People with the personality disorder of psychopathy display antisocial and aggressive behaviour in association with affective and interpersonal traits such as shallow affect, manipulation of others, and lack of guilt and empathy for victims (Reference ClecklyCleckly, 1941; Reference HareHare, 1991). Such individuals present significant problems to society: for example, they do not show reduced rates of offending in response to therapy, and are four times more likely to have violently reoffended 1 year after release from prison compared with non-psychopathic offenders (Reference Hemphill, Hare and WongHemphill et al, 1998; Reference Shine and HobsonShine & Hobson, 2000).
People with psychopathy show evidence of both cognitive and emotional dysfunction, which may contribute to their behaviour (Reference Tunstall, Fahy, McGuire, Fu, Murray and RussellTunstall et al, 2003). For example, adults with psychopathy and children with psychopathic traits have selective impairments in recognising distress cues (e.g. facial and vocal expressions of fear and sadness), but show normal recognition of other facial and vocal expressions of emotions such as happiness (Blair et al, Reference Blair, Colledge and Mitchell2001a , Reference Blair, Mitchell and Richell2002; Reference Stevens, Charman and BlairStevens et al, 2001). Also, people with psychopathy show reduced autonomic responsiveness to facial expressions of fear and sadness, but normal responses to other expressions of primary emotion, such as happy faces (Reference Blair, Jones and ClerkBlair et al, 1997). Thus, it has been proposed that in psychopathy individuals do not process facial and other signals of distress as aversive, and this in turn leads to lack of empathy, failure to inhibit behaviour that engenders distress in others, and impaired moral socialisation (Blair, Reference Blair1995, Reference Blair2003). However, nobody has directly examined brain function when psychopathic individuals implicitly (unconsciously) process facial emotion. We previously reported that in healthy populations limbic and visual cortical regions are activated during facial emotion processing tasks (Reference Surguladze, Brammer and YoungSurguladze et al, 2003). To test the theory that people with psychopathy have a selective impairment in processing distress cues, we used event-related functional magnetic resonance imaging (fMRI) to examine neural responses when people with psychopathy and a control group viewed expressions of distress (fearful faces) and expressions of positive emotion (happy faces). We tested the main hypothesis that compared with controls, the psychopathy group would show reduced activation in limbic and visual cortical regions involved in processing fearful faces, but no differences when processing happy faces.
METHOD
Participants
We studied 15 right-handed adult male volunteers of normal IQ. Six had a score of 25 or above on the Hare Psychopathy Checklist–Revised (PCL–R; Reference HareHare, 1991): mean PCL–R score 29.33, range 25–34; mean age 36 years, s.d.=9; full-scale IQ (FSIQ 90, s.d.=3). Nine were healthy men from the general population (mean age 27 years, s.d.=5; FSIQ 120, s.d.=18). In the UK it is accepted practice to define psychopathy as a score of 25 or above on the PCL–R (Cooke, Reference Cooke1995a ,Reference Cooke b , Reference Cooke1996, Reference Cooke and MichieCooke & Michie, 1999). The Wechsler Adult Intelligence Scale–Revised (Reference WechslerWechsler, 1981) was used to measure IQ. All participants in both groups were unmedicated; all were screened to exclude comorbid psychiatric illness (e.g. schizophrenia, major depression) and neurological and extracerebral disorders that might affect brain function (e.g. epilepsy or hypertension). People with psychopathy were recruited from forensic mental health services in south-east London (South London and Maudsley National Health Service Trust) and south-west London (St George's Healthcare NHS Trust). Four of the six volunteers in the psychopathy group were detained under the Mental Health Act 1983 under hospital orders (section 37/41), although at the time of scanning one of the four was under supervision in the community. Another was detained under section 3 of the Mental Health Act under the category of psychopathy. One of the volunteers had been discharged from detention under section 37/41 but continued to be supervised in the community. All were repeat offenders with multiple offence types. Index offences included attempted murder, manslaughter, attempted rape, multiple rape with strangulation, false imprisonment and indecent assault, and grievous bodily harm. None of the psychopathy group met the criteria for a substance misuse or dependence disorder within the 6 months prior to scanning, with the exception of one person who fulfilled criteria for harmful use of cocaine. Results were analysed with and without the inclusion of this individual (see Discussion). Ethical approval was obtained from the ethical committee of the South London and Maudsley Trust and Institute of Psychiatry, and the St George's Healthcare Trust. After a complete description of the study to the participants, written informed consent was obtained. The participants were familiarised with the stimuli and task procedures before scanning.
Functional neuroimaging task
Each volunteer participated in two 6 min experiments employing event-related fMRI. Participants were presented with facial expressions of happiness in one experiment and fear in the other experiment, at two intensities (low and prototypic) and also with neutral expressions, from a standardised series of prototypical facial expressions posed by ten different volunteers (Reference Young, Calder and SprengelmeyerYoung et al, 2002; Reference Surguladze, Brammer and YoungSurguladze et al, 2003). In each experiment, all stimuli were presented in a pseudo-randomised order while avoiding successive presentation of expressions of the same emotional intensity. Each stimulus type (i.e. intensity of expression regardless of face identity) was preceded by similar numbers of each of the other two stimulus types, to minimise the effect of the preceding stimulus type upon neural responses to the stimulus of interest. In summary, there were ten faces with three levels of intensity, each of which was presented twice to give a total of 60 stimuli per experiment. The duration of the interstimulus interval varied from 3 s to 8 s according to a Poisson distribution to prevent participants predicting the timing of the next stimulus presentation, with an average interval length of 4.9 s. During the interstimulus interval participants viewed a fixation cross. In subsequent analyses the fixation cross was used as the baseline stimulus in each of the experiments.
Participants were requested to decide upon the gender of each face and to press one of two buttons accordingly with the right thumb; for a full description of the experimental design and stimulus parameters, see Surguladze et al (Reference Surguladze, Brammer and Young2003). In pre-scan testing all participants were able to identify the gender of the faces correctly.
Image acquisition
Magnetic resonance images were acquired using a GE Signa 1.5 T system (General Electric, Milwaukee, Wisconsin, USA) with an operating console and software (Advanced Nuclear Magnetic Resonance, Woburn, Massachusetts, USA) for gradient echo echoplanar imaging (EPI) at the Maudsley Hospital, London. A quadrature birdcage headcoil was used for radio frequency transmission and reception. An inversion recovery EPI data-set was acquired at 43 near-axial 3 mm thick planes parallel to the anterior–posterior commissural line: time to echo (TE) 73 ms, time to inversion (TI) 180 ms, time to recovery (TR) 16 s, in-plane resolution 1.72 mm, interslice gap 0.3 mm, matrix size 128 ×128 pixels. This higher-resolution EPI data-set provided whole brain coverage and was later used to register the fMRI data-sets acquired from each individual in standard stereotactic space. In total 180 T 2-weighted images depicting blood oxygen level dependent (BOLD) contrast were acquired at each of 16 near-axial noncontiguous 7 mm thick planes parallel to the intercommissural line: TE 40 ms, TR 2 s, in-plane resolution 3.44 mm, interslice gap 0.7 mm, matrix size 64×64 pixels.
Neuroimaging data analysis
Individual brain activation maps
Data were analysed with software developed at the Institute of Psychiatry, London, using a non-parametric approach (for a full description and references, see http://www.brainmap.it). Experimental responses were analysed by convolving each contrast of interest–neutral and emotional expressions v. baseline (the fixation cross) and prototypic emotional v. neutral – with two gamma variate functions (peak responses at 4 s and 8 s). The best fit between the weighted sum of these convolutions and the time series at each voxel was computed using the constrained BOLD effect model of Friman et al (Reference Friman, Borga and Lundberg2003). Following computation of the model fit, a goodness-of-fit statistic was computed: this consisted of the ratio of the sum of squares of deviations from the mean image intensity (over the whole time series) due to the model to the sum of squares of deviations due to the residuals (SSQ ratio). Following computation of the observed SSQ ratio at each voxel, the data were permuted by the wavelet-based method (Reference Bullmore, Long and SucklingBullmore et al, 2001), from which activation of voxels and clusters can be detected at any desired type 1 error rate (Reference Bullmore, Suckling and OvermeyerBullmore et al, 1999).
Within-group comparisons of experimental responses to each contrast of interest (neutral and emotional responses v. baseline, and emotional expressions v. neutral) were then computed separately for the psychopathy and control groups. The observed and permuted SSQ ratio maps for each individual were transformed into the standard space (Reference Talairach and TournouxTalairach & Tournoux, 1988) using the two-stage warping procedure described in detail elsewhere (Reference Brammer, Bullmore and SimmonsBrammer et al, 1997). Group activation maps were then computed by determining the median SSQ ratio at each voxel (over all individuals) in the observed and permuted data maps (medians are used to minimise outlier effects). The distribution of median SSQ ratios over all intracerebral voxels from the permuted data were then used to derive the null distribution of SSQ ratios, which can be thresholded to produce group activation maps at any desired voxel or cluster-level type 1 error rate. In the two-level clustering procedure (described in detail by Reference Bullmore, Suckling and OvermeyerBullmore et al, 1999), the first (voxelwise) thresholding is carried out at an uncorrected P value of 0.05 to give the maximum allowable sensitivity. In order to eliminate the resulting false-positive activations, a second, cluster-level thresholding step is carried out, and the threshold of this second step is adjusted to give an expectation of less than one false-positive cluster over the whole brain. As the cluster level threshold is set at the whole brain level, the normal, voxelwise issue of multiple comparisons does not apply.
Here we report results from the group activation maps of prototypic (100%) expressions v. neutral from the ‘fear’ and ‘happy’ experiments for both the psychopathy and control groups, in which neutral faces were used as non-emotive control stimuli. We predicted that the psychopathy group would show a normal pattern of increased limbic and visual cortical response to happy faces compared with neutral faces, but would fail to show increased responses to fearful faces compared with neutral faces.
Between-group contrasts
Comparisons of responses between groups or experimental conditions was performed using non-parametric analysis of variance (ANOVA). Data were fitted at each intracerebral voxel at which all participants have non-zero data using a linear model of the type Y=a+bX+e, where Y is the vector of SSQ for each individual, X is the contrast matrix for the particular intercondition/group contrasts required, a is the mean effect across all individuals in the various conditions/groups, b is the computed group/condition difference and e is a vector of residual errors. The model is fitted by minimising the sum of absolute deviations rather than the sums of squares to reduce outlier effects. The null distribution of b is computed by permuting data between conditions/groups (assuming the null hypothesis of no effect of experimental condition or group membership) and refitting the above model. Group difference maps are computed as described above at voxel or cluster level by appropriate thresholding of the null distribution of b. This is a standard method for tests of this kind and it gives exact P values with minimum assumptions (Reference EdgingtonEdgington, 1995).
We tested the main hypothesis that compared with controls, the psychopathy group would show reduced activation in limbic and visual regions involved in processing fearful faces, but no difference when processing happy faces. Hence we undertook a two-way ANOVA to determine voxel- and cluster-wise between-group differences in BOLD signal to each of the two different facial expression–baseline contrasts for fear and happiness.
In addition, we carried out a two-group (control, psychopathy)×two-condition (neutral, prototypic emotion) ANOVA to determine voxel- and cluster-wise between-group differences in BOLD signal change to neutral and prototypically fearful faces and neutral and prototypically happy faces. Group×condition analysis tested for interaction effects – that is, differences in the effect of changes from neutral to prototypic emotion on neural response in healthy controls and people with psychopathy. We tested the subsidiary hypothesis that selective deficits in fear processing in the psychopathy group would produce significant between-group differences in change in neural response from neutral to emotion expressions for facial fear, but not for facial happiness.
RESULTS
There was no significant difference in response accuracy (percentage correct) or reaction times (seconds) in either experiment. For fearful faces, the mean accuracy was 98% (s.d.=2) in the psychopathy group and 97% (s.d.=7) in the control group; the mean reaction time in the psychopathy group was 0.87 s (s.d.=0.21) and in the control group it was 0.9 s (s.d.=0.18). For happy faces, the mean accuracy was 99% (s.d.=1) in the psychopathy group and 98% (s.d.=5) in the control group; the mean reaction time in the psychopathy group was 0.92 s (s.d.=0.21) and in the control group it was 0.95 s (s.d.=0.25).
Facial fear experiment
Between-group comparisons
A two-way ANOVA of the fear–baseline contrast in each group (Table 1) revealed that compared with people with psychopathy, control participants showed areas of significantly greater activation in the cerebellum and fusiform gyrus (Brodmann area 19) bilaterally, and in the left postcentral gyrus (BA 4) (see Data supplement 1a to the online version of this paper). No area was activated more in people with psychopathy than in healthy controls.
Table 1 ‘Fear’ experiment: results reported only for contrasts that reveal significant differences between groups or conditions

| Comparison | Size1 | Talairach coordinates | P | BA | Cerebral region | ||
|---|---|---|---|---|---|---|---|
| (x) | (y) | z) | |||||
| Between group comparisons2 | |||||||
| Two-way ANOVA of neural response to fearful faces Control group>psychopathy group | 130 | 32 | -63 | -24 | 0.001 | Cerebellum | |
| 223 | 29 | -81 | 18 | 19 | Fusiform gyrus3 | ||
| 81 | -40 | -67 | -24 | 0.008 | Cerebellum | ||
| 163 | -32 | -78 | -18 | 19 | Fusiform gyrus3 | ||
| 106 | -40 | -22 | 48 | 0.0002 | 3 | Postcentral gyrus | |
| Control and psychopathy group × condition analysis | 79 | 26 | -74 | -24 | 0.005 | Cerebellum | |
| 193 | 32 | -70 | -18 | 19 | Fusiform gyrus3 | ||
| 82 | -40 | -22 | 42 | 0.002 | 3 | Postcentral gyrus | |
| Within group comparisons | |||||||
| Control group: fearful faces > neutral faces4 | 132 | 40 | 15 | 20 | 0.0003 | 46 | Middle frontal gyrus |
| 119 | 29 | -59 | 31 | 0.0003 | 39 | Angular gyrus | |
| 110 | -40 | -22 | 48 | 0.0003 | 3 | Postcentral gyrus | |
| 70 | 32 | -63 | -24 | 0.0008 | Cerebellum | ||
| 25 | -40 | -74 | -13 | 0.004 | 19 | Fusiform gyrus | |
| 23 | 22 | -81 | -13 | 0.003 | 19 | Fusiform gyrus | |
| Control group: neutral faces>fearful faces | 43 | 4 | 0 | 54 | 0.0005 | 6 | Premotor cortex, SMA |
| Psychopathy group: fearful faces>neutral faces5 | 55 | 40 | 15 | -2 | 0.001 | 13 | Insula |
| 78 | 25 | -67 | 26 | 0.0003 | 7 | Precuneus | |
| Psychopathy group: neutral faces>fearful faces | 75 | 40 | -63 | -24 | 0.0003 | Cerebellum | |
| 223 | 32 | -59 | -18 | 37 | Fusiform gyrus3 | ||
| 42 | -36 | -74 | -18 | 0.0003 | 19 | Fusiform gyrus | |
| 123 | -36 | -74 | -7 | 19 | Inferior occipital gyrus3 | ||
| 75 | -36 | -30 | 42 | 0.0003 | 40 | Inferior parietal lobule | |
A two-group (control, psychopathy) ×two-condition (neutral, fear) ANOVA (Fig. 1a) revealed an interaction in a cluster including active areas in the right cerebellum and fusiform gyrus (BA 19) and in the postcentral gyrus (BA 3).
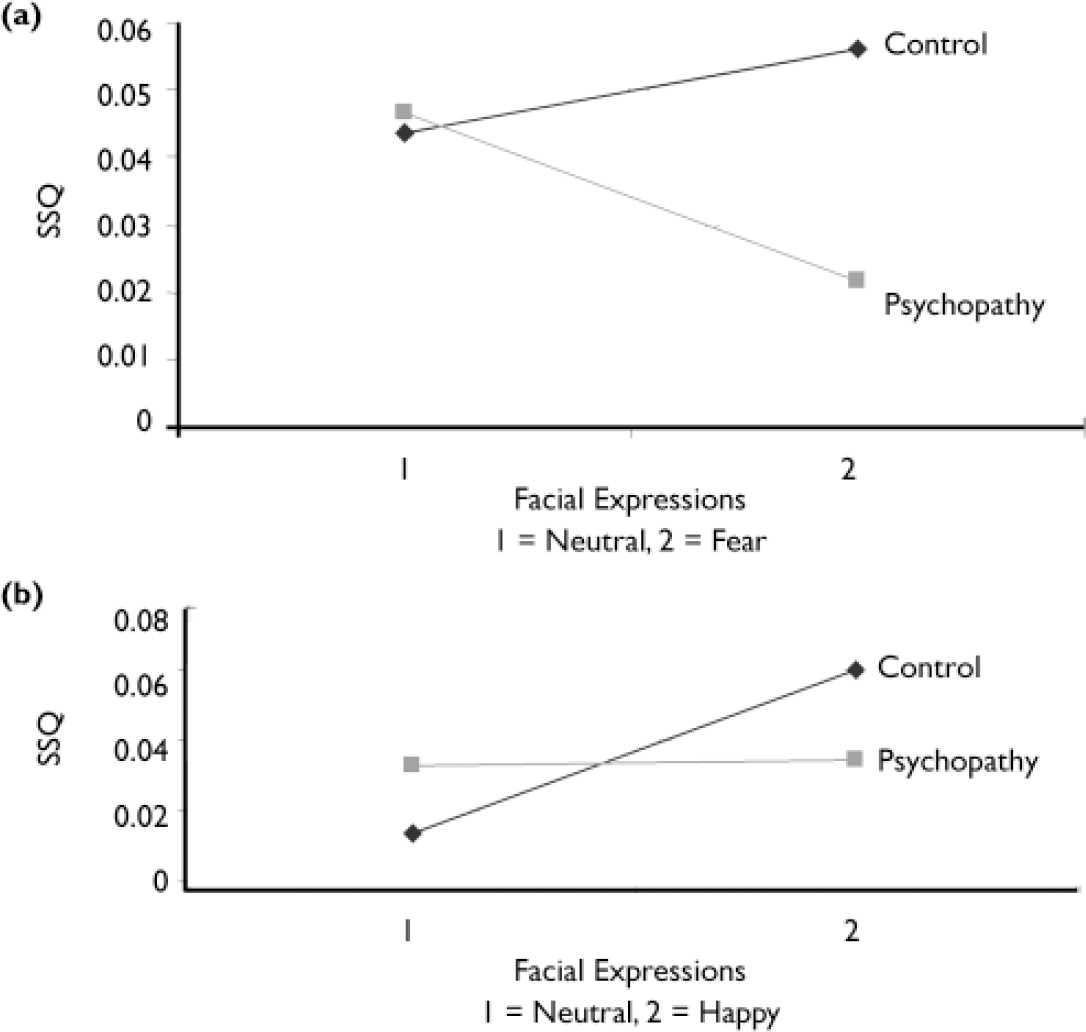
Fig. 1 Interaction between group and expression: (a) fearful v. neutral: right cerebellum and fusiform gyrus; (b) happy v. neutral: right lingual, middle occipital and fusiform gyri (SSQ, sum of squares ratio – see text).
Within-group comparisons
Healthy control participants demonstrated greater activation to fearful faces compared with neutral faces in clusters that included areas in the right fusiform gyrus (BA 19), cerebellum, middle frontal gyrus (BA 46), inferior frontal gyrus (BA 9) and precuneus (BA 31), and the left fusiform gyrus (BA 19) and postcentral gyrus (BA 3) (see Data supplement 2a to the online version of this paper). Greater activation to neutral faces compared with fearful faces was demonstrated in the right premotor cortex and supplementary motor area (BA 6) and the left anterior cingulate gyrus (BA 24).
Participants with psychopathy demonstrated greater activation to fearful faces compared with neutral faces in clusters that included areas in the right insula (BA 13) and precuneus (BA 7) (see Data supplement 2b to the online version of this paper). Greater activation to neutral faces compared with fearful faces was demonstrated in clusters including areas in the right cerebellum and fusiform gyrus (BA 37), and the left fusiform gyrus (BA 19), inferior occipital gyrus (BA 19) and inferior parietal lobule (BA 40) (see Data supplement 2c to the online version of this paper).
Facial happiness experiment
Between-group comparisons
A two-way ANOVA of the happy–baseline contrast in each group revealed that compared with people with psychopathy, control participants showed areas of significantly greater activation in the right fusiform gyrus (BA 19) and left lingual gyrus (BA 18), cerebellum and precentral gyrus (BA 4) (see Data supplement 1b to the online version of this paper, and Table 2). There was no area in which activation was greater in people with psychopathy compared with the control group.
Table 2 ‘Happy’ experiment: results reported only for contrasts that reveal significant differences between groups or conditions

| Comparison | Size1 | Talairach coordinates | P | BA | Cerebral region | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Between group comparisons2 | |||||||
| Two-way ANOVA of neural response to happy faces | |||||||
| Control group > psychopathy group: main effect of group analysis | 321 | 32 | -81 | -13 | 0.0003 | 19 | Fusiform gyrus |
| 613 | -7 | -89 | -18 | 18 | Lingual gyrus3 | ||
| 443 | -29 | -63 | -24 | Cerebellum3 | |||
| 70 | -32 | -18 | 42 | 0.005 | 4 | Precentral gyrus | |
| Control and psychopathy group × condition analysis | 58 | 25 | -70 | -13 | 0.004 | 18 | Lingual gyrus |
| 113 | 32 | -81 | -7 | 18 | Middle occipital gyrus3 | ||
| 103 | 25 | -70 | -18 | 19 | Fusiform gyrus3 | ||
| Within-group comparison4 | |||||||
| Control group: happy faces > neutral faces | 256 | 32 | -74 | -18 | 0.0003 | 19 | Fusiform gyrus |
| 293 | 25 | -78 | -24 | Cerebellum3 | |||
| 47 | -22 | -70 | 20 | 0.0008 | 31 | Precuneus | |
| 58 | -32 | -22 | 48 | 0.0003 | 3 | Postcentral gyrus | |
| 31 | -36 | -52 | -35 | 0.0008 | Cerebellum | ||
| 223 | -18 | -85 | -18 | 18 | Fusiform gyrus3 | ||
| 22 | -29 | -56 | -24 | 0.002 | Cerebellum | ||
| 83 | -7 | -74 | -7 | 18 | Lingual gyrus3 | ||
| Control group: neutral faces > happy faces | 37 | -22 | 7 | 4 | 0.001 | 69 | Putamen |
| Psychopathy group: happy faces > neutral faces | 122 | 36 | -67 | -24 | 0.0003 | Cerebellum | |
| 63 | 22 | -74 | -18 | 19 | Fusiform gyrus3 | ||
| 71 | -40 | -78 | -13 | 0.0009 | 19 | Fusiform gyrus | |
| 41 | 32 | -63 | 42 | 0.001 | 19 | Precuneus | |
| 45 | 7 | 0 | 42 | 0.001 | 24 | Anterior—middle cingulate gyrus | |
| 49 | -22 | -67 | 42 | 0.002 | 7 | Superior parietal lobule | |
The two-group (control, psychopathy)×two-condition (neutral, happy) ANOVA revealed an interaction in a cluster including active areas in the right lingual gyrus (BA 18), middle occipital gyrus (BA 18) and fusiform gyrus (BA 19) (Table 2; Fig. 1b).
Within-group comparisons
The control group demonstrated greater activation to happy faces compared with neutral faces in clusters that included areas in the right fusiform gyrus (BA 19) and cerebellum; the left cerebellum, fusiform gyrus (BA 18) and lingual gyrus (BA 18); the left postcentral gyrus (BA 3) and precentral gyrus (BA 4), and the precuneus (BA 31) (see Data supplement 3a to the online version of this paper). Greater activation to neutral faces compared with happy faces was demonstrated in the left putamen.
The psychopathy group demonstrated greater activation to happy faces compared with neutral faces in clusters that included areas in the right cerebellum and fusiform gyrus (BA 19); left fusiform gyrus (BA 19) and middle occipital gyrus (BA 19); right precuneus (BA 19), anterior cingulate gyrus (BA 24) and medial frontal gyrus (BA 6); and left superior parietal lobule (BA 7) and precuneus (BA 7) (see Data supplement 3b to the online version of this paper). No area was more active in response to neutral faces compared with happy faces.
DISCUSSION
We carried out a cross-sectional event-related fMRI study of brain activation when people with psychopathy and healthy control participants implicitly processed fearful, happy and neutral facial expressions. We tested the hypothesis that, compared with the control group, the psychopathy group would show reduced activation in limbic and visual cortical regions when processing fearful faces, but not when processing happy faces. Between-group comparisons revealed that compared with controls, the psychopathy group showed significantly reduced activation in the fusiform and extrastriate cortices, and in other brain regions when processing both facial emotions. Hence, the psychopathy group showed reduced neural responses not only to facial expressions of distress (fear), but also to expressions of positive emotion (happiness).
However, response pattern differed by emotion type. Group×condition analysis revealed a cluster in the right lingual and fusiform cortices in which the control group showed a greater increase in activation than the psychopathy group when processing happy faces compared with neutral faces (see Fig. 1b and Data supplement 3 to the online version of this paper). In contrast, when processing fearful faces compared with neutral faces, the control group showed increased activation in the right cerebellum and fusiform gyrus (see Fig. 1a) but the psychopathy group showed decreased activation in these regions.
Further evidence that response pattern differs with emotion type was provided by separate within-group contrasts of fearful faces compared with neutral faces, and happy faces compared with neutral faces. For example, both participants with psychopathy and control participants activated overlapping brain regions when processing happy faces compared with neutral faces, including bilateral fusiform and extrastriate cortices. In contrast, participants in control and psychopathy groups activated different regions when processing fearful faces compared with neutral faces – for example, control participants activated the fusiform gyrus bilaterally, whereas those in the psychopathy group did not. Instead, the latter showed decreased rather than increased bilateral fusiform gyrus activation to fearful faces compared with neutral faces.
In summary, people with psychopathy show reduced visual cortical activation in response to both fearful and happy faces compared with controls. However, they also show a normal pattern of increased visual cortical responses to happy faces compared with neutral faces, in contrast to an atypical pattern of decreased visual cortical responses to fearful faces compared with neutral faces.
Prior neuroimaging studies have consistently shown increased fusiform and extrastriate cortical activation in response to happy v. neutral expressions (Reference Surguladze, Brammer and YoungSurguladze et al, 2003). Hence, our results suggest that the neural substrates for processing facial expressions of happiness are functionally intact in psychopathic disorder, although less responsive than those of controls. However, prior studies of facial emotion recognition in children with psychopathic traits did not report differences in recognition of happy faces compared with controls (Reference Blair, Colledge and MurrayBlair et al, 2001b ). Hence, the neural hyporesponsiveness to happy faces we found in our psychopathy group may not be associated with significant impairments of behaviour. We did not directly study this issue, however. Further studies are required to investigate the relationship between emotion recognition and brain function when people with psychopathy process happy facial expressions.
Prior neuroimaging studies in healthy individuals have also consistently demonstrated increased visual cortical activation in response to fearful faces (Morris et al, Reference Morris, Frith and Perrett1996, Reference Morris, Friston and Buchel1998; Reference Vuilleumier, Richardson and ArmonyVuilleumier et al, 2004). Hence, findings of reduced rather than increased visual cortical response to fearful faces compared with neutral faces in psychopathic individuals suggest an atypical pattern of facial fear processing in people with this disorder.
In healthy people, visual cortical activation in response to fearful faces is boosted by feedback modulation from the amygdala (Reference Vuilleumier, Richardson and ArmonyVuilleumier et al, 2004). Hence, reduced rather than increased visual cortical response to fearful faces compared with neutral faces in psychopathy may reflect differences in amygdala function in people with this disorder. This would support suggestions that amygdala dysfunction underpins selective deficits in processing facial expressions of distress in adults with psychopathy and children with psychopathic traits; including recognition of fearful and sad faces (Reference Blair, Colledge and MurrayBlair et al, 2001b ) and reduced autonomic responsiveness to distress cues (Reference Blair, Jones and ClerkBlair et al, 1997). We did not find significant between-group differences in amygdala function; this might be due to our small sample size, or to other factors (see below). Nevertheless, others reported that people with psychopathy have low resting skin conductance and reduced aversive conditioning relative to people without this disorder, suggesting reduced amygdala activity (Reference Hare and QuinnHare & Quinn, 1971; Reference HareHare, 1982; Reference PatrickPatrick, 1994; Reference Birbaumer, Veit and LotzeBirbaumer et al, 2005). Also, people with psychopathy show reduced amygdala activity during aversive conditioning and when processing negative valence words (Reference Kiehl, Smith and HareKiehl et al, 2001; Reference Birbaumer, Veit and LotzeBirbaumer et al, 2005). Furthermore, there is an association between reduced amygdaloid volume and increased levels of psychopathy (Reference Tiihonen, Hodgins and VaurioTiihonen et al, 2000). In addition, in a non-psychiatric population of college students, high scorers on the emotional–interpersonal factor of a trait measure of psychopathy (the Psychopathy Personality Inventory) showed reduced amygdala activation during a facial affect recognition task compared with low scorers (Reference Gordon, Baird and EndGordon et al, 2004). Thus, we plan in future studies, and in larger samples, to investigate the ‘connectivity’ of amygdala and cortical facial emotion processing areas.
Facial fear processing, aversive conditioning and socialisation
The differences we found when people with psychopathy process facial emotion may contribute to their clinical phenotype. For example, failure to recognise and emotionally respond to facial and other signals of distress may underlie failure to inhibit behaviour that engenders distress in others during social interactions; or, more generally, may underlie the lack of emotional empathy observed in this population (Blair, Reference Blair1995, Reference Blair2003). Also, reduced affective responses to facial expressions of distress may lead to failure to form conditioned associations between representations of behaviour that engendered distress and aversive arousal, so contributing to impaired moral socialisation (Blair, Reference Blair1995, Reference Blair2003). Further, generalised impairment of aversive conditioning may make individuals with psychopathy less anxious when anticipating the consequences of their actions, and less responsive to punishment occurring as a result of their actions (Reference BlairBlair, 2001; Reference VidingViding, 2004).
Limitations of the study
There were several potential limitations to our study, including sample size, the failure to directly detect amygdala activity in within-group and between-group contrasts, and the inclusion in the psychopathy group of one person with a recent history of harmful use of cocaine. Nevertheless, we employed a conservative analysis method to reduce the risk of type 1 errors, so that our findings are likely to reflect true activations. Hence, the activations we report are likely to remain even if increasing sample size reveals additional active brain areas.
Our failure to find activation of the amygdala in any of our contrasts between the psychopathy and control groups may be due to our small sample size and hence power limitations. However, we previously detected differential amygdala activation in the fear–neutral contrast in a healthy control group (n=9) using the same paradigm (Reference Surguladze, Brammer and YoungSurguladze et al, 2003). Similarly, differential amygdala activation has been detected in psychopathy during an emotion processing task in the same number of people (n=6) (Reference Muller, Sommer and WagnerMuller et al, 2003). Also, not all previous studies of facial fear perception in healthy controls have demonstrated amygdala activation (Reference Sprengelmeyer, Rausch and EyselSprengelmeyer et al, 1998; Reference Lange, Williams and YoungLange et al, 2003). However, there may be scope for optimising magnetic resonance acquisition parameters in future studies (e.g. by using a smaller slice thickness, or slice-dependent variations in echo time; Reference Stocker, Kellerman and SchneiderStocker et al, 2006) to increase the likelihood of detecting amygdala activation, given its central importance to theories of social cognition in general and psychopathy in particular.
One person in the psychopathy group had displayed significant use of cocaine in the 6 months prior to scanning. However, our results did not differ when his data were dropped from the analysis.
Hence, people with psychopathy have biological differences from controls when they implicitly process facial emotion. The underlying biological substrates for processing facial expressions of happiness are functionally intact, although less responsive than those of controls. In contrast, people with psychopathy display an atypical pattern of response to fearful faces compared with neutral faces, including decreased activation of the fusiform and extrastriate cortical regions. This may partly account for impaired recognition of and reduced autonomic responsiveness to expressions of fear, and impairments of empathy. Further studies are required to elucidate how these abnormalities arise and how they affect social behaviour and socialisation.
Acknowledgements
We gratefully acknowledge the participation of all our volunteers, and the comments of three anonymous referees.






eLetters
No eLetters have been published for this article.