Right-handedness, left cerebral dominance for language and normal cerebral asymmetry are considered to be secondary to a dominant allele, the ‘right-shift factor’ (Reference AnnettAnnett, 1998). In schizophrenia, several studies reported an excess of non-right-handedness (Reference Shan-Ming, Flor-Henry and DayiShan-Ming et al, 1985), decreased language lateralisation on the dichotic listening paradigm (Reference Bruder, Kayser and TenkeBruder et al, 1999) and decreased (Reference Petty, Barta and PearlsonPetty et al, 1995) or reversed (Reference Barta, Pearlson and BrillBarta et al, 1997) anatomical asymmetry. It has been postulated that the genetic mechanism underlying normal left hemispheric dominance is altered in schizophrenia (Reference Crow, Ball and BloomCrow et al, 1989). Were this to be true, the discovery of the ‘right-shift factor’ may also identify a locus where genetic aberrations predispose for schizophrenia (Reference CrowCrow, 1999). However, literature on decreased cerebral dominance in schizophrenia is equivocal. The aim of the present study was therefore to review quantitatively studies on handedness, language lateralisation on the dichotic listening paradigm and anatomical asymmetry in schizophrenia.
METHOD
Literature search
Studies were retrieved that dealt with lateralisation in schizophrenia, published between January 1980 (introduction of Diagnostic and Statistical Manual of Mental Disorders (3rd edn), DSM-III) and December 1999. Only English-language publications from international journals and book chapters were selected.
Inclusion
The included studies had to meet the following criteria.
-
(a) Patients had a diagnosis of schizophrenia, according to the criteria of DSM-III, DSM-III-R, DSM-IV, Research Diagnostic Criteria (RDC), International Classification of Disease ICD-9 or ICD-10. Studies on patients with schizophrenia-spectrum disorder (schizoaffective and schizophreniform disorder or schizotypal personality disorder) were not included.
-
(b) Studies had compared patients with schizophrenia to healthy controls who were not at increased risk for schizophrenia or psychosis. For an additional analysis on hand preference, we also included studies using non-schizophrenia psychiatric and neurological patients as control groups.
-
(c) Studies reported data on handedness, language lateralisation as measured with the dichotic listening paradigm, or anatomical asymmetry of the planum temporale, Sylvian fissure, temporal horn of the lateral ventricle or superior temporal gyrus.
-
(d) Studies had reported sufficient statistical information, i.e. frequencies, means and standard deviations, exact F or t values, or exact P values.
Analyses
After computing effect sizes for each study (Reference Hedges and OlkinHedges & Olkin, 1985), meta-analytic methods were applied in order to obtain a combined effect size, which indicated the magnitude of the association across all studies (cf Reference Aleman, Hijman and de HaanAleman et al, 1999). In addition, Stouffer's Z, weighted for sample size, provided an indication of the significance of the difference between the patient and the comparison group. Finally, a homogeneity statistic (Q) was calculated, to assess heterogeneity of results across studies (Reference Shadish, Haddock, Cooper and HedgesShadish & Haddock, 1994). If heterogeneity reached significance, further analyses were carried out to examine potential moderators on the effect size. In order to investigate such potential moderators, correlations (Pearson coefficients, two-tailed) were calculated between the effect size of the studies and available variables.
Handedness
Prevalences of mixed- and left-handedness were grouped together as ‘non-right-handedness’. For each study, odds ratios were calculated from the prevalence of non-right-handedness in schizophrenia or pre-schizophrenia subjects and in the comparison subjects. Odds ratios were combined by applying the method of logarithmic odds ratio meta-analysis (Reference Shadish, Haddock, Cooper and HedgesShadish & Haddock, 1994).
Dichotic listening studies
Effect sizes (Reference Hedges and OlkinHedges & Olkin, 1985) were calculated from the ‘right-ear advantages’ (score right ear minus score left ear) of patients and controls, from the ‘laterality index’ (score right ear minus score left ear, divided by score right ear plus score left ear) or from F values.
Anatomical asymmetry
Brain torque
Only two studies on brain torque (Reference Luchins, Morihisa and WeinbergerLuchins et al, 1981; Guerguerian & Lewine, 1998) reported means and standard deviations of an asymmetry index for patients and controls, while others gave frequencies of abnormal frontal and occipital asymmetry. On these studies, meta-analysis was performed using the ‘difference rate’, i.e. the difference between the proportion of individuals with absent or reversed asymmetry in the patient group and in the control group. For the meta-analyses on asymmetry of the planum temporale, Sylvian fissure, temporal horn of the lateral ventricle and superior temporal gyrus, effect sizes were computed from the mean size (and standard deviation) of left and right structures. The measurements concerned absolute structure sizes, not corrected for total brain volume, and total structure sizes (not only grey matter volumes). When studies gave separate data for men and women, these were included as two independent effect sizes, thereby increasing the total number of effect sizes (K).
Each study was used for three meta-analyses. In the first, asymmetry of the structure was calculated for the control subjects. In the second, asymmetry was calculated for the patients. The third meta-analysis was conducted on the difference of the two d values of each group and compared asymmetry in patients to that in controls. In this way, differences in overall brain size between patients and controls could not influence the outcome, as every structure was compared to its contralateral homologue first, after which the resulting standardised asymmetry indices were compared between the groups. Differences in measurement technique between the studies were largely controlled for, as well as the statistic results from within-study comparisons.
When studies that measured the superior temporal gyrus provided separate data on anterior, middle and posterior segments, these were pooled.
All studies had included predominantly right-handed subjects, except for Holinger et al (Reference Holinger, Shenton and Wible1999), who measured superior temporal gyral volumes in left-handed patients with schizophrenia and left-handed controls. To increase homogeneity among studies, this study was excluded in the meta-analysis, but included for the calculation of a possible correlation between handedness distribution and asymmetry.
RESULTS
Handedness
Sixteen cross-sectional studies that assessed handedness in patients with schizophrenia and healthy subjects were included (Reference Taylor, Dalton and FlemingerTaylor et al, 1980; Reference Chaugule and MasterChaugule & Master, 1981; Reference Piran, Bigler and CohenPiran et al, 1982; Reference Kameyama, Niwa, Hiramatsu, Flor-Henry and GruzelierKameyama et al, 1983; Reference Manschreck and AmesManschreck & Ames, 1984; Reference MerrinMerrin, 1985; Reference Shan-Ming, Flor-Henry and DayiShan-Ming et al, 1985; Reference Green, Satz and SmithGreen et al, 1989; Reference Nelson, Satz and GreenNelson et al, 1993; Reference Clementz, Jacono and BeiserClementz et al, 1994; Reference Cannon, Byrne and CassidyCannon et al, 1995; Reference Green and RiegGreen & Rieg, 1995; Reference O'Callaghan, Buckley and MadiganO'Callaghan et al, 1995; Reference Taylor and AmirTaylor & Amir, 1995; Reference Malesu, Cannon and JonesMalesu et al, 1996; Reference Orr, Cannon and GilvarryOrr et al, 1999).
Meta-analysis on these studies showed that the prevalence of non-right-handedness was significantly higher in patients with schizophrenia than in healthy subjects (Table 1). The magnitude of the odds ratios of each study is shown in Fig. 1.
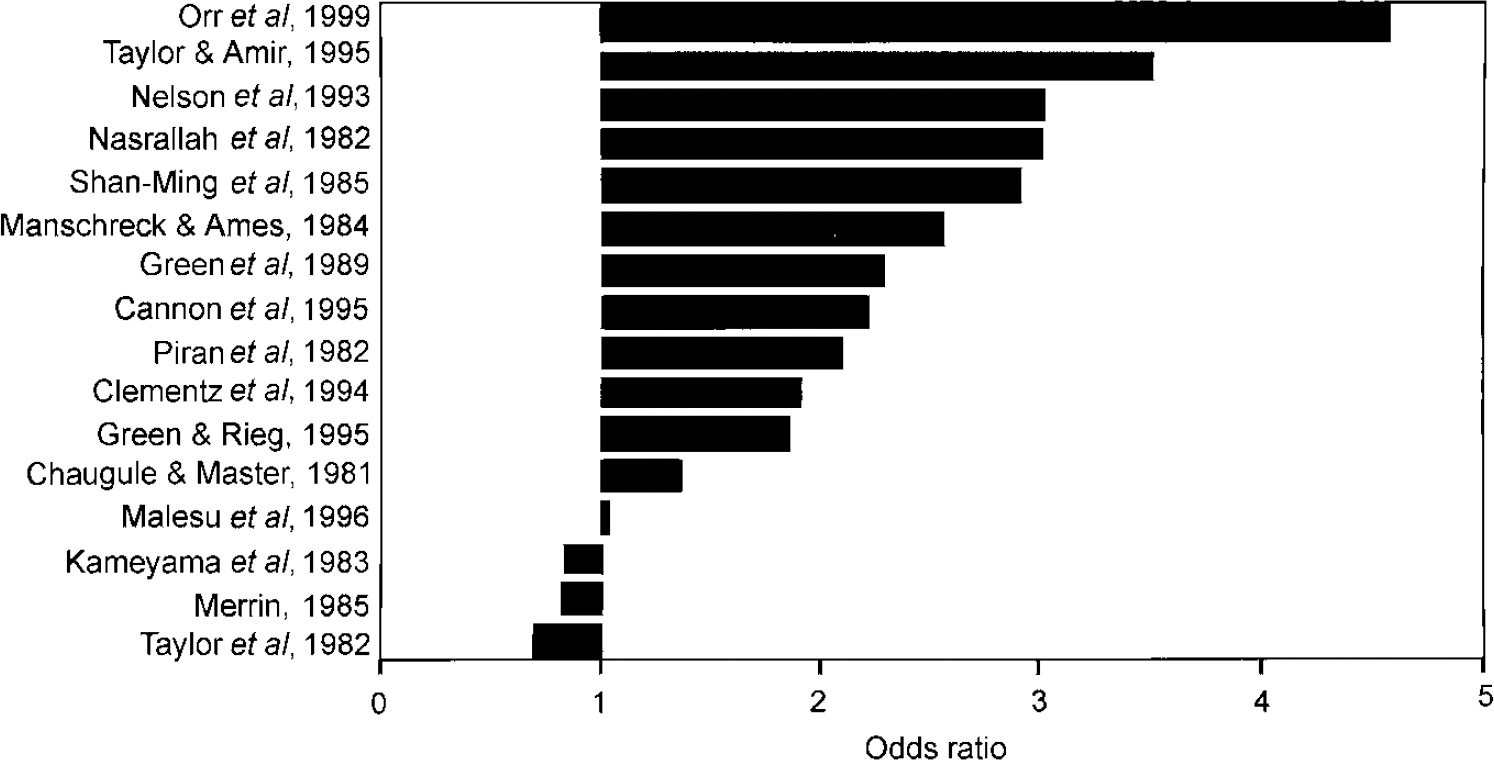
Fig. 1 Odds ratios of non-right-handedness in schizophrenia.
Table 1 Summary of meta-analyses of difference in cerebral dominance between patients with schizophrenia and comparison subjects
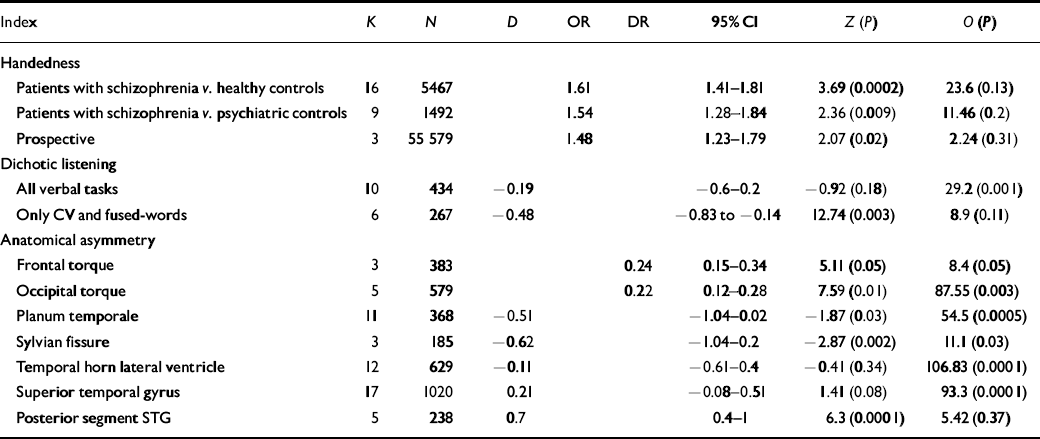
| Index | K | N | D | OR | DR | 95% Cl | Z (P) | O (P) |
|---|---|---|---|---|---|---|---|---|
| Handedness | ||||||||
| Patients with schizophrenia v. healthy controls | 16 | 5467 | 1.61 | 1.41-1.81 | 3.69 (0.0002) | 23.6 (0.13) | ||
| Patients with schizophrenia v. psychiatric controls | 9 | 1492 | 1.54 | 1.28-1.84 | 2.36 (0.009) | 11.46 (0.2) | ||
| Prospective | 3 | 55 579 | 1.48 | 1.23-1.79 | 2.07 (0.02) | 2.24 (0.31) | ||
| Dichotic listening | ||||||||
| All verbal tasks | 10 | 434 | -0.19 | -0.6-0.2 | -0.92 (0.18) | 29.2 (0.001) | ||
| Only CV and fused-words | 6 | 267 | -0.48 | -0.83 to - 0.14 | 12.74 (0.003) | 8.9 (0.11) | ||
| Anatomical asymmetry | ||||||||
| Frontal torque | 3 | 383 | 0.24 | 0.15-0.34 | 5.11 (0.05) | 8.4 (0.05) | ||
| Occipital torque | 5 | 579 | 0.22 | 0.12-0.28 | 7.59 (0.01) | 87.55 (0.003) | ||
| Planum temporale | 11 | 368 | -0.51 | -1.04-0.02 | -1.87 (0.03) | 54.5 (0.0005) | ||
| Sylvian fissure | 3 | 185 | -0.62 | -1.04-0.2 | -2.87 (0.002) | 11.1 (0.03) | ||
| Temporal horn lateral ventricle | 12 | 629 | -0.11 | -0.61-0.4 | -0.41 (0.34) | 106.83 (0.0001) | ||
| Superior temporal gyrus | 17 | 1020 | 0.21 | -0.08-0.51 | 1.41 (0.08) | 93.3 (0.0001) | ||
| Posterior segment STG | 5 | 238 | 0.7 | 0.4-1 | 6.3 (0.0001) | 5.42 (0.37) |
Three prospective follow-up studies measured hand preference in children of large birth cohorts, who were screened for schizophrenia in adult life (Reference David, Malmberg and LewisDavid et al, 1995; Reference Crow, Done and SackerCrow et al, 1996; Reference Cannon, Jones and MurrayCannon et al, 1997). From these, only one study (Reference Crow, Done and SackerCrow et al, 1996) provided quantitative indices on the degree of handedness. In the meta-analysis of these studies, pre-schizophrenic subjects were significantly more often non-right-handed than were the general population (Table 1).
Additional analysis was performed on nine studies where the comparison group consisted of non-schizophrenia psychiatric or neurological patients (Reference Chaugule and MasterChaugule & Master, 1981; Reference Piran, Bigler and CohenPiran et al, 1982; Reference Manschreck and AmesManschreck & Ames, 1984; Reference MerrinMerrin, 1985; Reference Shan-Ming, Flor-Henry and DayiShan-Ming et al, 1985; Reference Clementz, Jacono and BeiserClementz et al, 1994; Reference Taylor and AmirTaylor & Amir, 1995; Reference Malesu, Cannon and JonesMalesu et al, 1996; Reference Orr, Cannon and GilvarryOrr et al, 1999). The results indicated that the prevalence of non-right-handedness was significantly higher in schizophrenia subjects than in other psychiatric and neurological patients (Table 1).
Dichotic listening studies
Meta-analysis was conducted on 10 dichotic listening studies that compared patients with schizophrenia to healthy controls (Reference Hatta, Ayetani and YoshizakiHatta et al, 1984; Reference Wexler, Giller and SouthwickWexler et al, 1991; Reference Carr, Wale and DewisCarr et al, 1992; Reference Ragland, Goldberg and WexlerRagland et al, 1992; Reference Seidman, Pepple and FaraoneSeidman et al, 1993; Reference Grosh, Docherty and WexlerGrosh et al, 1995; Reference Sakuma, Hoff and DeLisiSakuma et al, 1996; Reference Oie, Rund and SundetOie et al, 1998; Reference Bruder, Kayser and TenkeBruden et al, 1999; Reference L⊘berg, Hughdahl and GreenLøberg et al, 1999). The right-ear advantage was not significantly decreased in schizophrenia, with significant heterogeneity among studies (Table 1). The magnitude of the effect size of each study is represented in Fig. 2.
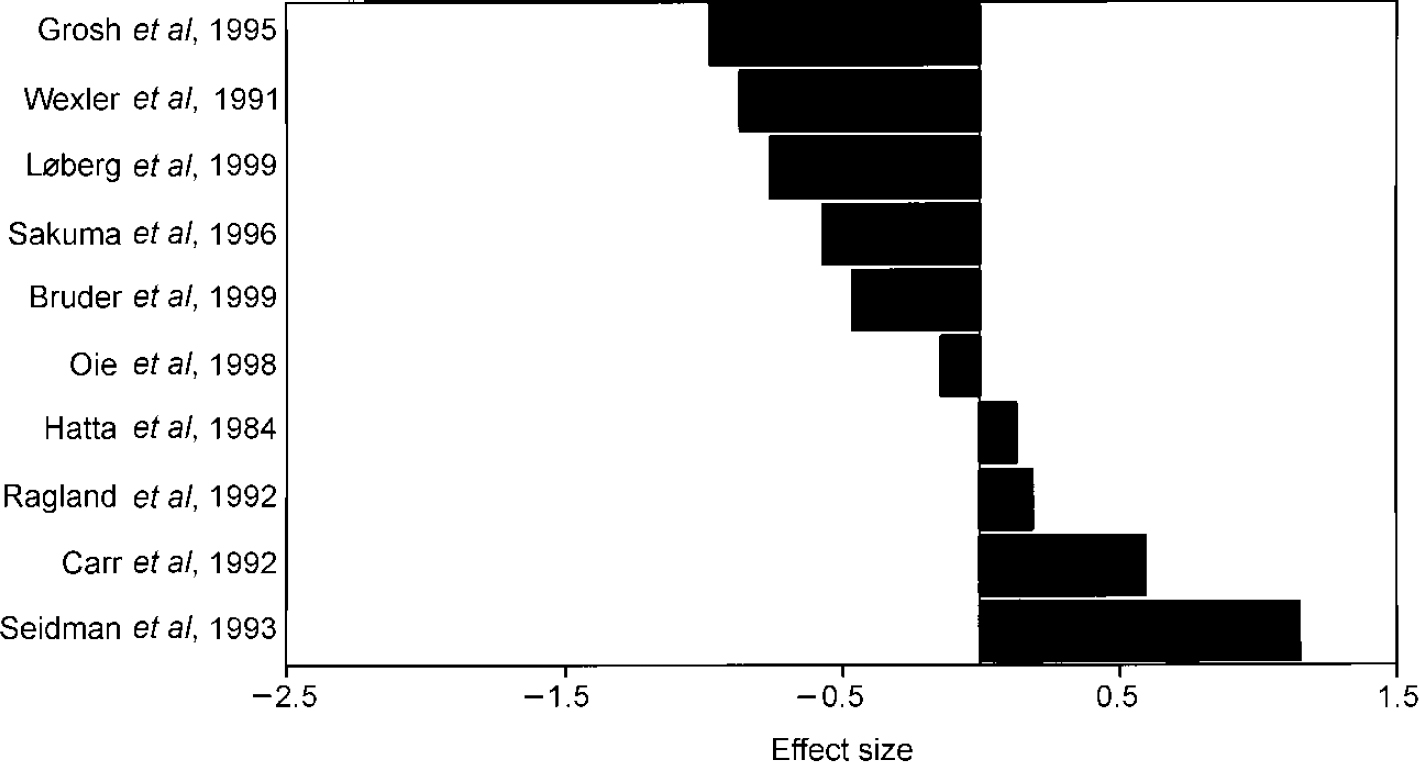
Fig. 2 Effect sizes of dichotic listening studies in schizophrenia (patients—controls).
A possible cause for the inhomogeneity in these studies may be the difference in verbal tasks the studies used to elicit a right-ear advantage. Four different tasks were used: the triad task, the fused-word task, the consonant—vowel task and the word-monitoring task. The fused-word and the consonant—vowel tasks are considered to reflect cerebral dominance most accurately (see Discussion). When only studies that used the fused-word and the consonant-vowel tasks were included, the right-ear advantage was significantly lower in schizophrenia and studies were no longer heterogeneous (Table 1).
Anatomical asymmetry
Brain torque
Meta-analyses were conducted on the frequency of abnormal asymmetry of the frontal lobe (Reference Andreasen, Dennert and OlsenAndreasen et al, 1982; Reference Jernigan, Zatz and MosesJernigan et al, 1982; Reference Falkai, Schneider and GreveFalkai et al, 1995b ) and the occipital lobe (Reference Andreasen, Dennert and OlsenAndreasen et al, 1982; Reference Jernigan, Zatz and MosesJernigan et al, 1982; Luchins & Meltzer, Reference Luchins and Meltzer1983, Reference Luchins and Meltzer1986; Reference Falkai, Schneider and GreveFalkai et al, 1995b ). The results showed that the frequency of abnormal asymmetry was significantly higher in schizophrenia for both the frontal and the occipital lobe, but studies were heterogeneous (Table 1). The small number of studies allowed no further investigation of potential moderators.
Planum temporale
Meta-analysis was performed on 10 studies that measured the planum temporale in schizophrenia (Rossi et al, Reference Rossi, Stratta and Mattel1992, Reference Rossi, Serio and Stratta1994; Reference Kleinschmidt, Falkai and HuangKleinschmidt et al, 1994; Reference Falkai, Bogerts and SchneiderFalkai et al, 1995a ; Reference Kulynych, Vladar and JonesKulynych et al, 1995; Reference Petty, Barta and PearlsonPetty et al, 1995; Reference Jacobsen, Giedd and VaituzisJacobsen et al, 1996; Reference Barta, Pearlson and BrillBarta et al, 1997; Reference Frangou, Sharma and SigmudssonFrangou et al, 1997; Reference Kwon, McCarley and HirayasuKwon et al, 1999). In the meta-analysis, significant asymmetry favouring the left hemisphere was found for the healthy comparison subjects, but not for the patients (Table 2). Studies were homogeneous for the comparison subjects, but heterogeneous for the patients, subjects, but heterogeneous for the patients, indicating that difference in measurement technique is not a factor. Possible moderators may be found in characteristics of the patient samples. Only three variables were reported frequently enough to calculate correlations, but no significant result emerged: gender distribution n=10, r=-0.25, NS; handedness distribution n=9, r=0.38, NS; duration of illness n=6, r=-0.29, NS.
Table 2 Summary of meta-analyses of difference in size between left and right hemispheric structures in controls and patients
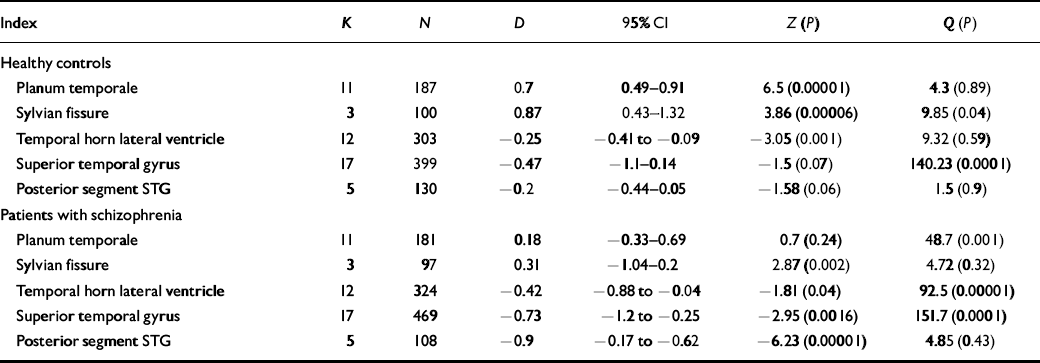
| Index | K | N | D | 95% CI | Z (P) | Q (P) |
|---|---|---|---|---|---|---|
| Healthy controls | ||||||
| Planum temporale | 11 | 187 | 0.7 | 0.49-0.91 | 6.5 (0.00001) | 4.3 (0.89) |
| Sylvian fissure | 3 | 100 | 0.87 | 0.43-1.32 | 3.86 (0.00006) | 9.85 (0.04) |
| Temporal horn lateral ventricle | 12 | 303 | -0.25 | -0.41 to ‒0.09 | -3.05 (0.001) | 9.32 (0.59) |
| Superior temporal gyrus | 17 | 399 | -0.47 | -1.1-0.14 | -1.5 (0.07) | 140.23 (0.0001) |
| Posterior segment STG | 5 | 130 | -0.2 | -0.44-0.05 | -1.58 (0.06) | 1.5 (0.9) |
| Patients with schizophrenia | ||||||
| Planum temporale | 11 | 181 | 0.18 | -0.33-0.69 | 0.7 (0.24) | 48.7 (0.001) |
| Sylvian fissure | 3 | 97 | 0.31 | -1.04-0.2 | 2.87 (0.002) | 4.72 (0.32) |
| Temporal horn lateral ventricle | 12 | 324 | -0.42 | -0.88 to ‒0.04 | -1.81 (0.04) | 92.5 (0.00001) |
| Superior temporal gyrus | 17 | 469 | -0.73 | -1.2 to ‒0.25 | -2.95 (0.0016) | 151.7 (0.0001) |
| Posterior segment STG | 5 | 108 | -0.9 | -0.17 to ‒0.62 | -6.23 (0.00001) | 4.85 (0.43) |
In the meta-analysis directly comparing patients and controls, asymmetry of the planum temporale was significantly reduced in patients (Table 1). The magnitude of the difference in effect sizes of asymmetry between patients and controls is shown in Fig. 3.
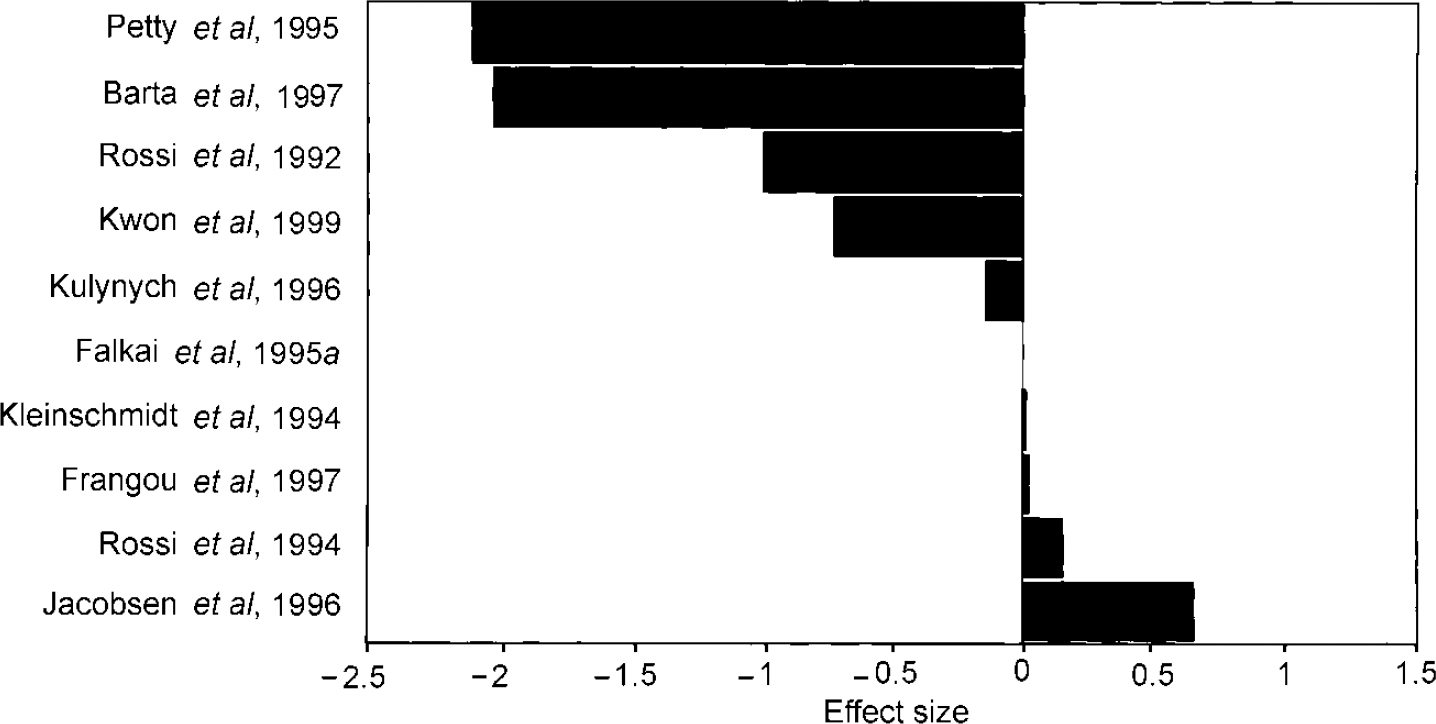
Fig. 3 Difference in asymmetry (patients—controls) of the planum temporale.
Sylvian fissure
In the meta-analysis on studies on asymmetry of the Sylvian fissure (Reference Falkai, Bogerts and GreveFalkai et al, 1992; Reference Hoff, Riordan and O'DonnellHoff et al, 1992; Reference Bartley, Jones and TorreyBartley et al, 1993), both controls and patients showed significant asymmetry favouring the left hemisphere (Table 2), but studies were heterogeneous for both groups. The small number of studies allowed no further investigation of potential moderators. When asymmetry of the Sylvian fissure was directly compared between patients with schizophrenia and healthy subjects, patients showed significantly decreased asymmetry (Table 1).
Temporal horn of the lateral ventricle
Eight studies on asymmetry of the temporal horn of the lateral ventricle were included (Reference Bogerts, Ashtari and DegreefBogerts et al, 1990; Reference Dauphinais, DeLisi and CrowDauphinais et al, 1990; Reference DeLisi, Stritzke and HolanDeLisi et al, 1991; Reference Zipursky, Marsh and LimZipursky et al, 1994; Reference Flaum, O'Leary and SwayzeFlaum et al, 1995; Reference Becker, Elmer and SchneiderBecker et al, 1996; Reference Marsh, Harris and LimMarsh et al, 1997; Reference Pearlson, Barta and PowersPearlson et al, 1997). In healthy as well as in schizophrenia subjects, rightward asymmetry was found (Table 2). Studies were homogeneous for the analysis of control subjects, but heterogeneous in the patients' analysis (Table 2). Correlations between asymmetry in patients and possible moderators were not significant: gender distribution n=8, r=-0.44, NS; handedness distribution n=5, r=0.27, NS; duration of illness n=6, r=-0.7, NS. In the meta-analysis directly comparing patients and controls, asymmetry of the temporal horn was not significantly different between schizophrenia and healthy subjects (Table 1).
Superior temporal gyrus
Meta-analysis was performed on 15 studies measuring superior temporal gyrus in schizophrenia subjects and healthy controls (Reference Barta, Pearlson and PowersBarta et al, 1990; Reference Zipursky, Marsh and LimZipursky et al, 1994; Reference Flaum, O'Leary and SwayzeFlaum et al, 1995; Reference Menon, Barta and AylwardMenon et al, 1995; Reference Vita, Dieci and GiobbioVita et al, 1995; Reference Kulynych, Vladar and JonesKulynych et al, 1996; Reference Frangou, Sharma and SigmudssonFrangou et al, 1997; Reference Marsh, Harris and LimMarsh et al, 1997; Reference Pearlson, Barta and PowersPearlson et al, 1997; Reference Reite, Sheeder and TealeReite et al, 1997; Reference Hirayasu, Shenton and SalisburyHirayasu et al, 1998; Reference Jacobsen, Giedd and CastelianosJacobsen et al, 1998; Reference Bryant, Buchanan and VladarBryant et al, 1999; Reference Havermans, Honig and VuurmanHavermans et al, 1999; Reference Highley, McDonald and WalkerHighley et al, 1999).
The results showed that the superior temporal gyrus was larger in the right hemisphere in schizophrenia subjects, while controls showed a trend towards asymmetry in the same direction (Table 2). Studies were heterogeneous for both groups and thus differences in measurement technique may have acted as moderators. The only study on post-mortem brains (Reference Highley, McDonald and WalkerHighley et al, 1999) found larger volumes of the superior temporal gyrus in the left hemisphere in controls, while 13 of 14 magnetic resonance imaging (MRI) studies found larger volumes in the right hemisphere in controls. However, when the post-mortem study was excluded from analysis, heterogeneity among studies remained high. Correlations between the effect size of asymmetry in controls and other potential moderators were not significant (slice and gap thickness n=15, r=-0.039, NS; sample size n=15, r=-0.22, NS; gender distribution n=15, r=-0.29, NS; handedness distribution n=15, r=0.32, NS). Correlations between potential moderators and the effect size of asymmetry in patients were not significant either (slice and gap thickness n=15, r=0.15, NS; sample size n=15, r=-0.18, NS; gender distribution n=15, r=-0.27, NS; handedness distribution n=15, r=-0.29, NS; duration of illness n=5, r=-0.51, NS).
In the meta-analysis directly comparing patients and controls, the patients with schizophrenia tended to have increased asymmetry of the superior temporal gyrus favouring the right hemisphere, but statistical significance was not reached (Table 1).
Posterior segment of the superior temporal gyrus
A separate analysis on five studies (Reference Menon, Barta and AylwardMenon et al, 1995; Reference Kulynych, Vladar and JonesKulynych et al, 1996; Reference Pearlson, Barta and PowersPearlson et al, 1997; Reference Hirayasu, Shenton and SalisburyHirayasu et al, 1998; Reference Jacobsen, Giedd and CastelianosJacobsen et al, 1998) was performed to assess asymmetry of the posterior segment of the superior temporal gyrus. Rightward asymmetry was found for the patients, while the controls showed a trend towards asymmetry in the same direction (Table 2). In the meta-analysis directly comparing patients and controls, rightward asymmetry of the posterior segment of the superior temporal gyrus was significantly larger in patients (Table 1).
DISCUSSION
The purpose of this study was to summarise present literature on cerebral dominance in schizophrenia quantitatively. The resulting analyses showed that functional asymmetry is decreased in schizophrenia, which was reflected in an increased prevalence of non-right-handedness and decreased language lateralisation in dichotic listening studies that applied the fused-word or consonant-vowel task. Decreased structural asymmetry in schizophrenia was found for brain torque, the planum temporale and Sylvian fissure, but not for the temporal horn of the lateral ventricle.
Hand preference
In the meta-analysis on handedness in cross-sectional studies, schizophrenia subjects were more frequently non-right-handed than healthy persons. However, these studies mainly included hospitalised patients with schizophrenia, thus tending to overrepresent severe cases. Prospective cohort studies do not have this limitation, since they are population-based. In the meta-analysis on three prospective cohort studies, the prevalence of non-right-handedness in subjects that later developed schizophrenia was significantly increased.
However, many diseases that involve subtle brain damage may be accompanied by increased prevalence of non-right-handedness (Reference Satz and GreenSatz & Green, 1999). To investigate the specificity of increased non-right-handedness in schizophrenia, an additional analysis was conducted comparing schizophrenia patients with non-schizophrenia psychiatric and neurological patients. In that analysis, non-right-handedness was still significantly increased in schizophrenia, suggesting that a specific cerebral lesion cannot explain the increased prevalence of non-right-handedness in schizophrenia. Thus, a more fundamental, possibly genetic mechanism may be involved. Interestingly, several studies report increased non-right-handedness in healthy relatives of patients with schizophrenia (Reference Hallett, Quinn and HewittHallett et al, 1986; Reference Chapman and ChapmanChapman & Chapman, 1987; Reference Orr, Cannon and GilvarryOrr et al, 1999), suggesting a genetic cause for decreased cerebral dominance in schizophrenia (Reference Crow, Ball and BloomCrow et al, 1989).
Dichotic listening studies
In the analysis of dichotic listening studies, the right-ear advantage was not significantly decreased in schizophrenia, while heterogeneity among studies was high. This may be attributable to the differences in verbal tasks used to elicit a right-ear advantage. For the triad task and the word-monitoring task, subjects have to respond to all stimuli on either ear. Schizophrenia subjects generally have a lower performance on these tasks than healthy subjects (Reference Hatta, Ayetani and YoshizakiHatta et al, 1984; Reference Carr, Wale and DewisCarr et al, 1992; Reference Seidman, Pepple and FaraoneSeidman et al, 1993; Reference Sakuma, Hoff and DeLisiSakuma et al, 1996). On several items of these tasks, controls may have 100% correct response, in which case no perceptual asymmetry is measured. These ceiling effects in the control, but not in the schizophrenia group, may cause relatively low ear asymmetry in the control group. For the consonant-vowel and the fused-word tasks, subjects were asked to respond only to the most clearly perceived item, thereby avoiding the problem of ceiling effects. When only studies that applied the consonant-vowel or fused-word task were included, patients with schizophrenia showed a significantly decreased right-ear advantage and heterogeneity disappeared. A decreased right-ear advantage was also reported in healthy parents (Reference Grosh, Docherty and WexlerGrosh et al, 1995) and children (Reference Hallett, Quinn and HewittHallett et al, 1986) of patients with schizophrenia, supporting the hypothesised genetic origin of decreased lateralisation in schizophrenia.
Anatomical asymmetry
In the meta-analysis of anatomical studies, the direction of brain torque was more frequently inverted, while the planum temporale and Sylvian fissure showed reduced asymmetry in schizophrenia. The decreased temporo-parietal asymmetries probably reflect decreased language dominance, since planum temporale and Sylvian fissure asymmetries are strongly related to cerebral dominance (Reference Gerschlager, Lalouschek and LehrnerGerschlager et al, 1998). Shapleske et al (Reference Shapleske, Rossell and Woodruff1999) published an extensive review on the planum temporale that also contained a meta-analysis on the planum temporale in schizophrenia. The present study, using more stringent inclusion criteria and including several recently published studies, confirms Shapleske et al's finding of decreased asymmetry of the planum temporale in schizophrenia.
Crow et al (Reference Crow, Ball and Bloom1989) reported that the temporal horn of the lateral ventricle was larger in the right hemisphere in the normal control group of their post-mortem study, probably owing to the more extended language-related cortex at the dominant side. The present results from the meta-analysis on healthy subjects confirm this finding. However, while Crow reported reduced asymmetry of the temporal horn in schizophrenia, the present meta-analysis found no significantly decreased asymmetry in schizophrenia.
Asymmetry of the superior temporal gyrus is also frequently used as an indication of language lateralisation (Reference Pearlson, Barta and PowersPearlson et al, 1997; Reference Highley, McDonald and WalkerHighley et al, 1999; Reference Holinger, Shenton and WibleHolinger et al, 1999; Reference Levitan, Ward and CattsLevitan et al, 1999). However, direction and magnitude of asymmetry of this structure is not well established in healthy subjects. In this meta-analysis, controls showed a trend towards asymmetry favouring the right hemisphere, while the superior temporal gyrus was found to be greater on the right in patients. The trend towards rightward asymmetry in healthy subjects is surprising since it partly overlaps with Wernicke's area. However, the superior temporal gyrus also incorporates primary and secondary auditory cortex that generally shows rightward asymmetry, reflecting right hemispheric dominance for non-verbal sounds (Reference Zattore, Evans, Meyers and GjeddeZattore et al, 1992). The posterior segment of the superior temporal gyrus might be a better candidate to reflect cerebral dominance for language, since it mainly consists of language-related hetero-modal cortex (Reference PearlsonPearlson, 1997). However, in the current meta-analysis, this segment also showed a trend towards asymmetry favouring the right hemisphere in healthy subjects. Therefore, neither the superior temporal gyrus, nor its posterior segment appears suited for the assessment of cerebral dominance for language.
Limitations
The presented paper included only studies on language lateralisation that used the dichotic listening paradigm. We were unable to retrieve enough visual half-field studies using language stimuli to allow for meta-analysis. Data of functional imaging studies are considered to be too diverse regarding technical assessment and statistical analysis to allow for direct comparison in a meta-analysis (Reference Lange, Moonen and BandettiniLange, 1999). A second limitation of the study is our choice to include only studies on patients with strict diagnosis of schizophrenia, excluding studies that used the broad DSM-II criteria for schizophrenia and studies that used patients with schizophrenia-spectrum disorders. Another reason that several studies could not be included was the absence of healthy control groups. By applying these strict inclusion criteria, the total number of studies was lower, but the contrast between the experimental and the comparison group was maximal.
Clinical implications
The reported excess of non-right-handedness and decreased right-ear advantage in healthy relatives of patients with schizophrenia suggest a genetic cause underlying the decreased cerebral lateralisation in schizophrenia. If this were true, a deviation of the genetic mechanism underlying cerebral dominance, the hypothesised ‘right-shift factor’ may cause a vulnerability to schizophrenia (Reference AnnettAnnett, 1999; Reference CrowCrow, 1999). This implies that the search for genes predisposing for schizophrenia may focus on loci that have a role in the establishment of cerebral dominance. In addition, indicators of decreased cerebral dominance in individuals, such as non-right-handedness, decreased right-ear advantage on the dichotic listening paradigm or decreased asymmetry of the planum temporale may help to identify subjects at increased risk for schizophrenia.
In sum, when literature on handedness, dichotic listening studies and asymmetry of language-related structures is reviewed quantitatively, compelling evidence emerged for decreased cerebral dominance in schizophrenia.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ Finding the locus of the gene for cerebral dominance may unravel the genetic predisposition for schizophrenia.
-
▪ Mixed- and left-handed subjects may be at increased risk of schizophrenia.
-
▪ The dichotic listening paradigm or measurement of planum temporale asymmetry may help identify subjects at increased risk of schizophrenia.
LIMITATIONS
-
▪ Visual half-field studies using language stimuli were not included.
-
▪ Functional imaging studies with language activation were not included.
-
▪ Narrow definitions were used for inclusion in the schizophrenia and healthy groups.








eLetters
No eLetters have been published for this article.