There is mounting evidence that Tourette syndrome is an inherited developmental alteration of synaptic neurotransmission within the cortico-striatal-thalamic-cortical circuitry (CSTC). Abnormalities in the striatum and in medial temporal structures have repeatedly been found in imaging studies using nuclear medicine techniques. To investigate morphological alterations in vivo, a number of region-based volumetric analyses of structural magnetic resonance imaging (MRI) data in patients of various ages with Tourette syndrome have been performed, but these differ considerably in their core findings with respect to basal ganglia volumes (Reference Gerard and PetersonGerard & Peterson, 2003). In our study the whole-brain three-dimensional MRI analysis technique of voxel-based morphometry, which avoids the biases inherent in operator-dependent techniques, was applied in boys with Tourette syndrome to test the specific hypothesis that differences exist between patients with this disorder and healthy controls in the striatum and medial temporal areas.
METHOD
The protocol was approved by the ethics committee of the University of Ulm, and written informed consent was obtained from the parents of all participants. Fourteen patients (mean age 12.5 years) were investigated; only boys were included to control for possible gender effects. The criteria for a definite diagnosis of Tourette syndrome according to the Tourette Syndome Classification Study Group (1993) were applied to all patients. Stimulants were the only medication used by the patients (n=4). For clinical characterisation, the German version of the Yale Global Tic Severity Scale (YGTSS; Reference SteinhausenSteinhausen, 2002) was applied. Attention-deficit hyperactivity disorder (ADHD) comorbidity was assessed using the DSM–IV criteria checklist (American Psychiatric Association, 1994); the mean value was 6.6 (s.d.=5.6). To provide an age-matched MRI control database, MRI scans of 15 healthy boys (mean age 13.4 years) without any neurological or psychiatric diagnosis were acquired. Patients and controls were well matched for age, handedness and IQ.
High-resolution, whole-head three-dimensional MRI data for all patients and controls were collected on the same 1.5 T clinical scanner (Siemens, Erlangen, Germany) and processed in the same way using methods implemented in Statistical Parametric Mapping software (SPM2; http://www.fil.ion.ucl.ac.uk/spm). Voxel-based morphometry was performed according to the principles described by Good et al (Reference Good, Johnsrude and Ashburner2001) as the ‘optimised’ protocol. First, all images were normalised by use of an affine-only procedure and a template built from children aged 5–18 years by researchers from the Imaging Research Center at Cincinnati Children's Hospital Medical Center; see http://www.irc.chmcc.org). After creation of customised tissue probability maps, the MRI data were segmented, cleaned, normalised to the study-specific template, segmented and cleaned a second time, then modulated by the Jacobian determinants and finally smoothed (6 mm isotropic Gaussian kernel) (Reference Good, Johnsrude and AshburnerGood et al, 2001).
Within the framework of the general linear model, the grey-matter images of the Tourette syndrome group were statistically compared with the normal database in a parametric group analysis. Data were analysed in SPM for effects of diagnosis, including individual total grey-matter volume, age and individual scores on the DSM–IV criteria checklist for ADHD as covariates of no interest and individual YGTSS values as the covariate of interest. To detect whether each voxel in the patient data had a greater or lesser grey-matter volume than in controls, the appropriate two contrasts were calculated. For general analysis of effects in areas with an a priori hypothesis – the basal ganglia (striatum) and mesiotemporal regions – significance was set at P<0.001, uncorrected for multiple comparisons, and then a small-volume correction was made using a 10 mm radius volume of interest (Reference Wilke, Kowatch and Del BelloWilke et al, 2004).
RESULTS
In the optimised voxel-based morphometry analysis, both contrasts ‘patients>controls’ and ‘controls>patients’ yielded significant results at a threshold of small-volume corrected P<0.001. Patients were found to have regional increases in grey-matter volumes in the area of the ventral putamen bilaterally (x, y, z=25, 13, (-5) and (-20), 17, (-6) respectively) (Fig. 1(A)). In the search for syndrome-associated regions of decreased grey-matter volumes, significant mediotemporal voxel clusters were localised in the bilateral hippocampal area (x, y, z=27, (–19), (–17) and (–20), (–19), (–21) respectively (Fig. 1(B)). In the covariance analysis including disease severity, these two areas of increased and of decreased grey-matter density were both found to covary significantly with YGTSS at P<0.001.
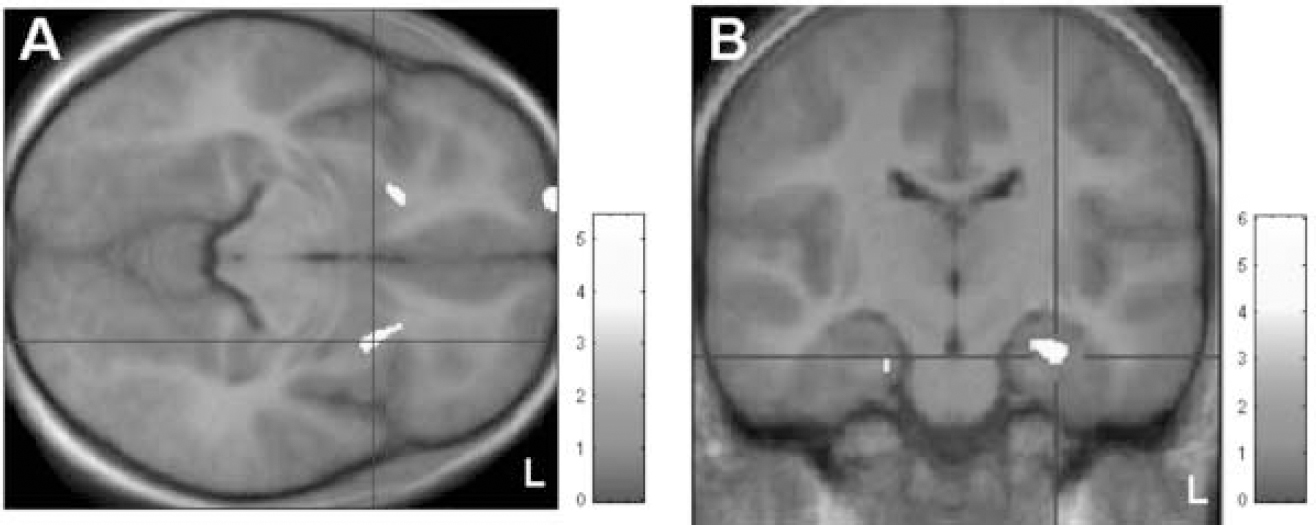
Fig. 1 Voxel-based morphometric analysis. (A) Patients>controls: areas showing significantly increased grey matter (at small-volume corrected P<0.001) localised in the ventral putamen bilaterally; clusters are superimposed on the study-specific template in axial view. (B) Controls>patients: display of clusters showing significantly decreased grey matter (at small-volume corrected P<0.001), superimposed on the study-specific template, in coronal view. Z-scores are indexed by the grey-scale bar.
DISCUSSION
The morphological changes in striatal and left mediotemporal (hippocampal) areas detected correspond to integral components of the CSTC involving its temporolimbic associations. These results are consistent with functional neuroimaging data, since early functional imaging studies identified different parts of CSTC (including temporolimbic, sensorimotor, orbitofrontal and association pathways) to be altered with respect to metabolism and blood flow (Reference PetersonPeterson, 2001). Later radioligand studies of presynaptic/synaptic function and the post-synaptic dopaminergic system achieved inconsistent findings, with partly decreased, partly increased and partly normal striatal bindings (cf. Reference Gerard and PetersonGerard & Peterson, 2003). Our data do not provide evidence that the frequent comorbidity of Tourette syndrome and ADHD, which was present but mild in our patients according to DSM–IV checklist criteria, was relevant for the involvement of limbic pathways, since the individual ADHD values were covaried out.
The change in putamen volume is in general agreement with previous investigations; note that the putaminal volume changes in our study were local increases in grey matter. Previous investigations of functional networks in Tourette syndrome using [15H2O]–positron emission tomography (PET) or functional MRI (Reference Peterson, Skudlarski and AndersonPeterson et al, 1998; Reference Stern, Silbersweig and CheeStern et al, 2000) suggested that basal ganglia seem to be overactive (disinhibited), in accordance with a more recent study using [11C]dihydrotetrabenazine–PET to quantify striatal monoaminergic innervation in this syndrome which showed increased binding in the ventral striatum (Reference Albin, Koeppe and BohnenAlbin et al, 2003). Thus, it seems plausible that in the course of the disorder the increased activity might be mirrored by increased signal properties due to neuronal volume changes, at least in a subset of patients. It has to be kept in mind that children and not adults were investigated in our study and that previous volumetric studies in affected children mostly found no reduction of basal ganglia volumes, although a large-scale volumetric study reported reduced volumes of the caudate nuclei, but not of lenticular nuclei (Reference Peterson, Thomas and KanePeterson et al, 2003).
The ventral striatum which we found to be altered in our patient cohort is thought of as the part of the basal ganglia participating in temporolimbic pathways of the CSTC. We found the left hippocampal gyrus to be a part of the limbic system significantly decreased in volume, a finding not described previously in Tourette syndrome or in ADHD to our knowledge. The hippocampal cortex is a polymodal convergence area and a core component of the limbic system loops of the CSTC (Reference PetersonPeterson, 2001). This constellation implies that two elements of this pathway were found to be altered in volume in our study and that the temporolimbic CSTC pathway seems to be important for Tourette-related symptoms. This finding is strongly supported by the significant covariance of tic severity and the striatal and hippocampal volume changes.
Using voxel-based morphometric analysis as a sensitive and unbiased comparison of regional brain morphology, we found evidence for grey-matter changes in temporolimbic CSTC pathways which might be the morphological correlate of a dopaminergic hyperfunction. Our finding of decreased hippocampal grey-matter volumes needs to be correlated with neuropsychological deficits in patients with Tourette syndrome in future studies. The results of this type of analysis may be useful in recognising regions that are of particular relevance to a specific disorder, even when the neuromorphometric abnormalities are subtle.
Acknowledgement
We thank Mrs Sonja Fuchs for help with acquisition of the MRI data.




eLetters
No eLetters have been published for this article.