Studies of brain morphology in schizophrenia have provided overwhelming evidence of abnormal structural anatomy in people with the disorder. Two of the most consistently demonstrated structural abnormalities in schizophrenia are thalamic and hippocampal volume reduction. Reference Crespo-Facorro, Roiz-Santiáñez, Pelayo-Terán, Rodríguez-Sánchez, Pérez-Iglesias and González-Blanch1,Reference Shenton, Dickey, Frumin and McCarley2 The point in illness development at which these abnormalities arise, and their relationship to established risk factors, has however been more difficult to ascertain.
Cannabis is an environmental factor for which there is considerable evidence of risk-modifying effects. Reference Arseneault, Cannon, Witton and Murray3 Although there have been no consistent reports of brain structural abnormalities in association with use of this drug by the healthy population, Reference Martín-Santos, Fagundo, Crippa, Atakan, Bhattacharyya and Allen4 studies have reported grey matter loss in association with cannabis use in people with established schizophrenia (e.g. Rais et al Reference Rais, Cahn, Van Haren, Schnack, Caspers and Hulshoff Pol5 ). Furthermore, an earlier analysis of baseline data from the Edinburgh High-Risk Study (EHRS) found that people who are well but at genetically high risk of schizophrenia also exhibited structural brain abnormalities in association with cannabis use. Reference Welch, McIntosh, Job, Whalley, Moorhead and Hall6 In the current study we sought to examine the effects of cannabis on longitudinal thalamic and amygdala-hippocampal complex volumes within this high-risk population.
Method
Participants
Data were collected on people at elevated risk of schizophrenia as part of the EHRS. Details of this recruitment process have been described previously. Reference Hodges, Byrne, Grant and Johnstone7 In brief, individuals with schizophrenia, with a family history of schizophrenia and with relatives in adolescence/early adulthood, were identified from hospital case records. We then approached relatives aged 16–25 who were at high risk of schizophrenia: those who agreed to participate were given a detailed clinical, neuropsychological and brain imaging assessment. Assessments were repeated after approximately 2 years in consenting participants who had enrolled in the first 2 years of the study. As part of this repeat assessment, use of alcohol, tobacco and illicit drugs (including cannabis) in the interim period was ascertained by self-report. Exposures in this period were dichotomised as follows: cannabis use during this period or not; alcohol use exceeding UK government recommendations during this period (greater than 14 units/week for women and 21 units/week for men) or not; ecstasy use during this period or not; amphetamine use during this period or not; tobacco smoker during this period or not. The choice of a dichotomous rather than continuous measure of drug and alcohol use reflected the manner in which drug/alcohol use was recorded at the data collection stage.
Rationale for focusing on the thalami and amygdala-hippocampal complex
Previous reports from the EHRS have indicated that people who are clinically well but at genetically high risk of schizophrenia have reduced thalamic volume compared with controls, Reference Lawrie, Whalley, Abukmeil, Kestelman, Donnelly and Miller8 and studies have established thalamic reduction as a measure of genetic liability to psychosis. Reference McDonald, Bullmore, Sham, Chitnis, Suckling and MacCabe9,Reference McIntosh, Job, Moorhead, Harrison, Whalley and Johnstone10 Further thalamic reductions occur between the vulnerability state and frank psychosis. Reference Chan, Di, McAlonan and Gong11
In keeping with the possibility that cannabis may contribute to these changes, there are data suggesting that the thalamus is directly influenced by exposure to the drug. Cannabinoid 1 (CB1) receptors are expressed in this brain region, with highest levels of binding in the mediodorsal and anterior complex nuclei, Reference Sví$znenská, Dubový and $Snulcová12 regions which connect to cortical association areas consistently implicated in schizophrenia. Reference Shenton, Dickey, Frumin and McCarley13 Changes in thalamic regional cerebral blood flow secondary to cannabis consumption have also been reported, Reference Mathew, Wilson, Coleman, Turkington and DeGrado14,Reference O'Leary, Block, Koeppel, Flaum, Schultz and Andreasen15 and animal studies find that cannabis consumption has robust and reproducible effects on the function of several thalamic subregions. Reference Freedland, Whitlow, Miller and Porrino16 We believe that the data outlined above provide a strong rationale for examining the effect of cannabis consumption on thalamic volume in this population, and this is the primary focus of this study. It is the case, however, that the amygdala-hippocampal complex has also been shown to undergo volume loss during transition from at-risk state to schizophrenia, Reference Lawrie, McIntosh, Hall, Owens and Johnstone17 and expresses a high density of CB1 receptors. Reference Eggan and Lewis18 For these reasons, as well as to ascertain whether any thalamic volume loss observed in the thalami was specific to this structure, we also examined the effect of cannabis consumption on volume of the amygdala-hippocampal complex. We have not extended the analysis to other structures, as this would have led to issues of multiple comparisons.
Magnetic resonance imaging and analysis
Each participant underwent magnetic resonance imaging (MRI) scanning on a 1 T Siemens (Erlangen, Germany) Magnetom scanner both at baseline (T 1) and follow-up (T 2). Details of image acquisition and processing have been given elsewhere. Reference Whalley, Kestelman, Rimmington, Kelso, Abukmeil and Best19 Image processing used the software package Analyze version 7.5 for UNIX (Mayo Foundation, Rochester, Minnesota, USA) to outline the thalamus and ascertain its volume. Reference Whalley, Kestelman, Rimmington, Kelso, Abukmeil and Best19
The thalami were outlined by naturalistic boundaries, as described in previous studies. Reference Whalley, Kestelman, Rimmington, Kelso, Abukmeil and Best19,Reference Lawrie, Whalley, Abukmeil, Kestelman, Donnelly and Miller20 All thalamic nuclei were grouped together, as MRI resolution makes distinction between the individual thalamic nuclei difficult. Reference Whalley, Kestelman, Rimmington, Kelso, Abukmeil and Best19 Additionally, given the technical difficulties inherent in separating the amygdala from the hippocampus at 1 T, the amygdala-hippocampal complex was also traced as a unitary structure. Volumetric image processing was done by three investigators. The intraclass correlation coefficient between raters for the thalami was 0.84. For the amygdala-hippocampal complex it was 0.82.
Statistical analysis
Demographics and clinical variables were compared between high-risk subgroups that did and did not consume cannabis in the interim period, with the independent t-test and the χ2-test. Additionally, baseline whole-brain and region of interest volumes were compared between these two subgroups using the independent t-test.
Any differential thalamic or amygdala-hippocampal complex volume change in high-risk individuals exposed and not exposed to cannabis in the interim period was examined for right and left structures separately, using repeated-measures analysis of variance and looking for cannabis exposure×time interactions. Structure volume at T 1 and T 2 were entered as the dependent variable, gender and exposure status to cannabis, alcohol, tobacco, ecstasy and amphetamines were included as fixed factors, and age at first assessment and time span between assessments at T 1 and T 2 were entered as covariates. Standardised residuals were checked for normality. To facilitate interpretation of the findings, mean rate of change in structure volume was also calculated from the raw data. A small number of participants had used illicit drugs other than those listed above. Repeated-measures analysis was therefore re-run excluding any individuals with a history of use of any substance other than alcohol, cannabis, tobacco, ecstasy and amphetamines. None of the participants who did not consume cannabis between the two time points had used either ecstasy or amphetamines, whereas substantial numbers of individuals using cannabis had used one of these drugs. Given this imbalance of variables, and the evidence that ecstasy in particular may be specifically thalamotoxic, Reference de Win, Jager, Booij, Reneman, Schilt and Lavini21 repeated-measures analysis was also run excluding any participants who had used either of these two drugs. All analyses were conducted in SPSS (version 14) for Windows.
It was conceivable that change occurring in structure volume in association with cannabis consumption was simply part of a more generalised effect. To ascertain whether any volume changes were specific to the structures investigated, repeated-measures analysis was re-run with addition of rate of change of whole-brain volume ([WBV2 – WBV1]/time between assessments) included as an additional covariate.
Results
Scans were obtained at both time points in 66 individuals at familial high risk of schizophrenia. Substance misuse data were available for 57 of these individuals of whom 25 consumed cannabis between the two assessments.
Demographic and clinical characteristics of these individuals who did and did not consume cannabis in the interim period are shown in Table 1. In addition to the substances detailed in Table 1, two participants used opiates, two cocaine and three lysergic acid diethylamide (LSD) in the period between scans; in all cases these people were in the cannabis exposure group. At baseline there was no significant difference in whole-brain, thalamic or amygdala-hippocampal complex volumes when comparing individuals who did or did not use cannabis in the interim period. Additionally, the two groups were reasonably balanced at baseline in terms of age, gender and scores on the Rust Inventory of Schizotypal Cognitions, a measure of mild positive psychotic symptoms. Reference Rust22
The mean raw absolute volume change in the structures of interest together with mean rate of volume change ([structure volume at T 2 – structure volume at T 1]/time between scans) in both the cannabis exposed and non-exposed groups is shown in Table 2. Cannabis exposure×time interactions are also shown, after inclusion of the covariates detailed in the accompanying text. As can be seen, cannabis exposure is associated with bilateral thalamic volume loss, this effect being significant on the left (F = 4.47, P = 0.04) and highly significant on the right (F= 7.66, P= 0.008). Analysis was repeated, excluding the seven participants who consumed illicit drugs other than cannabis, ecstasy or amphetamines in the period of interest; the cannabis exposure× time interaction remained significant on both the right- and left-hand side. It was also re-run excluding the 14 people who had used either ecstasy or amphetamine in the period of interest; again, the cannabis exposure×time interaction remained significant bilaterally. Additionally, the primary analysis was also re-run with the inclusion of rate of change of whole-brain volume as an additional covariate. Once again, the cannabis exposure×time interaction remained significant on both sides. In contrast to the effects seen in the thalami, those in the amygdala-hippocampal complex were non-significant.
Discussion
The present study finds thalamic volume reduction over time in a population at high familial risk of schizophrenia who consume cannabis. This volume loss is not observed in individuals at high familial risk of schizophrenia who remain cannabis-free during the inter-scan interval.
Evidence for a role in the aetiology of schizophrenia is (arguably) stronger for cannabis than for any other putative environmental risk factor. It has also been demonstrated that the rate of grey matter loss in people recently diagnosed with schizophrenia is accelerated in those who consume the drug, Reference Rais, Cahn, Van Haren, Schnack, Caspers and Hulshoff Pol5 an
Table 1 Demographic and clinical characteristics of individuals at high familial risk of schizophrenia who did and did not consume cannabis between T 1 and T 2
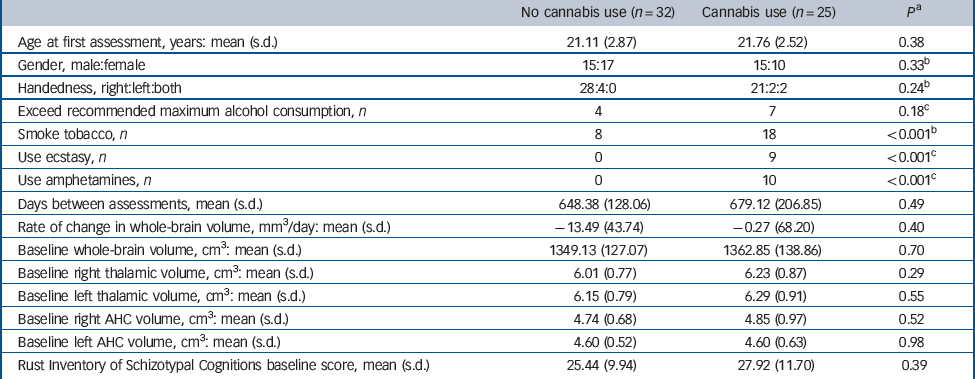
| No cannabis use (n = 32) | Cannabis use (n = 25) | P a | |
|---|---|---|---|
| Age at first assessment, years: mean (s.d.) | 21.11 (2.87) | 21.76 (2.52) | 0.38 |
| Gender, male:female | 15:17 | 15:10 | 0.33 b |
| Handedness, right:left:both | 28:4:0 | 21:2:2 | 0.24 b |
| Exceed recommended maximum alcohol consumption, n | 4 | 7 | 0.18 c |
| Smoke tobacco, n | 8 | 18 | <0.001 b |
| Use ecstasy, n | 0 | 9 | <0.001 c |
| Use amphetamines, n | 0 | 10 | <0.001 c |
| Days between assessments, mean (s.d.) | 648.38 (128.06) | 679.12 (206.85) | 0.49 |
| Rate of change in whole-brain volume, mm3/day: mean (s.d.) | –13.49 (43.74) | –0.27 (68.20) | 0.40 |
| Baseline whole-brain volume, cm3: mean (s.d.) | 1349.13 (127.07) | 1362.85 (138.86) | 0.70 |
| Baseline right thalamic volume, cm3: mean (s.d.) | 6.01 (0.77) | 6.23 (0.87) | 0.29 |
| Baseline left thalamic volume, cm3: mean (s.d.) | 6.15 (0.79) | 6.29 (0.91) | 0.55 |
| Baseline right AHC volume, cm3: mean (s.d.) | 4.74 (0.68) | 4.85 (0.97) | 0.52 |
| Baseline left AHC volume, cm3: mean (s.d.) | 4.60 (0.52) | 4.60 (0.63) | 0.98 |
| Rust Inventory of Schizotypal Cognitions baseline score, mean (s.d.) | 25.44 (9.94) | 27.92 (11.70) | 0.39 |
AHC, amygdala-hippocampal complex.
a Independent t-test.
b Chi-squared test.
c Fisher's exact test.
observation in keeping with reports that cannabis consumption may affect the course of established schizophrenia. Reference Linszen, Dingemans and Lenior23,Reference Zammit, Moore, Lingford-Hughes, Barnes, Jones and Burke24 Ours are, however, the first longitudinal data to demonstrate that this association between structure volume loss and exposure to cannabis is present in people at genetically high risk of developing schizophrenia who are currently not psychotic. This observation may be important in understanding the link between cannabis exposure and the subsequent development of schizophrenia.
Importance of the thalamus
The structure in which we observe this progressive cannabis-associated volume loss, the thalamus, is believed to function as an information-processing and relay station. It is a conduit for bidirectional flow of signals between cortical and subcortical regions, links different cortical regions via transthalamic pathways and is a point of convergence for frontostriatal and cerebellothalamo-cortical circuits. Reference Byne, Hazlett, Buchsbaum and Kemether25 Given this role of interconnecting diverse brain regions, it would be expected that thalamic lesions would have widespread consequences. It may also be expected, given that schizophrenia is increasingly regarded as a disorder of connectivity, Reference Friston and Frith26 that these may resemble schizophrenia. This is indeed what has been demonstrated by studies examining the consequences of thalamic vascular insults; such lesions resulting
Table 2 Comparison of structure volume changes between scans in individuals at high familial risk of schizophrenia, exposed and not exposed to cannabis

| Absolute change (scan 2–scan 1), mm3: mean (s.d.) | Rate of change ([scan 2–scan 1]/time), mm3/day: mean (s.d.) | Exposure × time interaction a | |||
|---|---|---|---|---|---|
| No cannabis exposure | Cannabis exposure | No cannabis exposure | Cannabis exposure | ||
| Right thalamus | 32.84 (509.35) | –264.48 (621.70) | 0.06 (0.80) | –0.36 (0.92) | F = 7.66, P < 0.01 |
| Left thalamus | 26.66 (667.58) | –181.39 (621.70) | 0.07 (1.00) | –0.22 (1.01) | F = 4.47, P = 0.04 |
| Right amygdala-hippocampal complex | 147.77 (563.94) | 175.31 (432.19) | 0.18 (0.95) | 0.19 (0.66) | F = 0.71, P = 0.40 |
| Left amygdala-hippocampal complex | –121.37 (488.70) | –4.43 (384.77) | –0.23 (0.81) | –0.03 (0.59) | F = 0.01, P = 0.92 |
a Investigation of exposure × time interactions has been undertaken with inclusion of the following covariates: gender, age at first assessment, time between scans and use of other substances in inter-scan period (alcohol exceeding government recommendations, tobacco use, use of ecstasy and use of amphetamines each being included as separate factors).
in a variety of schizophrenia-like presentations, with negative symptoms being particularly prominent. Reference Byne, Hazlett, Buchsbaum and Kemether25
The prediction that thalamic dysfunction will result in impairment of proper communication between different brain regions is central to many of the models proposed to explain the pathophysiology of schizophrenia. Andreasen et al, for example, proposed that a disruption in prefrontal-thalamic-cerebellar circuitry may contribute to the cognitive disturbances that are characteristic of the condition. Reference Andreasen, Paradiso and O'Leary27,Reference Andreasen, Nopoulos, O'Leary, Miller, Wassink and Flaum28 In keeping with this, functional imaging has previously demonstrated that this network is indeed disrupted in the EHRS cohort, Reference Whalley, Simonotto, Flett, Marshall, Ebmeier and Owens29 a population known to exhibit subtle impairments in the cognitive domains affected in schizophrenia. Reference Byrne, Hodges, Grant, Owens and Johnstone30,Reference O'Connor, Harris, McIntosh, Owens, Lawrie and Johnstone31 Other neural models have focused on the connections the thalamus has with the dorsolateral prefrontal cortex and the potential impact of thalamic dysfunction on working memory. Reference Carlsson and Carlsson32,Reference Jones33 Still others have emphasised the potential role of the thalamus in impaired sensory gating. Reference Mega and Cummings34 Intriguingly, neuropsychological testing has demonstrated that within a population with schizophrenia, thalamic volume is positively associated with global neuropsychological performance as well as scores in the specific domains of motor, language and executive functioning. Reference Coscia, Narr, Robinson, Hamilton, Sevy and Burdick35
Although diverse, the models outlined have a common implication: given the extensive connections of the thalamus, even relatively minor abnormalities of this structure could potentially have dramatic consequences. Even though a genetic propensity to schizophrenia is itself associated with reduced thalamic volume, further volume reductions occur between the vulnerability state and frank psychosis. Reference Chan, Di, McAlonan and Gong11 By demonstrating that cannabis use by a vulnerable population is associated with thalamic volume loss, the current findings raise the possibility that this drug may be a factor which increases the likelihood of such abnormalities arising. This may lead to a worsening of previously subtle symptomatology and ultimately influence the risk of transition to schizophrenia.
Self-medication
Although substantial data suggest that cannabis use is a risk factor for schizophrenia, a frequently posited non-causative explanation for its association with the condition is the self-medication hypothesis. In applying this explanation to the current data, it would be argued that the experience of schizophrenic-type symptomatology leads to both cannabis use and thalamic volume loss. We believe such an explanation unlikely, it being undermined by the fact that individuals in this study were all well between the two assessment points. Although some participants in the EHRS did show transient or partial psychotic symptoms, there was no significant difference in ratings on the Rust Inventory of Schizotypal Cognitions at baseline between those who did and did not use cannabis between the scan points. The possibility that biological processes associated with the development of psychotic symptoms is driving both the thalamic volume loss and cannabis use (and hence the association between the two) also seems unlikely given previous findings from the EHRS demonstrating no thalamic volume change when those with or without psychotic symptoms are compared. Reference Lawrie, Whalley, Abukmeil, Kestelman, Miller and Best36 Consequently, we feel that the most likely explanation for the effects seen is that they are indeed secondary to cannabis exposure, the current data therefore strengthening the supposition that cannabis can play an aetiological role in the development of schizophrenia.
Strengths and limitations
A limitation of this study is that we did not examine the impact of cannabis use in a non-high-risk comparator group, raising the possibility that the effects observed are a normal consequence of cannabis use rather than specific to those at elevated genetic risk of schizophrenia. Unfortunately, too few of the control participants in the EHRS (only four) consumed cannabis in the interim period for such an analysis to be statistically meaningful. As discussed in the introduction, however, there is little evidence of cannabis-associated brain structural abnormalities in the healthy population. Reference Martín-Santos, Fagundo, Crippa, Atakan, Bhattacharyya and Allen4 It therefore seems highly unlikely that the effects we observe would also be seen in a population not at genetically high risk of schizophrenia.
Further limitations of the study are that it was inadequately powered to establish whether thalamic volume loss in association with cannabis use predicts subsequent schizophrenia (although we do know that seven of the high-risk participants did develop the condition at some point after the second scan), or to explore dose–response effects.
We were also unable to establish whether volume loss was attributable to changes in specific thalamic nuclei. This was outwith the scope of this particular research, but clearly would be of significant interest. Not least, it would suggest the regions in which we may expect to find the cannabis-associated abnormalities of brain connectivity that may be central in understanding how thalamic abnormalities contribute to the symptomatology of schizophrenia. Previous research using a statistical modelling approach to analyse shape reported that inward deformations of the anterior and posterior thalamus are more pronounced in people with schizophrenia than their unaffected relatives (who were intermediate between those with schizophrenia and healthy controls). Reference Harms, Wang, Mamah, Barch, Thompson and Csernansky37 Additionally, post-mortem studies of people who had schizophrenia have observed reduced volume and/or neuronal numbers in the mediodorsal nucleus, anterior nucleus and pulvinar. Reference Cronenwett, Csernansky, Kendall and Alexander38,Reference Young, Manaye, Liang, Hicks and German39 These findings have generally been in agreement with structural MRI studies. Reference Sim, Cullen, Ongur and Heckers40 Clearly, more accurate localisation of the regions of thalamic volume loss secondary to cannabis use, together with identification of any cannabis-associated thalamocortical connectivity changes, will be an important focus of future work in this field. As this paper demonstrates, negative findings when such studies are undertaken in the normal cannabis-using population does not preclude the possibility that significant effects are seen in populations with a genetic loading for schizophrenia.
Funding
This work was funded by the UK Medical Research Council and the Dr Mortimer and Theresa Sackler Foundation.





eLetters
No eLetters have been published for this article.