The ease and reliability of eliciting first-rank symptoms (Reference Schneider and HamiltonSchneider, 1959) have given them an important role in defining a concept of schizophrenia in both research and clinical practice. Psychopathological explanations (Reference FrithFrith, 1992; Reference SimsSims, 1995) have been matched by neurobiological (Reference NasrallahNasrallah, 1985; Reference TrimbleTrimble, 1990; Reference SpenceSpence, 1996) theories. First-rank symptoms might be expected to have aetiological and therefore genetic significance. In a small sample of twins, McGuffin et al (Reference McGuffin, Farmer and Gottesmann1984) reported the heritability of nuclear symptoms to be zero, although in the same study they found the most heritable category was that defined by Research Diagnostic Criteria (RDC; Reference Spitzer, Endicott and RobinsSpitzer et al, 1978), which also depend on the presence of a number of first-rank symptoms. Here, in siblings with schizophrenia and schizoaffective disorder, we investigate whether first-rank symptoms represent more than one component of psychopathology and whether that component(s) is familial and therefore possibly heritable. Previously, we examined positive, negative and disorganised symptoms in a set of these multiply affected families (Reference Loftus, DeLisi and CrowLoftus et al, 1998).
METHODS
Families with two or more ill siblings were recruited in the UK, the USA and the Republic of Ireland as part of a multi-centre genetic linkage project. Sources of recruitment were the local psychiatric services, the National Schizophrenia Fellowship (NSF) and Schizophrenia - a National Emergency (SANE) in the UK and Republic of Ireland, and the National Alliance for Mental Illness (NAMI) in the USA. Identification of families, clinical evaluations and diagnostic procedures were similar in all locations (details of this population have been published elsewhere : Reference Garner, Kelly and CardonGarner et al, 1996; Reference Dann, DeLisi and DevotoDann et al, 1997; Reference Loftus, DeLisi and CrowLoftus et al, 1998). The total number of families assessed was 102, comprising 51 from the USA, 32 from Northwick Park in north-west London, 14 from Oxford and five in Dublin. After giving a complete description of the study to the subjects, their written informed consent was obtained. Each ill sibling was interviewed using structured diagnostic interviews (the Schedule for Affective Disorders and Schizophrenia (SADS; Reference Endicott and SpitzerEndicott & Spitzer, 1978) or the Diagnostic Interview for Genetic Studies (DIGS; Reference Nurnberger, Blehar and KaufmannNurnberger et al, 1994)). For each sibling all available hospital case notes were obtained. Collateral information was collected from at least one well member of each family (usually a parent), using a structured interview, the Relative Psychiatric History Questionnaire (Gershon ES, National Institute of Mental Health, USA; unpublished). Siblings were included if they met DSM-III-R criteria for schizophrenia or schizoaffective disorder (American Psychiatric Association, 1987) based on the above sources of information. Each sibling was diagnosed independently by two trained diagnosticians. In cases where there was disagreement, consensus was reached by consultation with a third person. All clinical procedures were identical in both centres, and diagnostic reliability studies had been periodically performed, with κ statistic scores ranging between 0.85 and 0.90 for primary diagnoses.
Altogether, the above information was reviewed for 230 siblings. A total of 45 siblings were excluded from the study : 35 due to a lack of adequate case notes, and four because they failed to meet criteria for DSM-III-R schizophrenia or schizoaffective disorder; this resulted in three of their co-siblings also being excluded. Three additional siblings were excluded because of a history of organic complications such as head injury, encephalitis and low IQ, which, with inadequate information, rendered diagnosis difficult. Another six families were excluded because first-rank symptoms were documented in the case notes as being present but no further details were given. The final number of families included was 75, comprising 171 siblings. There were two families with four ill siblings and nine families with three ill siblings. A family of three siblings formed three sibling pairs (sib-pairs) and a family of four formed six sib-pairs. Case notes, DIGS or SADS interviews and information from relatives were reviewed to determine the presence or absence of first-rank symptoms in each of the 171 siblings. Table 1 shows the demographic characteristics, including ethnicity, of the analysed sample.
Table 1 Demographic details
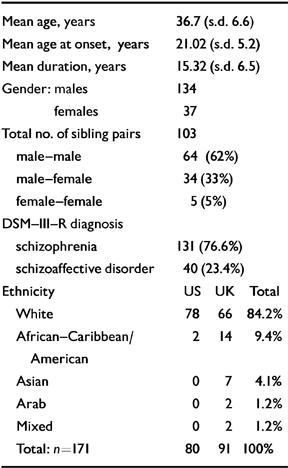
| Mean age, years | 36.7 (s.d. 6.6) |
| Mean age at onset, years | 21.02 (s.d. 5.2) |
| Mean duration, years | 15.32 (s.d. 6.5) |
| Gender : males | 134 |
| females | 37 |
| Total no. of sibling pairs | 103 |
| male-male | 64 (62%) |
| male-female | 34 (33%) |
| female-female | 5 (5%) |
| DSM-III-R diagnosis | |
| schizophrenia | 131 (76.6%) |
| schizoaffective disorder | 40 (23.4%) |
| Ethanicity | US UK Total |
| White | 78 66 84.2% |
| African-Caribbean/American | 2 14 9.4% |
| Asian | 0 7 4.1% |
| Arab | 0 2 1.2% |
| Mixed | 0 2 1.2% |
| Total : n=171 | 80 91 100% |
Individual first-rank symptoms were rated as : 0=absent, 1=present. A narrow definition of first-rank symptoms was employed. All ratings were performed by one observer (J.L.) and were based on a lifetime history of the illness. First-rank symptoms rated were : audible thoughts; running commentary; third-person auditory hallucinations; thought withdrawal, thought insertion and thought broadcasting; delusional perception and delusions of control (including made feelings, made drives and somatic passivity).
The data were then analysed in two different ways, using SPSS V6.1 :
-
(a) by exploratory factor analysis; all factor analyses were performed using orthogonal rotation (VARIMAX) in order to simplify the interpretation of factors; only factors with eigenvalue > 1 were considered;
-
(b) by Spearman rank correlation of factor regression scores between members of sibling pairs; post hoc X2 tests of observed against expected concordance were applied to individual symptoms.
RESULTS
Table 2 shows the distribution of first-rank symptoms in the whole sample, while Table 3 shows the distribution between siblings 1 and 2. Delusional perception was omitted from further analysis, as it was present in only 2.4% of the total sample (n=5).
Table 2 Distribution of first-rank symptoms (n=206)
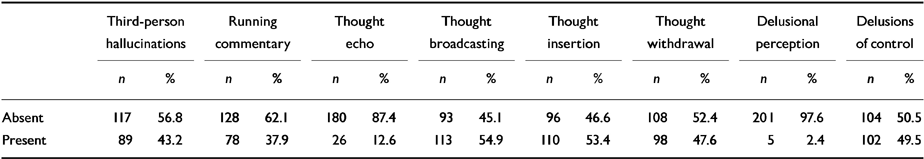
| Third-person hallucinations | Running commentary | Thought echo | Thought broadcasting | Thought insertion | Thought withdrawal | Delusional perception | Delusions of control | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| Absent | 117 | 56.8 | 128 | 62.1 | 180 | 87.4 | 93 | 45.1 | 96 | 46.6 | 108 | 52.4 | 201 | 97.6 | 104 | 50.5 |
| Present | 89 | 43.2 | 78 | 37.9 | 26 | 12.6 | 113 | 54.9 | 110 | 53.4 | 98 | 47.6 | 5 | 2.4 | 102 | 49.5 |
Table 3 shows that the distribution of first-rank symptoms was similar in both sibling 1 and sibling 2 (sibling 1 is defined as the elder member of the sib-pair).
Table 3 Distribution of first-rank symptoms between siblings
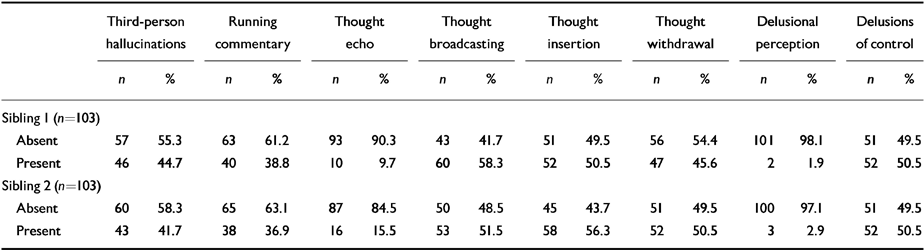
| Third-person hallucinations | Running commentary | Thought echo | Thought broadcasting | Thought insertion | Thought withdrawal | Delusional perception | Delusions of control | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| Sibling I (n=103) | ||||||||||||||||
| Absent | 57 | 55.3 | 63 | 61.2 | 93 | 90.3 | 43 | 41.7 | 51 | 49.5 | 56 | 54.4 | 101 | 98.1 | 51 | 49.5 |
| Present | 46 | 44.7 | 40 | 38.8 | 10 | 9.7 | 60 | 58.3 | 52 | 50.5 | 47 | 45.6 | 2 | 1.9 | 52 | 50.5 |
| Sibling 2 (n=103) | ||||||||||||||||
| Absent | 60 | 58.3 | 65 | 63.1 | 87 | 84.5 | 50 | 48.5 | 45 | 43.7 | 51 | 49.5 | 100 | 97.1 | 51 | 49.5 |
| Present | 43 | 41.7 | 38 | 36.9 | 16 | 15.5 | 53 | 51.5 | 58 | 56.3 | 52 | 50.5 | 3 | 2.9 | 52 | 50.5 |
Factor analyses revealed very similar patterns of correlation between the individual symptoms in the whole sample (n=206) and when siblings 1 and 2 were considered separately (n=103) (Table 4).
Table 4 Exploratory factor analyses : rotated component matrix
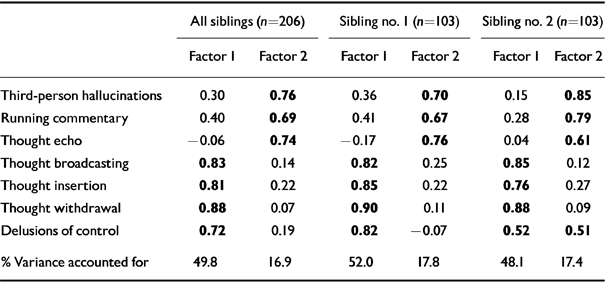
| All siblings (n=206) | Sibling no. 1 (n=103) | Sibling no. 2 (n=103) | ||||
|---|---|---|---|---|---|---|
| Factor 1 | Factor 2 | Factor 1 | Factor 2 | Factor 1 | Factor 2 | |
| Third-person hallucinations | 0.30 | 0.76 | 0.36 | 0.70 | 0.15 | 0.85 |
| Running commentary | 0.40 | 0.69 | 0.41 | 0.67 | 0.28 | 0.79 |
| Thought echo | -0.06 | 0.74 | -0.17 | 0.76 | 0.04 | 0.61 |
| Thought broadcasting | 0.83 | 0.14 | 0.82 | 0.25 | 0.85 | 0.12 |
| Thought insertion | 0.81 | 0.22 | 0.85 | 0.22 | 0.76 | 0.27 |
| Thought withdrawal | 0.88 | 0.07 | 0.90 | 0.11 | 0.88 | 0.09 |
| Delusions of control | 0.72 | 0.19 | 0.82 | -0.07 | 0.52 | 0.51 |
| % Variance accounted for | 49.8 | 16.9 | 52.0 | 17.8 | 48.1 | 17.4 |
In the whole sample, the analysis yielded two factors which accounted for about 67% of the total variance. Factor 1 was characterised by thought broadcasting (0.83), thought insertion (0.81), thought withdrawal (0.88) and delusions of control (0.72), and accounted for 49.8% of the variance. Third-person auditory hallucinations (0.76), running commentary (0.69) and thought echo (0.74) loaded significantly on factor 2, which explained 16.9% of the total variance.
Factor analysis on siblings 1 and 2 again provided a two-factor rotated matrix, very similar to that yielded by the whole sample but for the fact that delusions of control had a moderate loading on factors 1 and 2 in the second sibling (0.52 and 0.51 respectively).
Regression scores were calculated for both factors, and Spearman rank correlations were used to determine the association of factor scores within sib-pairs. A significant correlation was observed for factor 1 which, as previously indicated, included thought broadcasting, thought withdrawal, thought insertion and delusions of control (r=0.21, P=0.03). For factor 2 the comparable correlation coefficient was not significant (r=0.04, P=0.62).
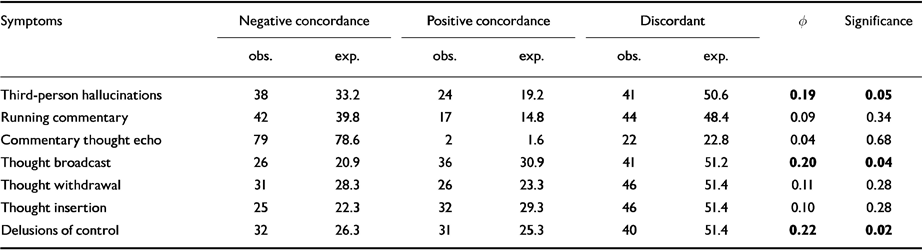
Post hoc X 2 analyses were used to look for significant correlations between the individual symptoms in factors 1 and 2 (Table 5). Levels of statistical association for individual symptoms between sib-pairs were significant only for three first-rank symptoms : third-person auditory hallucinations (P=0.05), delusions of control (P=0.02) and thought broadcasting (P=0.04).
Table 5 Concordance for individual symptoms in sibling pairs : significant correlation between individual symptoms using χ2 test (Φ)
| Symptoms | Negative concordance | Positive concordance | Discordant | Φ | Significance | |||
|---|---|---|---|---|---|---|---|---|
| obs. | exp. | obs. | exp. | obs. | exp. | |||
| Third-person hallucinations | 38 | 33.2 | 24 | 19.2 | 41 | 50.6 | 0.19 | 0.05 |
| Running commentary | 42 | 39.8 | 17 | 14.8 | 44 | 48.4 | 0.09 | 0.34 |
| Commentary thought echo | 79 | 78.6 | 2 | 1.6 | 22 | 22.8 | 0.04 | 0.68 |
| Thought broadcast | 26 | 20.9 | 36 | 30.9 | 41 | 51.2 | 0.20 | 0.04 |
| Thought withdrawal | 31 | 28.3 | 26 | 23.3 | 46 | 51.4 | 0.11 | 0.28 |
| Thought insertion | 25 | 22.3 | 32 | 29.3 | 46 | 51.4 | 0.10 | 0.28 |
| Delusions of control | 32 | 26.3 | 31 | 25.3 | 40 | 51.4 | 0.22 | 0.02 |
DISCUSSION
Summary of findings
The findings suggest that certain first-rank symptoms are as familial or heritable as other individual symptoms of schizophrenia and comprise two psychopathologically distinct components. Replication would help validate the importance of first-rank symptoms in the various diagnostic criteria for schizophrenia.
Principal components analysis revealed a two-factor structure of first-rank symptoms that exhibited a degree of stability across the sub-samples of siblings. The first component (representing close to 50% of the variance) groups the primary experiences of thought alienation (insertion, withdrawal and broadcast) with delusions of control. The second component (representing less than 20% of the variance) encompasses the hallucinatory phenomena (third-person voices, running commentary, thought echo). These appear to be relatively independent components of the core syndrome, and may reflect separable anomalies of function. It has been suggested (Reference CrowCrow, 1998) that nuclear symptoms are deviations in the transition from thought to speech, and from speech to meaning. One possibility to be considered is that factor 1 identifies anomalies in the former category, i.e. in the generation of speech from thought (which can be conceived as a prefrontal and motor function), whereas factor 2 identifies a disturbance in the process by which meaning is extracted from a message received from another person, a process which presumably takes place in the region of the occipito-parieto-temporal junction. It may thus be possible to assign the factors to each of the two areas of hetero-modal association cortex that relate to motor (anterior) and sensory (posterior) functions in the brain, respectively.
Comparison with similar studies
Our findings demonstrate that a factor comprising thought insertion, thought broadcasting, thought withdrawal and delusions of control is significantly correlated in sib-pairs with schizophrenia or schizo-affective disorder. There is some agreement between the results yielded by factor analysis and those obtained from X 2 measures of association of individual symptoms. Similar studies have not always found significant correlations for first-rank symptoms. In the study by Cardno et al (Reference Cardno, Jones and Murphy1999), factor analysis of OPCRIT symptoms (Reference McGuffin, Farmer and HarveyMcGuffin et al, 1991) yielded a factor described as first-rank which had high loadings for persecutory delusions (0.52), bizarre delusions (0.63), delusions of passivity (0.73) and thought insertion (0.62). Correlations between sib-pairs for this factor were nonsignificant. In another study, Cardno et al (Reference Cardno, Jones and Murphy1999) found a modest association for broadly defined positive formal thought disorder, grandiose delusions and delusions of influence, although the authors expressed concern that multiple statistical testing might have generated false positives. With the Major Symptoms of Schizophrenia Scale, Kendler et al (Reference Kendler, Karkowski-Shuman and O'Neill1997) found significant sibling resemblance for eight of the nine items, although the correlation (r=0.16, P=0.02) for Schneiderian delusions was the least significant, and that for hallucinations was non-significant. Factor analysis of the same scale yielded a positive factor which had high loadings on hallucinations, any delusions and Schneiderian delusions with Pearson correlations between siblings that were significant (r=0.16, P=0.005). In the study of Burke et al (Reference Burke, Murphy and Bray1996) in the same sample of siblings, first-rank symptoms were subsumed under the headings ‘delusions’ and ‘hallucinations’. Again, correlations were significant for this factor. In Farmer et al's (Reference Farmer, McGuffin and Gottesmann1984) H and P subtypes, the former, including delusions of control, was identified as having the greater genetic loading. Although other studies have used different rating scales and have classified individual first-rank symptoms within wider symptom groups, there is some evidence from these studies that certain first-rank symptoms may be shared above chance expectation in sib-pairs.
Neurobiological theories of first-rank symptoms
Our findings suggest that, contrary to the conclusions of an earlier twin study (Reference McGuffin, Farmer and GottesmannMcGuffin et al, 1984), first-rank symptoms, when subgrouped, are related to whatever is familial or heritable in the predisposition to schizophrenic illness. In this study, the most significant correlation was for factor 1, which included passivity of thought and experience. Thus we speculate that certain first-rank symptoms are closer to the heritable core than others, and may reflect an underlying shared neurobiological abnormality.
Spence (Reference Spence1996) argues that first-rank symptoms reflect a breakdown in the barrier between conscious and unconscious processes, the latter assuming the nature of alien or persecutory experiences as they are not perceived as willed or generated by the self. Relevant to this view is the demonstration by Libet et al (Reference Libet, Gleason and Wright1983) that neuronal activity related to an action precedes the subject's conscious awareness of a decision to act by approximately 400-500 ms. Comparing the passivity phenomena of schizophrenia to symptoms seen in organic illnesses affecting the parietal lobe and right hemisphere, Spence et al (Reference Spence, Brooks and Hirsch1997) used positron emission tomography (PET) to investigate neural correlates. They found that patients with passivity phenomena exhibited reversible hyperactivation of the cingulate gyrus and right inferior parietal lobule, an area with afferent and efferent connections to various parts of the brain that include the sensory, prefrontal, premotor, cingulate and superior temporal cortices. The hyperactivation disappeared when the passivity phenomena remitted. These authors regarded their findings as providing some support for previous claims (Reference NasrallahNasrallah, 1985; Reference CuttingCutting, 1990) that the right hemisphere, in particular the right parietal lobe, may be responsible for ‘alienation’, defined not only as made movement but also made thoughts and affect. To explain passivity phenomena as a disturbance of external-internal spatial schemata, Spence et al (Reference Spence, Brooks and Hirsch1997) invoked the theories of Pandya & Yeterian (Reference Pandya and Yeterian1984) concerning the role of the cingulate gyrus and parietal lobe in mediating inward and outward attention respectively.
Like Nasrallah, Crow (Reference Crow1998) views the central problem as a defect in bihemispheric communication, but one that is due to incomplete functional differentiation, with the consequence that internally generated neural activity (e.g. thought) is perceived as having the character of a message from an independent source. Within the context of a bi-hemispheric theory of language, Crow (Reference Crow1998) proposes that a defect in the relationship between the linear sequencing of the dominant hemisphere and the spatial and two-dimensional organisation of concepts in the non-dominant hemisphere lies at the root of first-rank symptoms. A consequence is that the indexical reference frame of language is lost; the individual is no longer able to separate his/her own thoughts or feelings from those which are externally derived. This linguistic explanation parallels Spence's attempts to account for the loss or distortion of ego boundaries, and is consistent with Cutting's (Reference Cutting1997) argument that greater knowledge of inter-hemispheric interaction is a necessary prerequisite for progress in understanding the neurobiological basis of psychopathology.
Methodological limitations
Caution must, however, be exercised in interpreting the results of this and other sibpair studies. The design of this study does not enable one to disentangle genetic from environmental influences. Nevertheless, there is evidence from a number of behavioural genetic studies that both normal variation and psychopathology are genetically determined (Reference Plomin and DanielsPlomin & Daniels, 1987; Reference Reiss, Plomin, Hetherington, Hetherington, Reiss and PlominReiss et al, 1994). In these studies the familial or common environment was found to be relatively unimportant.
There are also limitations that are specific to this study. First, the ratings of case notes and interviews were performed by one person (J.L.), thus without interrater validity testing. Second, the study was mainly retrospective, i.e. ratings of first-rank symptoms were made from interviews and case notes collected as part of a project not specifically designed to study first-rank symptoms. However, narrow definitions were used, with the objective of increasing the accuracy of the ratings made on the available information.
CLINICAL IMPLICATIONS AND LIMITATIONS
CLINICAL IMPLICATIONS
-
▪ Nuclear symptoms are a familial component of predisposition to psychosis.
-
▪ Nuclear symptoms can be subdivided into thought withdrawal, insertion and broadcast, together with delusions of control (group I) and third-person auditory hallucinations, thought echo and running commentary (group 2).
-
▪ It is suggested that group I symptoms are anomalies of the asymmetry of frontal association cortex and group 2 of temporo-parietal-occipital association cortex.
LIMITATIONS
-
▪ Assessments of case notes and interviews were performed by one person (J.L.).
-
▪ First-rank symptoms were assessed retrospectively.
-
▪ The project was not designed specifically as a study of first-rank symptoms.








eLetters
No eLetters have been published for this article.