Post-traumatic stress disorder (PTSD) is a psychopathological response to a terrifying experience. Initially associated with combat, PTSD is observed in civilians following traumatic experiences, including violence, accident, natural disaster and life-threatening illness. Lifetime prevalence of PTSD in civilians is between 1% and 9% (Reference Helzer, Robins and McEvoyHelzer et al, 1987; Reference Breslau, Davis and AndreskiBreslau et al, 1991; Reference Davidson, Hughes and BlazerDavidson et al, 1991). Average duration is 3 to 5 years, with many patients experiencing PTSD for more than 10 years (Reference Kessler, Sonnega and BrometKessler et al, 1995). Selective serotonin reuptake inhibitors (SSRIs) such as sertraline, paroxetine and fluoxetine have shown efficacy for up to 3 months in the treatment of PTSD (Reference Connor, Sutherland and TuplerConnor et al, 1999; Reference Brady, Pearlstein and AsnisBrady et al, 2000; Reference Tucker, Zaninelli and YehudaTucker et al, 2001; Reference Martenyi, Brown and ZhangMartenyi et al, 2002). Sertraline has shown significant benefit during 24- to 28-week maintenance treatment of PTSD (Reference Davidson, Pearlstein and LondborgDavidson et al, 2001; Reference Londborg, Hegel and GoldsteinLondborg et al, 2001). However, few published studies have examined the efficacy of pharmacotherapies in preventing PTSD relapse, although considerable evidence supports pharmacotherapeutic maintenance treatment for major depression and anxiety, and panic disorders (Reference Montgomery, Dufour and BrionMontgomery et al, 1988; Reference Frank, Kupfer and PerelFrank et al, 1990; Reference Entsuah, Rudolph and HackettEntsuah et al, 1996; Reference Reimherr, Amsterdam and QuitkinReimherr et al, 1998; Reference Michelson, Pollack and LydiardMichelson et al, 1999). This study, conducted in Belgium, Bosnia, Croatia, Yugoslavia, Israel and South Africa, was designed to assess the efficacy of fluoxetine in preventing PTSD relapse for up to 6 months.
METHOD
Patient population
Participants were men and women aged 18-65 years who met DSM—IV criteria for PTSD (American Psychiatric Association, 1994) according to the Structured Clinical Interview for DSM—IV Axis I Disorders for Patients, Investigator Version (SCID—I modified; Reference First, Spitzer and GibbonFirst et al, 1997) and the Clinician-Administered PTSD Scale, Current Diagnostic Version (CAPS—DX; Reference Blake, Weathers and NagyBlake et al, 1995). To enrol, patients had to have a total score ≥50 on the CAPS—DX and a score ≥4 on the Clinical Global Impression of Severity (CGI—S) scale (Reference GuyGuy, 1976) at baseline (visit 2). Individuals with Montgomery—Åsberg Depression Rating Scale (MADRS; Reference Montgomery and AsbergMontgomery & Åsberg, 1979) scores >20 at baseline were ineligible for the study. Exclusion criteria included serious comorbid illness, concomitant psychotherapy, serious suicidal risk or risk to others, and diagnosis of an Axis I psychiatric disorder (defined by DSM—IV criteria) 5 years before the primary traumatic episode. Patients with lifetime diagnoses of bipolar disorder, obsessive—compulsive disorder (OCD) or schizophrenia were excluded. Those with a diagnosis of any Axis I psychiatric disorder or comorbidity following the primary traumatic episode, except generalised anxiety disorder, depression, panic disorder or social phobia, were also excluded. Patients with a history of alcohol or substance misuse following the primary traumatic episode were allowed to enrol if the misuse had resolved at least 6 months before study entry.
The study was conducted from June 1998 to August 2000 at 18 study centres in Belgium, Bosnia, Croatia, Yugoslavia, Israel and South Africa. The ethical review board for each site reviewed the study; written informed consent was obtained from all participants.
Study design
After a 1- to 2-week evaluation period, participants were randomised to 12 weeks' double-blind acute treatment with fluoxetine or placebo. Fluoxetine-treated patients initially received 20 mg/day. This dose could be increased by 20-mg increments at each of three titration points based on predefined response criteria (CGI—S≥3) to a maximum dosage of 80 mg/day. Acute response to fluoxetine v. placebo has been described elsewhere (Reference Martenyi, Brown and ZhangMartenyi et al, 2002).
After 12 weeks of acute treatment with fluoxetine or placebo, participants who responded to treatment by a 50% decrease in the eight-item Treatment Outcome PTSD (TOP-8) score (Reference Davidson and ColketDavidson & Colket, 1997) from baseline, a CGI—S score ≤2, and failing DSM—IV diagnostic criteria for PTSD continued in a 24-week relapse prevention phase. Those patients who had received fluoxetine were randomised either to continue without variation from final dosage in the acute phase or to placebo treatment. In order to maintain blinding, patients switching to placebo did not undergo a tapering regimen. This was possible because fluoxetine is associated with significantly fewer and less-severe discontinuation-emergent adverse events than are other SSRIs (Reference Rosenbaum, Fava and HoogRosenbaum et al, 1998).
Patients who responded to treatment with placebo during the acute treatment phase were continued on placebo during the relapse prevention phase to preserve blinding. Participants discontinued the trial if relapse criteria were met (40% increase in TOP-8 score and an increase in CGI—S score of ≥ 2 from the baseline at week 12 of acute treatment) at any time during the relapse prevention phase. Relapse could also be determined by the clinical judgement of the investigator.
Outcome measures
The primary efficacy measure for the relapse prevention phase was time to relapse based on the TOP-8 scale and the CGI—S scale. TOP-8 is an 8-item clinician-rated instrument measuring the presence and severity of PTSD symptoms in three major dimensions (intrusive, avoidant and hyperarousal symptoms). Each item is rated from 0 to 4, with higher numbers indicating greater severity.
Secondary assessments included the CAPS—DX total, intrusive, avoidance and hyperarousal scores; the Clinical Global Impression of Improvement (CGI—I) scale (Reference GuyGuy, 1976); and the Davidson Trauma Scale (DTS) total, intrusive, avoidance and hyperarousal sub-scores (Reference Davidson, Book and ColketDavidson et al, 1997). Changes in comorbid psychiatric disorders were measured using the MADRS, the Hamilton Rating Scale for Anxiety (HRSA; Reference HamiltonHamilton, 1959) and the Hopkins 90-Item Symptoms Checklist Revised (SCL-90-R; Reference DerogatisDerogatis, 1983; Reference Rief and FichterRief & Fichter, 1992). Both the DTS and the SCL-90-R are patient-rated scales; all others are clinician-rated.
Safety was assessed by evaluating treatment-emergent adverse events, discontinuations for adverse events, vital signs measurements and clinical laboratory tests. Adverse events were ascertained by non-probing enquiry and were recorded regardless of perceived causality. An event was considered treatment-emergent if it occurred for the first time or worsened during the relapse prevention phase of the study. Investigators assessed patient compliance at each visit by direct questioning and by counting returned medication. Patients were considered non-compliant if they missed more than 4 consecutive days or more than 10 cumulative days of study medication. Patients were also considered non-compliant if the ratio of the number of capsules taken to the number of capsules prescribed was less than 0.8 or more than 1.2.
Statistical methods
Time to relapse was evaluated by plotting Kaplan—Meier survival curves. A long-rank test was used to compare the time to relapse curves for the fluoxetine/fluoxetine and fluoxetine/placebo treatment groups. Analyses of change from baseline (week 12 of acute treatment) in TOP-8, MADRS, DTS, SCI-90-R, CGI—S and HRSA scores were conducted using a repeated-measures model with visit, treatment, investigator and visit-by-treatment interaction as effects in the model. The corresponding baseline score was included in the model as a covariate. An unstructured covariance matrix was fitted to the within-patient repeated measures. Change from baseline to each visit was tested between treatment groups using contrasts within the repeated-measures model. The comparison between groups of the difference from baseline to the last visit (week 36) was considered the primary comparison. Analysis of CGI—I was done in a similar manner using raw post-baseline values. For the CAPS total scores and sub-scores, which were collected at baseline (week 12), mid-point (week 24) and end-point (week 36 or discontinuation), analyses of the change from baseline to end-point (last observation carried forward, LOCF) were conducted using analysis of variance with treatment and investigator as effects in the model.
To investigate the possible effect of trauma type (combat-related v. non-combat-related trauma), a repeated-measures analysis of variance was conducted as described above, with the addition of trauma type in the model. In addition, the two-way interactions of trauma type by visit and by treatment were included along with the three-way interaction of treatment by trauma type and by visit.
Treatment differences in patient characteristics at baseline were assessed using Fisher's exact test for categorical variables and analysis of variance for continuous variables. The analysis of variance model included investigator and therapy. Treatment-emergent adverse events and treatment-emergent abnormal laboratory values were analysed using Fisher's exact test. The mean final dose of fluoxetine was summarised.
All analyses were based upon the intent-to-treat principle and were performed using SAS software (SAS Institute Inc., Version 6 (for MVS), Carey, NC, 1991). Tests of treatment effects were conducted at a two-sided alpha level of 0.05. Investigators with fewer than two randomised patients per treatment group were pooled for statistical analysis purposes.
RESULTS
Sample description
Participants were predominantly male (81%) and Caucasian (90%); 47% had been exposed to combat-related traumatic events. None reported onset of PTSD at a young age or childhood sexual abuse. Of the 226 participants randomised to fluoxetine during the 12-week acute treatment phase, 131 responders to treatment agreed to continue in the study. Of these, 69 were randomised to receive fluoxetine and 62 to receive placebo in the 24-week relapse prevention phase (Fig. 1). Demographic as well as disease characteristics following 12 weeks of acute fluoxetine treatment were similar in both groups (Table 1). Of the 75 participants assigned to placebo in the acute phase, 31 were responders and continued on placebo during the 24-week relapse prevention phase.

Fig. 1 Flow diagram of the study. Flx/Flx, fluoxetine/fluoxetine treatment; Flx/Plc, fluoxetine/placebo treatment; Plc/Plc, placebo/placebo treatment.
Table 1 Demographics and illness severity at baseline (following response to 12 weeks of acute fluoxetine treatment)1
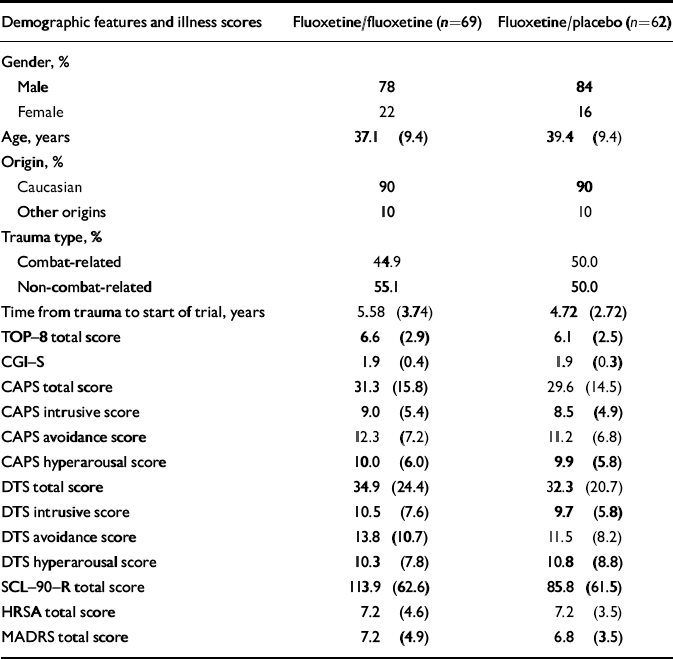
| Demographic features and illness scores | Fluoxetine/fluoxetine (n=69) | Fluoxetine/placebo (n=62) |
|---|---|---|
| Gender, % | ||
| Male | 78 | 84 |
| Female | 22 | 16 |
| Age, years | 37.1 (9.4) | 39.4 (9.4) |
| Origin, % | ||
| Caucasian | 90 | 90 |
| Other origins | 10 | 10 |
| Trauma type, % | ||
| Combat-related | 44.9 | 50.0 |
| Non-combat-related | 55.1 | 50.0 |
| Time from trauma to start of trial, years | 5.58 (3.74) | 4.72 (2.72) |
| TOP-8 total score | 6.6 (2.9) | 6.1 (2.5) |
| CGI-S | 1.9 (0.4) | 1.9 (0.3) |
| CAPS total score | 31.3 (15.8) | 29.6 (14.5) |
| CAPS intrusive score | 9.0 (5.4) | 8.5 (4.9) |
| CAPS avoidance score | 12.3 (7.2) | 11.2 (6.8) |
| CAPS hyperarousal score | 10.0 (6.0) | 9.9 (5.8) |
| DTS total score | 34.9 (24.4) | 32.3 (20.7) |
| DTS intrusive score | 10.5 (7.6) | 9.7 (5.8) |
| DTS avoidance score | 13.8 (10.7) | 11.5 (8.2) |
| DTS hyperarousal score | 10.3 (7.8) | 10.8 (8.8) |
| SCL-90-R total score | 113.9 (62.6) | 85.8 (61.5) |
| HRSA total score | 7.2 (4.6) | 7.2 (3.5) |
| MADRS total score | 7.2 (4.9) | 6.8 (3.5) |
Medication compliance was high for both groups at all time points. The mean exposure to the study drug was 157 days during the 6-month relapse prevention phase for fluoxetine/fluoxetine-treated patients. The mean final dose was 53 mg/day.
Efficacy
An analysis of time to relapse showed that fluoxetine was statistically significantly superior to placebo in relapse prevention (log-rank χ2=4.88, P=0.027) (Fig. 2).
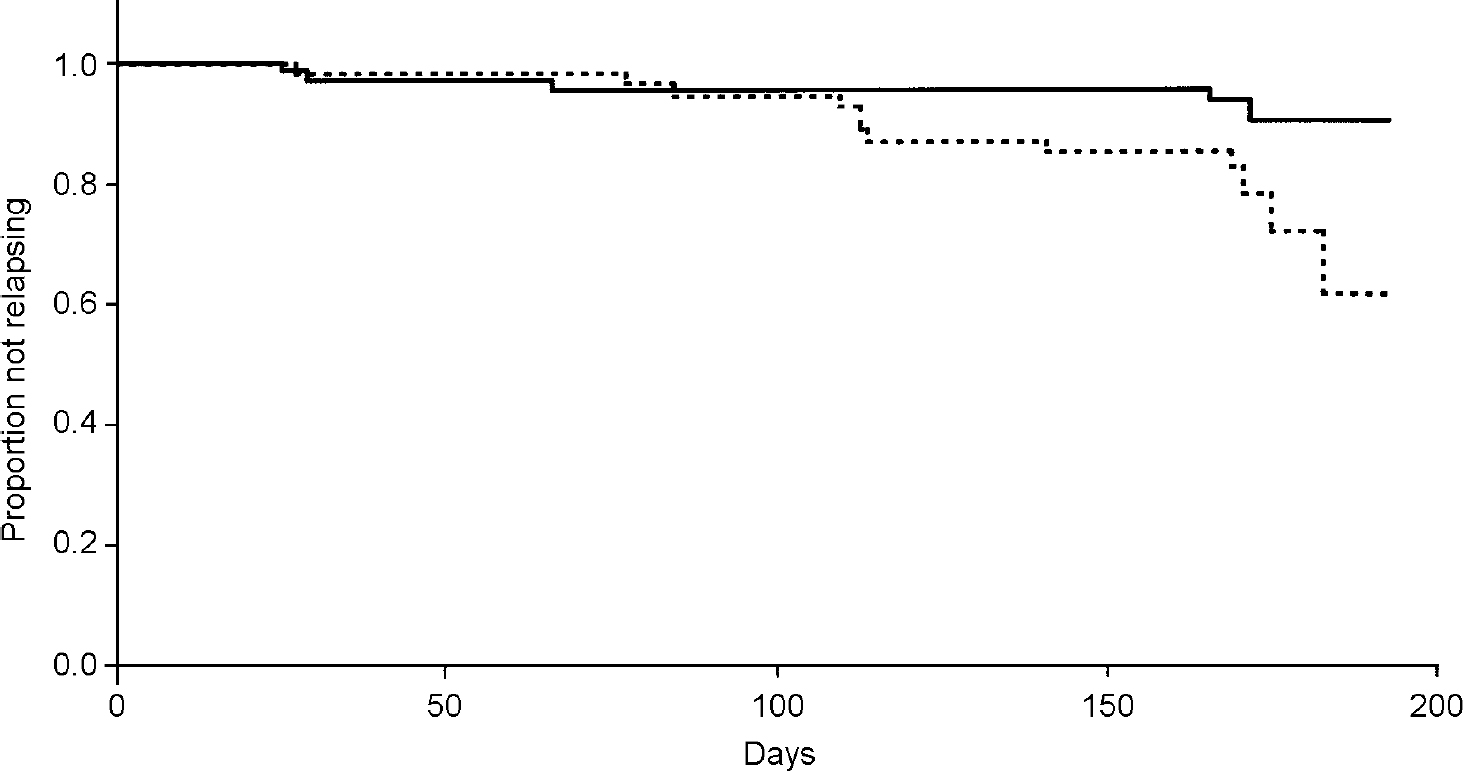
Fig. 2 Kaplan—Meier survival analysis of time to relapse. — fluoxetine/fluoxetine;---fluoxetine/placebo.
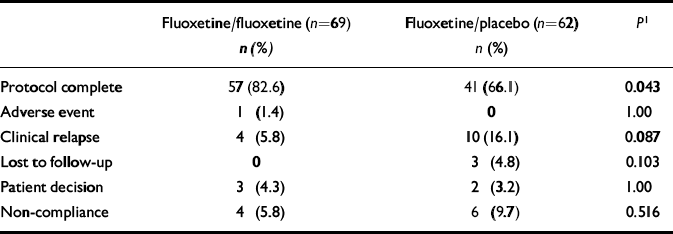
A higher percentage of fluoxetine/fluoxetine-treated patients (82.6%) completed the relapse prevention phase compared with fluoxetine/placebo-treated patients (66.1%) (Fisher's exact test, P=0.043). A higher percentage of fluoxetine/placebo-treated patients (16.1%) discontinued the study because of relapse compared with fluoxetine/fluoxetine-treated patients (5.8%) (P=0.087) (Table 2).
Table 2 Reasons for discontinuation
| Fluoxetine/fluoxetine (n=69) | Fluoxetine/placebo (n=62) | P 1 | |
|---|---|---|---|
| n (%) | n (%) | ||
| Protocol complete | 57 (82.6) | 41 (66.1) | 0.043 |
| Adverse event | 1 (1.4) | 0 | 1.00 |
| Clinical relapse | 4 (5.8) | 10 (16.1) | 0.087 |
| Lost to follow-up | 0 | 3 (4.8) | 0.103 |
| Patient decision | 3 (4.3) | 2 (3.2) | 1.00 |
| Non-compliance | 4 (5.8) | 6 (9.7) | 0.516 |
Fluoxetine/fluoxetine-treated patients had statistically significantly greater mean improvement in TOP—8 total score from baseline to end-point than did fluoxetine/placebo-treated patients (fluoxetine/fluoxetine, -1.8; fluoxetine/placebo +0.05; F=6.721,112, P=0.011) (Fig. 3). The effect size of 0.5, typically considered to be of medium size, implies that the median improvement in the fluoxetine/fluoxetine group exceeded the improvement of 69% of individuals in the fluoxetine/placebo group.
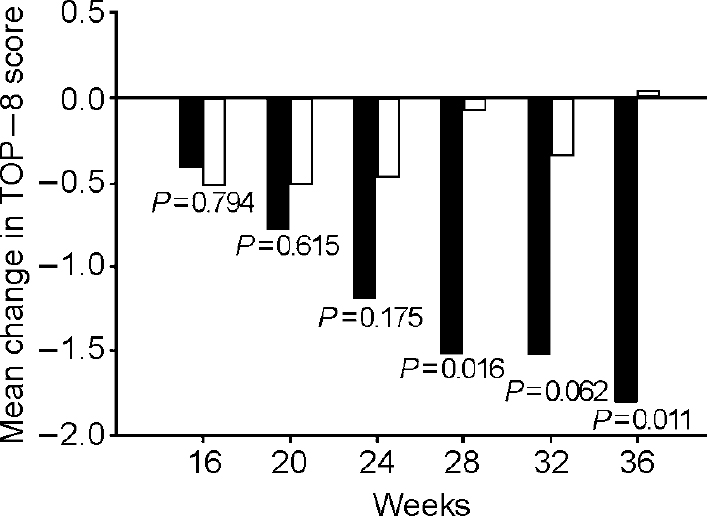
Fig. 3 Least square mean change from baseline (12 weeks of acute fluoxetine treatment) in Treatment Outcome PTSD (TOP-8) total score. Repeated measures model with visit, treatment, investigator and visit-by-treatment interaction as effects. Fluoxetine-treated patients who responded to 12 weeks' acute treatment were either continued on fluoxetine at the same dose or were switched to placebo. ▪, fluoxetine/fluoxetine; □, fluoxetine/placebo.
The CGI—S scores also showed statistically significant improvement for fluoxetine/fluoxetine-treated patients compared with fluoxetine/placebo-treated patients (F=8.391,112, P=0.005) (Table 3). In addition, fluoxetine-treated patients experienced greater improvement in CAPS score compared with placebo-treated patients. The difference between the two treatment groups was statistically significant for the avoidance sub-score (F=5.441,113, P=0.021) but was not statistically significant for the CAPS total score or the CAPS intrusive sub-score (total score: F=3.801,113, P=0.054; intrusive: F=3.111,113, P=0.080). The patient-rated SCL—90—R and DTS did not show statistically significant separations between treatment groups in total scores or any DTS sub-scores (Table 3). Fluoxetine/fluoxetine-treated patients experienced significantly greater improvement compared with fluoxetine/placebo-treated patients in symptoms of anxiety and depression as measured by the HRSA (F=6.731,112, P=0.011) and MADRS (F=5.131,112, P=0.026) scores (Table 3).
Table 3 Mean change from baseline (week 12 of acute treatment) to end-point in illness severity measures
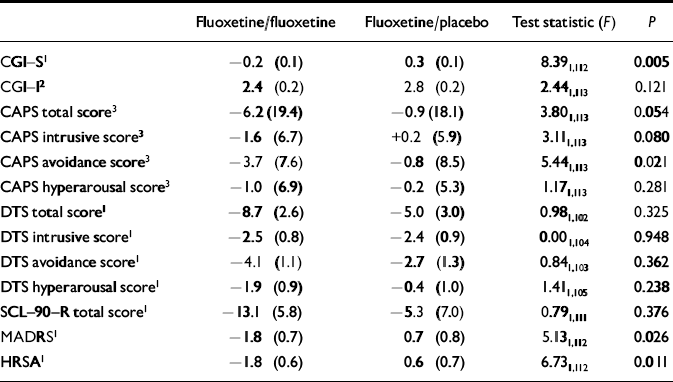
| Fluoxetine/fluoxetine | Fluoxetine/placebo | Test statistic (F) | P | |
|---|---|---|---|---|
| CGI-S1 | -0.2 (0.1) | 0.3 (0.1) | 8.391,112 | 0.005 |
| CGI-I2 | 2.4 (0.2) | 2.8 (0.2) | 2.441,113 | 0.121 |
| CAPS total score3 | -6.2 (19.4) | -0.9 (18.1) | 3.801,113 | 0.054 |
| CAPS intrusive score3 | -1.6 (6.7) | +0.2 (5.9) | 3.111,113 | 0.080 |
| CAPS avoidance score3 | -3.7 (7.6) | -0.8 (8.5) | 5.441,113 | 0.021 |
| CAPS hyperarousal score3 | -1.0 (6.9) | -0.2 (5.3) | 1.171,113 | 0.281 |
| DTS total score1 | -8.7 (2.6) | -5.0 (3.0) | 0.981,102 | 0.325 |
| DTS intrusive score1 | -2.5 (0.8) | -2.4 (0.9) | 0.001,104 | 0.948 |
| DTS avoidance score1 | -4.1 (1.1) | -2.7 (1.3) | 0.841,103 | 0.362 |
| DTS hyperarousal score1 | -1.9 (0.9) | -0.4 (1.0) | 1.411,105 | 0.238 |
| SCL-90-R total score1 | -13.1 (5.8) | -5.3 (7.0) | 0.791,111 | 0.376 |
| MADRS1 | -1.8 (0.7) | 0.7 (0.8) | 5.131,112 | 0.026 |
| HRSA1 | -1.8 (0.6) | 0.6 (0.7) | 6.731,112 | 0.011 |
When exploring the possible effect of trauma type (combat-related v. non-combat-related), a significant three-way interaction was detected between visit, treatment and trauma type (P=0.005). For the non-combat-related traumas, the mean change from baseline to last visit was -1.72 for the fluoxetine/fluoxetine group compared with -1.25 for the fluoxetine/placebo group (P=0.633). For the combat-related traumas, the mean change for the fluoxetine/fluoxetine group was -1.62 compared with +1.97 for the fluoxetine/placebo group (P=0.002). It should be noted that for patients with non-combat-related PTSD, placebo was associated with some improvement but for patients with combat-related PTSD, placebo was associated with a worsening of symptoms. Fluoxetine was associated with similar levels of improvement in both patient types.
Safety
There were no significant differences between the two groups in any vital sign measure or laboratory result.
The difference between treatment groups in the number of patients reporting one or more treatment-emergent adverse events was not statistically significant (fluoxetine/fluoxetine 39%; fluoxetine/placebo 24%; Fisher's exact test P=0.091). There were no statistically significant differences in the numbers of patients reporting any single event. The adverse events most commonly reported by fluoxetine/fluoxetine-treated patients were insomnia (15%), anxiety (6%) and headache (6%); those most commonly reported by fluoxetine/placebo-treated patients were insomnia (10%), headache (5%) and pain (5%). Two patients, both in the fluoxetine/fluoxetine treatment group, experienced serious adverse events requiring hospitalisation (back pain and traffic accident). Only the patient involved in the traffic accident discontinued the trial early.
DISCUSSION
Efficacy
Fluoxetine treatment for maintenance of improvement of PTSD symptoms is associated with significantly longer time to relapse, greater improvement in overall PTSD symptoms and significantly greater reduction in symptoms of comorbid disorders than is placebo treatment. There appears to be a delay between cessation of active treatment and worsening of symptoms; clinicians should be aware of the possibility of a relapse of symptoms in patients for a period of several months after the discontinuation of fluoxetine treatment.
Participants in the fluoxetine/fluoxetine treatment group continued to experience statistically significant improvement in mean TOP—8 score throughout the 24-week relapse prevention period and showed statistically significant better improvement at end-point than did fluoxetine/placebo-treated participants.
Improvement in illness severity, as demonstrated by the CGI—S scale, was also statistically significant (P=0.005).
Fluoxetine/fluoxetine-treated participants experienced significantly greater improvement compared with fluoxetine/placebo-treated participants in the CAPS avoidance sub-score. Both of the patient-rated scales (DTS and SCL—90—R) failed to show significant differences in the improvement of PTSD symptoms between the two treatment groups, possibly as a result of inconsistent patient self-rating.
Because comorbid psychiatric disorders such as anxiety and depression are commonly associated with PTSD, the HRSA and MADRS scores were collected throughout the relapse prevention phase to monitor changes in patients' comorbid symptoms. When compared with fluoxetine/placebo-treated participants, fluoxetine/fluoxetine-treated participants experienced significantly greater improvement in both HRSA and MADRS total scores.
The findings demonstrate the efficacy of pharmacotherapy with fluoxetine, an SSRI, in the prevention of PTSD relapse and continual improvement in PTSD symptoms for up to 6 months following response to 12 weeks of acute treatment. In addition, the study design excluded patients with comorbid major depression (patients with a MADRS score >20 were excluded), which differs from previous studies that allowed unlimited severity of depression (Reference Davidson, Pearlstein and LondborgDavidson et al, 2001; Reference Londborg, Hegel and GoldsteinLondborg et al, 2001). The results of this study, therefore, represent improvement and relapse prevention of PTSD rather than improvement and relapse prevention of a mixed state of PTSD and depression.
Safety
Safety and tolerability of fluoxetine in this study were comparable to previous studies of fluoxetine in PTSD and to fluoxetine trials for other indications. Fluoxetine was generally well tolerated, with no statistically significant differences between treatment groups in either the incidence of any individual adverse event, or the drop-out rate due to adverse events.
The mean fluoxetine dose at end-point, 53 mg/day, was consistent with fluoxetine doses in the upper range for the treatment of clinical depression and the recommended range for patients with OCD.
This study was designed to assess the effect of pharmacotherapy with fluoxetine for preventing PTSD relapse, and investigators were instructed to avoid providing any type of counselling or behavioural therapy to study participants during study visits. Numerous other studies, however, have shown that psychotherapy is effective in the treatment of individuals with PTSD, and various behavioural treatments have shown efficacy in the reduction of the core symptoms of PTSD (Reference BallengerBallenger, 1999). Results of the acute treatment phase of this trial show an increased placebo response for participants with dissociative symptoms at baseline, resulting in a statistically significant interaction between treatment group and participants with and without dissociative symptoms at baseline (Reference Martenyi, Brown and ZhangMartenyi et al, 2002). These results suggest that different populations of individuals with PTSD may respond favourably to psychotherapy compared with pharmacotherapy. Indeed, the combination of psychotherapy and pharmacotherapy may yield the most significant therapeutic effect and warrants further study.
One limitation of this study was the duration of acute therapy. Both large national surveys (Reference Kessler, Sonnega and BrometKessler et al, 1995) and a pharmacotherapy study with sertraline (Reference Davidson, Pearlstein and LondborgDavidson et al, 2001) suggest that PTSD may require a fairly lengthy acute treatment (in excess of 12 weeks) before maximum improvement of symptoms is achieved. In this study, the statistically significant continued improvement in PTSD symptoms (measured by the TOP-8 scale) after 12 weeks of acute therapy suggests that the full therapeutic effect of fluoxetine on the improvement of PTSD symptoms may not have been observed even after 9 months of therapy.
CLINICAL IMPLICATIONS
-
▪ Unlike previous studies of selective serotonin reuptake inhibitors and post-traumatic stress disorder (PTSD), this study focused on improvement and relapse prevention of PTSD rather than improvement and relapse prevention of a mixed state of PTSD and depression.
-
▪ Fluoxetine is efficacious in continuing to reduce PTSD symptoms and preventing PTSD relapse for up to 6 months.
-
▪ Optimal length of acute treatment of PTSD exceeds 3 months; current data suggest improvement continues for up to 9 months or more.
LIMITATIONS
-
▪ PTSD symptoms continued to improve during the relapse prevention period of this study, suggesting that a longer acute treatment phase should have been used before initiating the relapse prevention phase.
-
▪ The patient population for this study consisted largely of men of Caucasian origin with adult-onset PTSD. Further study will be needed to determine whether the results are similar in other PTSD populations.
-
▪ This study demonstrated the efficacy of fluoxetine for relapse prevention for up to 6 months of treatment. However, PTSD is a chronic condition and studies of therapy over longer periods are needed.
Acknowledgements
The authors thank the clinical investigators who generously agreed to participate in the study: Belgium — Professor Michel De Clercq (Cliniques Universitaires Saint-Luc Psychiatric Emergencies, Brussels) and Dr Michel Schittecatte (Hôpital Vincent Van Gogh Psychiatric Department, Marchienne Au Point); Bosnia and Herzegovina — Dr Slobodan Loga and Professor Ismet Ceric (Psihijatrijska Klinika, Sarajevo); Croatia — Dr Vera Folnegovic-Smalc (Psihijatrijska Bolnica Vrapce, Zagreb), Dr Miro Jakovljevic and Professor Ljubomir Hotujac (Klinicki Bolnicki Rebro Zagreb) and Professor Nikola Mandic (Klinicka Bolnica Osijek, Osijek); Israel — Professor Moshe Kotler (Beer Sheva Mental Health Center, Beer Sheva), Professor Arieh Shalev (Hadassah Medical Center, Jerusalem) and Professor Ronit Weizman (Ramat-Hen Mental Health Center, Tel-Aviv); Yugoslavia — Professor Miloje Preradovic (Vojnomedicinska Akademija, Beograd) and Dr Grozdanko Grbesa (Klinicki Center Nis, Nis); Republic of South Africa — Professor Dan Stein (Stikland Hospital, Bellville), Dr Ian Taylor (Park Drive Medical Centre, Port Elizabeth), Dr S. Brook (Gauteng), Dr Billy Marivate (Louise Pasteur Medical Centre, Pretoria) and Dr Prema Laban (Crompton Medical Centre-West, Pinetown).









eLetters
No eLetters have been published for this article.