disabilities at time points 16–18 years apart (Reference Reid and BallingerReid & Ballinger, 1995). They reported a correlation in problem behaviour prevalence at the two time points: the presented data indicated some movement into and out of the problem behaviour category, but details were not reported and predictors were not investigated.
The specific aims of our study were to determine the incidence of mental ill-health among adults with mild to profound intellectual disabilities, and investigate factors hypothesised to be related to incident mental ill-health. We are not aware of any such previous investigation.
METHOD
Participants
The adult intellectual disabilities population (aged 16 years and over) in Greater Glasgow, UK, was identified. The process identified all adults with intellectual disabilities who were registered with a general practitioner (family physician) in Greater Glasgow (all 631 of these doctors contributed to the ascertainment process); adults who were receiving support of any type paid for, or provided by, the social work department, including day services and support packages of any size; and adults using specialist intellectual disabilities health services. Hence, this included both adults who had spent all their lives in the community (82.4%) and adults who had previously spent some of their life in long-stay hospital accommodation (17.6%). At the time of the study, all long-stay hospital accommodation in the area had been closed. The rate of intellectual disabilities in adulthood was 3.33 per 1000 general population, which is comparable with ascertainment rates for the adult population with intellectual disabilities conducted elsewhere (Reference Farmer, Rohde and SacksFarmer et al, 1993; Reference McGrother, Thorp and TaubMcGrother et al, 2001; Reference Van Schrojenstein Lantman de-Valk, Wullink and van den Akkervan Schrojenstein Lantman de-Valk et al, 2006). Adults were recruited into a longitudinal cohort at the first time point (time 1) (Reference Cooper, Smiley and MorrisonCooper et al, 2007); measurements were repeated 2 years later (time 2).
Approval and consent
Ethics committee approval was gained. Consent was taken from each participant with capacity to decide to consent, or otherwise from their nearest relative, in keeping with the Adults with Incapacity (Scotland) Act.
Data collection process
Face-to-face interviews were completed with each person supported by their carer. Information was also collected from a relative. At both time 1 and time 2, following each interview, health data were discussed with a doctor. Individuals who had two or more symptoms, or one ‘high-risk’ symptom, at the interview on the Psychiatric Assessment Schedule for Adults with Developmental Disability (PAS–ADD) Checklist (Reference Moss, Prosser and CostelloMoss et al, 1998) each had a second face-to-face comprehensive psychiatric assessment conducted by the Glasgow University Centre for Excellence in Developmental Disabilities (UCEDD), which is run by two academics who are also qualified consultant psychiatrists specialised in working with adults with intellectual disabilities. In 67.8% of cases, one of the consultants collected the assessment information; in 32.2% of cases a non-consultant specialist psychiatrist conducted the assessment (81.3% of which were conducted by a specialist registrar who had passed the membership of the Royal College of Psychiatrists examinations and was in her final year of training to be eligible for consultant posts in intellectual disabilities psychiatry), rather than a consultant. In all cases the findings were case-conferenced by the consultant members of the research team to derive consultant-level diagnoses. At time 2, in addition, any episodes of mental ill-health that occurred between time 1 and time 2 were identified at the face-to-face interview by a series of semi-structured questions, and the PAS–ADD Checklist was completed for that episode at the interview with the person, supported by a carer. The same thresholds were used to identify people for the second face-to-face comprehensive psychiatric assessment by the UCEDD. Participants requiring diagnostic clarification of problem behaviours also received a comprehensive psychiatric assessment by the UCEDD, as did those who scored on items of mental ill-health on the C21st Health Check (Glasgow University Centre for Excellence in Developmental Disabilities, 2001). Medical and psychology case-notes were reviewed for all participants. Episodes of mental ill-health were classified according to the psychiatrists’ clinical opinion, the Diagnostic Criteria for Psychiatric Disorders for Use with Adults with Learning Disabilities/Mental Retardation (DC–LD; Royal College of Psychiatrists, 2001), the ICD–10 Diagnostic Criteria for Research (ICD–10–DCR; World Health Organization, 1993) and the revised DSM–IV (DSM–IV–TR; American Psychiatric Association, 2000) diagnostic criteria.
Instruments
The same instruments were used at both the time 1 and time 2 interviews.
PAS–ADD Checklist
The PAS–ADD Checklist is a screening tool for mental ill-health designed for use with adults with intellectual disabilities (Reference Moss, Prosser and CostelloMoss et al, 1998). However, when using the published threshold scores (which are 6 and above for the affective or neurotic disorder sub-scale, 5 and above for the possible organic condition sub-scale and 2 and above for the psychotic disorder sub-scale), the reported sensitivity of this tool is only about 66% (Reference Moss, Prosser and CostelloMoss et al, 1998; Reference SimpsonSimpson, 1999; Reference Sturmey, Newton and CowleySturmey et al, 2005). Simpson's extremely detailed study of the psychometric properties of the tool included receiver operating characteristic analyses for various possible ways of completing the PAS–ADD Checklist. These were completing it with the person's main carer, with two carers, or with day-centre staff, and for each of these scoring the PAS–ADD Checklist by counting items using the Likert scale, any positive item or a mid-point threshold for each item (i.e. a score of 2 or 3). This found that when the PAS–ADD Checklist was completed with the person's main carer and a threshold of any two positive items was used, the tool had a 100% sensitivity to detect people meeting criteria for an ICD–10 diagnosis with a false-positive rate of 58%, and 95% sensitivity to detect people meeting criteria for a DSM–IV diagnosis with a false-positive rate of 53%. As would be expected, both sensitivity and false-positive rate progressively reduced with increasing threshold score (Reference SimpsonSimpson, 1999). We wanted to maximise the detection of true positives, at the cost of false positives at this first stage of the process, as the two-stage process would mean that any false positives at stage 1 would be detected at stage 2 (the comprehensive psychiatric examination). Consequently we used the threshold of any two positive items across the whole scale, to trigger the second-stage full psychiatric assessment. Additionally, we used a threshold of only needing only one positive item if it was attempted suicide or talk of suicide, or any of the four psychosis items. We also added six new items after a pilot study with 50 persons. These were aimed at detecting mania and strengthening the psychosis sub-scale, and were specifically lability of mood; loss of social inhibitions/onset of inappropriate social behaviour; increased interest in sex/sexual indiscretions; excessive talking, laughing or singing; tearfulness; and thinking that people or the television are referring to the person or giving messages or instructions.
Demographic questionnaire
A semi-structured demography and supports questionnaire was designed specifically for the study, including postcode data to allocate individuals to quintiles of the Carstairs Deprivation Index, a Scottish area-based measure of socio-economic deprivation (Reference Carstairs and MorrisCarstairs & Morris, 1989).
Personal history questionnaire
A purpose-designed semi-structured past and personal history questionnaire was used to collect these data.
Vineland Scale
The Vineland Scale (Survey Form) (Reference Sparrow, Balla and CicchettiSparrow et al, 1984) was used to measure ability and skills.
C21st Health Check
Selections from the C21st Health Check were used, including problem behaviours, and mental ill-health items that identified participants requiring full psychiatric assessment even if they scored below the lowered threshold on the PAS–ADD Checklist.
Psychiatric assessment
Psychiatrist assessment followed a comprehensive semi-structured assessment format, which included using the Present Psychiatric State for Adults with Learning Disabilities (PPS–LD; Reference CooperCooper, 1997). This semi-structured schedule for use with adults with intellectual disabilities measures the comprehensive range of psychopathology required for classification by clinical, DC–LD, ICD–10–DCR and DSM–IV–TR criteria.
Physical health
At time 1 physical health was comprehensively measured using the full C21st Health Check, which includes measurement of vision and hearing.
Statistical analyses
Data were analysed using the Statistical Package for the Social Sciences version 11.5 for Windows. Potential bias among potential participants for whom consent was refused was examined, with regards to age, gender, level of ability, type of accommodation and support, and prevalence of mental ill-health at time 1. The 2-year incidence rate of mental ill-health was defined as the proportion of individuals with the onset of a new episode at any time in the 2-year period. For the common mental disorders, the standardised incidence ratio and 95% confidence interval were then calculated using published general population data. Two subgroups of incident outcomes were further investigated: incident episodes of mental ill-health (excluding problem behaviours, dementia and delirium), and incident episodes of problem behaviours. Dementia and delirium were excluded as their aetiology was postulated to differ from that of other types of mental ill-health. Problem behaviours were analysed separately because of ongoing debate regarding their nosological status, and because of comorbidity of problem behaviours and other types of mental ill-health, e.g. enduring problem behaviours plus incidence of depressive episode.
Multivariate logistic regression modelling was undertaken to assess potential risk factors for predicting which individuals would experience at least one incident episode of mental ill-health in the two subgroups defined above during the 2-year follow-up period. Four groups of factors (26 factors in total) were investigated for each of the outcomes:
-
(a) personal factors (six items): older age; female gender; more severe intellectual disabilities; Down syndrome; mental ill-health in the past; mental ill-health within a biological family member;
-
(b) past experiences (six items): death of parent/parental figure before age 19 years; divorce of parents before age 19 years; raised outside a family home before age 19 years; other adversity before age 19 years (compulsory removal from the family home, known abuse, neglect or exploitation, financial poverty, other traumatic experiences); known adult abuse, neglect or exploitation; previous long-stay hospital residence during adulthood;
-
(c) lifestyle and supports measured at time 1 (six items): type of accommodation/support (not living with a family carer); having no employment/day opportunities; Carstairs quintile (living in more deprived areas); single status; smoking; experiencing preceding life events;
-
(d) health and disabilities measured at time 1 (eight items): visual impairment; hearing impairment; bowel incontinence; urinary incontinence; impaired mobility; severe physical disabilities; epilepsy; special communication needs.
We conducted the analysis of each endpoint in discrete stages. Initially, the distribution of the outcomes of interest and each factor was assessed individually. Second, for each of the four subgroups of factors described above, a backwards stepwise method was used to determine the set of factors within the subgroup that were independently related to the outcome. Finally, the independently related factors from these four group-specific models were entered into a single global model and a backward stepwise method was again used to reach the final model for that outcome. Likelihood ratio tests were used in the stepwise procedures to determine statistical significance for removal of each factor (the removal criterion was set at 0.05). The two final models were checked for goodness of fit using the Hosmer–Lemeshow test, in which the study sample is divided into deciles of predicted risk and the numbers of observed and expected events compared using a χ2-test. Because of the small numbers of expected events in some deciles of predicted risk, the lowest risk groups were combined until the expected number of events exceeded 3 in all groups.
RESULTS
Cohort at time 2
At time 1 the cohort size was 1202. At time 2 the potential cohort size was 936, because of 54 deaths, 184 no longer satisfying the new requirements of the Adults with Incapacity (Scotland) Act for inclusion in research (through not having a nearest relative owing to death or loss of contact with family, or a welfare guardian) and 28 due to other circumstances such as serious physical ill-health. All 936 were invited to participate in the research at time 2, and 142 of the earlier participants (15.2%) and 143 (15.3%) of the nearest relatives declined. Hence, 651 (69.6%) participated at both assessments. There was no difference between participants and those for whom consent was not gained at time 2, in terms of time 1 age, gender, level of intellectual disabilities, type of accommodation/support or prevalence of mental ill-health (Table 1).
Table 1 Comparison of data collected at time 1 between participants at time 2 and those for whom consent to participate at time 2 was not gained

| Participants (n=651) | Non-participants (n=285) | P | |
|---|---|---|---|
| Age, years: mean (s.d.) | 43.6 (14.2) | 43.9 (14.4) | 0.764 |
| Gender, n (%) | |||
| Male | 355 (54.5) | 156 (54.8) | 0.953 |
| Female | 296 (45.5) | 129 (45.2) | |
| Type of living/support arrangement at time 1, n (%) | |||
| With family carer | 258 (39.7) | 113 (39.6) | 0.673 |
| Independent of support | 51 (7.8) | 28 (9.8) | |
| With paid carer support | 297 (45.7) | 122 (42.8) | |
| Congregate care setting | 44 (6.8) | 22 (7.7) | |
| Ability, n (%) | |||
| Mild intellectual disabilities | 254 (39.0) | 118 (41.4) | 0.127 |
| Moderate intellectual disabilities | 140 (21.5) | 73 (25.6) | |
| Severe intellectual disabilities | 126 (19.4) | 53 (18.6) | |
| Profound intellectual disabilities | 131 (20.1) | 41 (14.4) | |
| Prevalence of mental ill-health at time 1,1 n (%) | |||
| Including problem behaviours and autism | 243 (37.3) | 103 (36.1) | 0.729 |
| Excluding problem behaviours, including autism | 170 (26.1) | 74 (26.0) | 0.962 |
| Excluding problem behaviours and autism | 136 (20.9) | 56 (19.6) | 0.665 |
At time 2 the cohort comprised 355 (54.5%) men and 296 (45.5%) women, with mean age of 46.1 years (range 18.2–80.8). The level of intellectual disabilities was mild for 254 (39.0%), moderate for 140 (21.5%), severe for 126 (19.4%) and profound for 131 (20.1%). Most participants were single (628; 96.5%); 242 (37.2%) lived with a family carer, 294 (45.2%) lived in rented accommodation and held a single or shared tenancy agreement and received paid carer support in their home, 69 (10.6%) lived in a congregate care setting and 46 (7.1%) lived independently of any paid support. Of the participants living in rented accommodation, 32.8% were previously long-stay hospital residents, as were 31.1% of those living in a congregate care setting. Regarding daytime opportunities and occupation, 147 (22.6%) had none, of whom 24 were of retirement age (65 years or over).
Incidence of mental ill-health
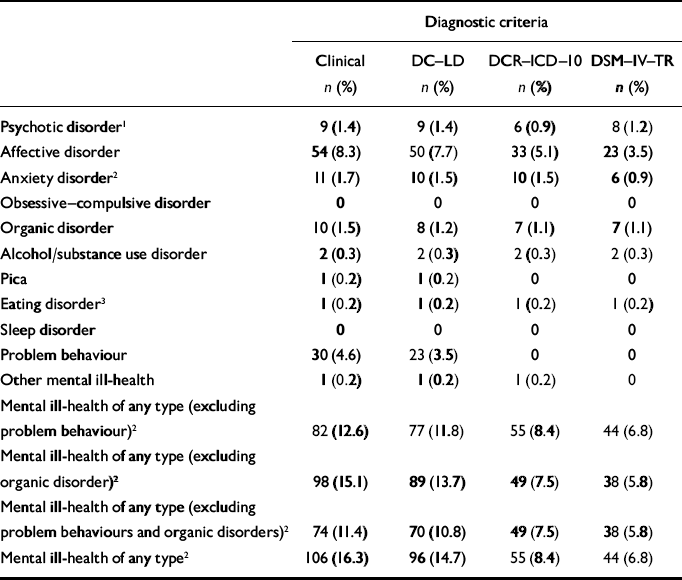
Table 2 reports incidence by diagnostic groups and total incidence by person rather than by episode (some people had more than one episode). Some people had incident episodes in two different diagnostic groupings, in which case both are included in the relevant diagnostic grouping (but the total incidence remains reported by person rather than by episode). The names of diagnostic groupings differ in the different diagnostic manuals (e.g. ‘schizophrenia, schizotypal and delusional disorders’ in ICD–10–DCR but ‘non-affective psychotic disorders’ in DC–LD), but the operationalised criteria within each manual have been strictly applied. The specific code numbers in each diagnostic grouping for each manual have been reported previously (Reference Cooper, Smiley and MorrisonCooper et al, 2007).
Table 2 Two-year incidence of mental ill-health by clinical, DC—LD, DCR—ICD—10 and DSM—IV—TR diagnostic criteria
| Diagnostic criteria | ||||
|---|---|---|---|---|
| Clinical n (%) | DC—LD n (%) | DCR—ICD—10 n (%) | DSM—IV—TR n (%) | |
| Psychotic disorder1 | 9 (1.4) | 9 (1.4) | 6 (0.9) | 8 (1.2) |
| Affective disorder | 54 (8.3) | 50 (7.7) | 33 (5.1) | 23 (3.5) |
| Anxiety disorder2 | 11 (1.7) | 10 (1.5) | 10 (1.5) | 6 (0.9) |
| Obsessive—compulsive disorder | 0 | 0 | 0 | 0 |
| Organic disorder | 10 (1.5) | 8 (1.2) | 7 (1.1) | 7 (1.1) |
| Alcohol/substance use disorder | 2 (0.3) | 2 (0.3) | 2 (0.3) | 2 (0.3) |
| Pica | 1 (0.2) | 1 (0.2) | 0 | 0 |
| Eating disorder3 | 1 (0.2) | 1 (0.2) | 1 (0.2) | 1 (0.2) |
| Sleep disorder | 0 | 0 | 0 | 0 |
| Problem behaviour | 30 (4.6) | 23 (3.5) | 0 | 0 |
| Other mental ill-health | 1 (0.2) | 1 (0.2) | 1 (0.2) | 0 |
| Mental ill-health of any type (excluding problem behaviour)2 | 82 (12.6) | 77 (11.8) | 55 (8.4) | 44 (6.8) |
| Mental ill-health of any type (excluding organic disorder)2 | 98 (15.1) | 89 (13.7) | 49 (7.5) | 38 (5.8) |
| Mental ill-health of any type (excluding problem behaviours and organic disorders)2 | 74 (11.4) | 70 (10.8) | 49 (7.5) | 38 (5.8) |
| Mental ill-health of any type2 | 106 (16.3) | 96 (14.7) | 55 (8.4) | 44 (6.8) |
The 2-year incidence rate for mental ill-health of any type was 16.3% (106 individuals); 82 individuals (12.6%) had an incident episode of mental ill-health excluding problem behaviours, of whom 74 (11.4%) had an incident episode of mental ill-health excluding problem behaviours, dementia and delirium; and 30 (4.6%) had an incident episode of problem behaviours. This rate is higher than that reported for the UK general population. Singleton & Lewis (Reference Singleton and Lewis2003) reported general population data on the incidence of common mental disorders. They used a sampling strategy to select 3536 persons from an original cohort of 8580 adults in England, Wales and Scotland to be reassessed 18 months after the initial assessments. Of the 3536 persons selected they were able to contact 3045, of whom assessments were completed with 2413. From the findings of that survey we would expect 8% of our cohort to have incident episodes of common mental disorders, i.e. 52 persons. Ninety-seven persons in the cohort had incident episodes that could be termed common mental disorders. The standardised incident ratio is therefore 1.87 (95% CI 1.51–2.28).
For mental ill-health of any type, the number of incident episodes per person was none for 545 (83.7%), one for 93 (14.3%), two for 11 (1.7%), three for 1 (0.2%) and four for 1 (0.2%). For 49 (46.2%) of the 106 participants with incident mental ill-health, the episode of mental ill-health had both incidence and recovery within the 2-year period; 57 had incidence and were still in episode at time 2.
Factors related to incidence of mental ill-health
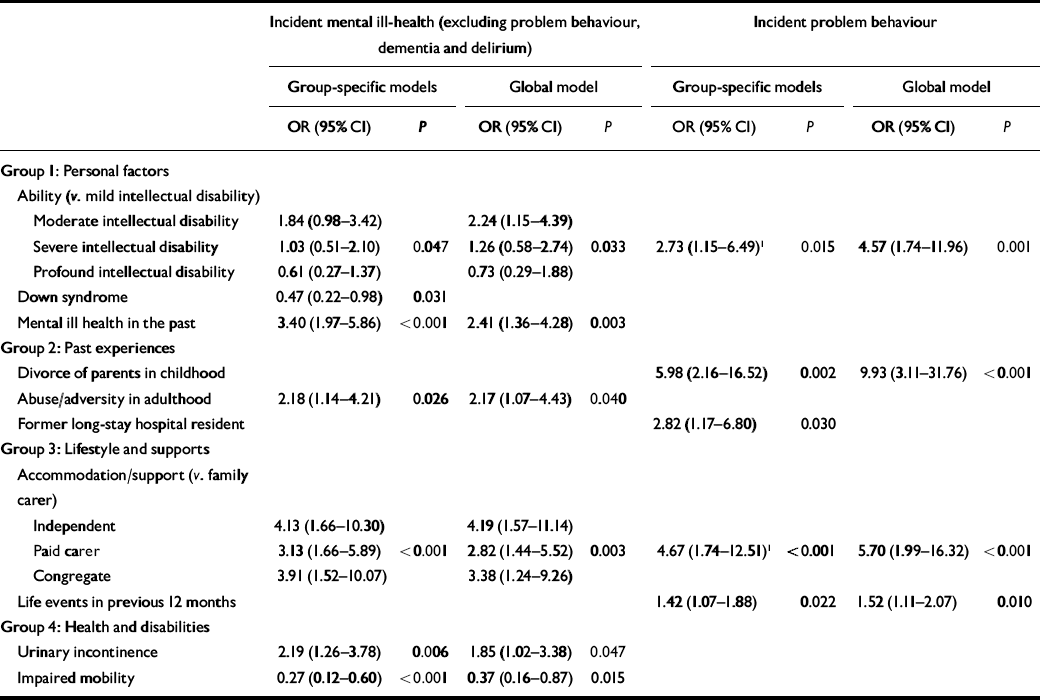
The results from the initial univariate analyses, exploring the relationship of each individual variable of interest with the two outcomes, are shown in a data supplement to the online version of this paper. For incident episodes of mental ill-health (excluding problem behaviours, dementia and delirium) at the second stage of analyses (the group-specific models), 1 participant had an incomplete data-set (but did not have incident mental ill-health) for personal factors; there was no incomplete data-set for past experiences; 3 participants had incomplete data-sets for lifestyle/supports, none of whom had incident mental ill-health; and 16 had incomplete data-sets for health/disabilities, of whom 1 had incident mental ill-health. At the third stage of analyses (the global model) 1 participant had an incomplete dataset, but did not have an incident episode. Table 3 displays the results. For the global model the Hosmer–Lemeshow statistic was χ2=1.86, d.f.=6, P=0.93, giving no indication of lack of fit.
Table 3 Factors independently related to incidence of mental ill-health and problem behaviours
| Incident mental ill-health (excluding problem behaviour, dementia and delirium) | Incident problem behaviour | |||||||
|---|---|---|---|---|---|---|---|---|
| Group-specific models | Global model | Group-specific models | Global model | |||||
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Group I: Personal factors | ||||||||
| Ability (v. mild intellectual disability) | ||||||||
| Moderate intellectual disability | 1.84 (0.98-3.42) | 2.24 (1.15-4.39) | ||||||
| Severe intellectual disability | 1.03 (0.51-2.10) | 0.047 | 1.26 (0.58-2.74) | 0.033 | 2.73 (1.15-6.49)1 | 0.015 | 4.57 (1.74-11.96) | 0.001 |
| Profound intellectual disability | 0.61 (0.27-1.37) | 0.73 (0.29-1.88) | ||||||
| Down syndrome | 0.47 (0.22-0.98) | 0.031 | ||||||
| Mental ill health in the past | 3.40 (1.97-5.86) | <0.001 | 2.41 (1.36-4.28) | 0.003 | ||||
| Group 2: Past experiences | ||||||||
| Divorce of parents in childhood | 5.98 (2.16-16.52) | 0.002 | 9.93 (3.11-31.76) | <0.001 | ||||
| Abuse/adversity in adulthood | 2.18 (1.14-4.21) | 0.026 | 2.17 (1.07-4.43) | 0.040 | ||||
| Former long-stay hospital resident | 2.82 (1.17-6.80) | 0.030 | ||||||
| Group 3: Lifestyle and supports | ||||||||
| Accommodation/support (v. family carer) | ||||||||
| Independent | 4.13 (1.66-10.30) | 4.19 (1.57-11.14) | ||||||
| Paid carer | 3.13 (1.66-5.89) | <0.001 | 2.82 (1.44-5.52) | 0.003 | 4.67 (1.74-12.51)1 | <0.001 | 5.70 (1.99-16.32) | <0.001 |
| Congregate | 3.91 (1.52-10.07) | 3.38 (1.24-9.26) | ||||||
| Life events in previous 12 months | 1.42 (1.07-1.88) | 0.022 | 1.52 (1.11-2.07) | 0.010 | ||||
| Group 4: Health and disabilities | ||||||||
| Urinary incontinence | 2.19 (1.26-3.78) | 0.006 | 1.85 (1.02-3.38) | 0.047 | ||||
| Impaired mobility | 0.27 (0.12-0.60) | <0.001 | 0.37 (0.16-0.87) | 0.015 | ||||
For incident episodes of problem behaviours at the second stage of analyses (the group-specific models), 1 participant had an incomplete data-set (but did not have incident problem behaviours) for personal factors; there was no incomplete data-set for past experiences; 5 participants had incomplete data-sets for lifestyle/supports, none of whom had incident problem behaviours; and 16 had incomplete data-sets for health/disabilities, but did not have incident problem behaviours. Type of accommodation/support was dichotomised to living with a family carer or not, and ability level was dichotomised to mild intellectual disabilities or moderate–profound intellectual disabilities, in view of numbers being too small to sub-categorise further. At the third stage of analyses (the global model) 3 participants had incomplete data-sets, none of whom had an incident episode. Table 3 displays the results. For the global model the Hosmer–Lemeshow statistic was χ2=1.11, d.f. 3, P=0.77, again giving no indication of inadequate fit.
In summary, factors at time 1 that were related to an incident episode of mental ill-health (excluding problem behaviours, dementia and delirium) being identified at time 2 were living in a congregate care setting, with paid carer support, or independently of care (i.e. not living with a family carer); urinary incontinence; not having impaired mobility; having a past psychiatric history; moderate rather than mild intellectual disabilities; and the experience of abuse, neglect or exploitation during adult life. Not living with a family carer was also related to incident episodes of problem behaviours, but other factors differed and included lower ability level, having experienced the divorce of parents in childhood, and a higher number of life events in the preceding 12-month period.
DISCUSSION
The high point prevalence of mental ill-health for adults with intellectual disabilities is accounted for by both incident cases and enduring episodes, with slightly more enduring than incident cases, particularly with regard to problem behaviours. Incidence is statistically significantly higher than that reported in the general population.
Some of the associations we found with incident mental ill-health are immutable, e.g. ability level. These may help identify high-risk groups within the population, who may warrant provision of early interventions and supports. Other factors may be amenable to interventions, e.g. urinary incontinence and not having immobility. Incontinence may be aetiological through a mediating effect of self-esteem, or alternatively a common underlying mechanism. This interaction between mental ill-health and incontinence has also been identified within the general population (Reference Perry, McGrother and TurnerPerry et al, 2006). People who lack mobility might have a greater level of individual care or personal interaction which has coincidental benefits for mental health, or limited mobility might preclude circumstances and experiences that might be adversive to mental health. This is the converse of findings in the general population (Reference Singleton and LewisSingleton & Lewis, 2003).
The type of accommodation and support the participant received at time 1 was related to incidence of mental ill-health, with individuals in settings other than family homes at higher risk. We do not know the reason why participants were living within a particular type of accommodation/support, but note that past psychiatric history and type of accommodation/support were independently predictive. This warrants further research attention, and engagement between professionals, service managers and paid carers.
We found adverse events (preceding life events, parental divorce in childhood, and adult abuse, neglect and exploitation) to be related to incident episodes. This suggests the need for greater support for individuals at the time of experiencing such adversities, and further research and development of clinical practice to determine ways and formats in which such support could be provided. We hope that our findings will help to raise awareness of the impact of such events on people with intellectual disabilities, and that the onset of problem behaviours (not only other types of mental ill-health) may have an emotional component.
Similarities with the general population include the findings for incontinence and adult abuse, and that preceding life events may predict problem behaviours (Reference Singleton and LewisSingleton & Lewis, 2003). There are also important differences, including incidence not being predicted by living in more deprived areas (Reference Lorant, Deliege and EatonLorant et al, 2003), not having any day-time occupation (Reference Singleton and LewisSingleton & Lewis, 2003), smoking status and epilepsy. The reversed trend for marital status compared with the general population was not statistically significant, hence conclusions cannot be drawn regarding it. We postulate that adults with intellectual disabilities who do not live with a family carer may not have the same lifestyle characteristics as the general population living in the same area, owing to being given accommodation in areas dissimilar from their place of origin, within which they acquired lifelong habits and preferences. In addition, the views and actions of closely involved relatives may have greater influence on them than those of their paid carers or local community. Such moves are often ‘placements’, determined by professionals on the basis of existing vacancies in the housing stock rather than by the individual, and made quickly owing to a sudden change in circumstances, for example following the death of a family carer or the breakdown of an existing care package. This differs from the general population who make choices for themselves in their own time regarding when to move and where to live. Other factors are of greater relevance for this population.
Our study is an important step forward in describing mental ill-health within the population with intellectual disabilities, and identifying differences from the general population in the factors that might predict incidence. People with intellectual disabilities are known to experience health inequalities compared with the general population (Reference Horwitz, Kerker and OwensHorwitz et al, 2001; US Office of the Surgeon General, 2002; NHS Health Scotland, 2004; Reference Cooper, Melville and MorrisonCooper et al, 2004; Reference Scheepers, Kerr and O'HaraScheepers et al, 2005). If public health interventions are focused only on areas of importance to the general population, they will fail to address the factors most relevant to the population of adults with intellectual disabilities. This is then likely to lead to a widening of the existing inequality gap. Our results are therefore important, as they are a first step towards stimulating more research in this area, and being able to influence the development of interventions, service design, public health strategy, and health and social care policy, to start to reduce health inequalities.
There is no published research regarding the incidence and predictors of mental ill-health for the intellectual disabilities population with which we could draw comparisons.
Strengths and limitations of the study
The study has several limitations. The data collection on abuse, neglect and exploitation is unlikely to have detected all survivors of these experiences, owing to surrounding secrecy. Although we interviewed the nearest relative of each participant as well as the participants themselves and the people who support them, it is possible that we missed some information on previous episodes of mental ill-health, which is often overlooked in this population. Although we have presented a standardised incidence ratio for common mental disorders, it is important to note that there are differences between our study and that of Singleton & Lewis (Reference Singleton and Lewis2003), due to different methods of assessment, different instruments and different diagnoses (the most common diagnosis reported by Singleton & Lewis was non-specific psychiatric morbidity, which they conceived to represent mixed anxiety depression, whereas problem behaviours were common in the cohort we report). The statistical relationships that we found do not necessarily mean that there is a cause and effect relationship between the time 1 variables and incidence. Our research objectives were not to derive a clinically useful predictive tool, but to assess a broad range of factors that are potentially associated with incidence of mental ill-health and problem behaviours in this population; we hope this will stimulate further research in this area, whether epidemiological or interventional.
Strengths of the study include the comprehensiveness of data collection and psychiatric assessment, the large cohort size and the longitudinal design. Cohort retention is less successful with the intellectual disabilities population than the general population (Reference Wadsworth, Mann and RodgersWadsworth et al, 1992; Reference Maughan, Collishaw and PicklesMaughan et al, 1999; Reference Richards, Maughan and HardyRichards et al, 2001), hence the high level of participation at time 2 is a further strength. The two time points are close enough to reduce the likelihood of missing interim-period data, which is an important consideration in study design, given the population's known poor access to services when ill, the high job mobility of paid carers, and the limitations in communication skills and retention of information of many people with intellectual disabilities.
Urinary incontinence and ability level were retained within the statistical models at stages 2 and 3, even though the univariate analyses at stage 1 reported the relationship between these factors and incident mental ill-health to be greater than 0.05 when analysed individually. This is likely to be due to associations between some of the variables. Specifically, urinary incontinence and impaired mobility are associated (occurring more often in people with more severe intellectual disabilities), and people with Down syndrome are more likely to have moderate and severe intellectual disabilities than mild or profound intellectual disabilities, compared with the population with intellectual disabilities in general. We found both impaired mobility and Down syndrome were protective against incident mental ill-health, accounting for these findings. It is likely that several of the factors we investigated are interdependent.
Future research
Longitudinal studies to understand why a person's type of accommodation and support affect mental health are indicated. Longer-term outcomes and predictors of recovery from or persistence of mental ill-health require further investigation. Our results support the need for research to lead to trials on continence management, focusing resources on those living without family carers, training paid carers in the early detection and warning signs of mental ill-health, and on mental health screening programmes and early intervention trials for people in higher-risk groups for incidence of mental ill-health.
Acknowledgements
We are grateful to Patricia Peters, who provided secretarial support, Andrew Williamson and the staff of the primary care liaison team. We thank study participants and their paid and family carers. The research at time 1 was funded by the Greater Glasgow Health Board and the West of Scotland Research and Development Mental Health Programme. At time 2 the research was funded by the Chief Scientist Office, Scottish Executive Health Department (reference CZH/4/96).






eLetters
No eLetters have been published for this article.