The diagnosis of autism spectrum disorder (ASD) is based on developmental history and the presence of abnormal behaviours. The clinical picture is heterogeneous and the aetiology is unknown. One hypothesis that has been advanced, to account for the cognitive style in autism, suggests that it is best described as an extreme variant of male intelligence. Reference Asperger, Firth and Firth1–Reference Baron-Cohen3 Proponents of the ‘extreme male brain’ theory also have suggested that an increased androgen exposure in utero may contribute to the development of ASD in both genders. Reference Baron-Cohen2–Reference Knickmeyer and Baron-Cohen4 This is supported by an association between higher testosterone levels in amniotic fluid and poorer social skills at age four Reference Knickmeyer, Baron-Cohen, Raggatt and Taylor5 and higher scores on autism rating scales. Reference Auyeung, Baron-Cohen, Ashwin, Knickmeyer, Taylor and Hackett6 Hormonal studies of children with ASD have shown elevated androgens, especially in girls, Reference Geier and Geier7 and females with ASD often report androgen-related conditions in adulthood. Reference Ingudomnukul, Baron-Cohen, Wheelwright and Knickmeyer8 However, somewhat contradictory to the androgen hypothesis for ASD is the frequently observed androgynous appearance in both genders, the strikingly youngish look in many middle-aged individuals with ASD, and a high-pitched voice in a substantial proportion of males with ASD.
Based on the above observations, the aim of this study was to assess physical measures, supposedly related to androgen influence, in individuals with and without ASD, hence shedding further light on possible biological underpinnings of the disorder.
Method
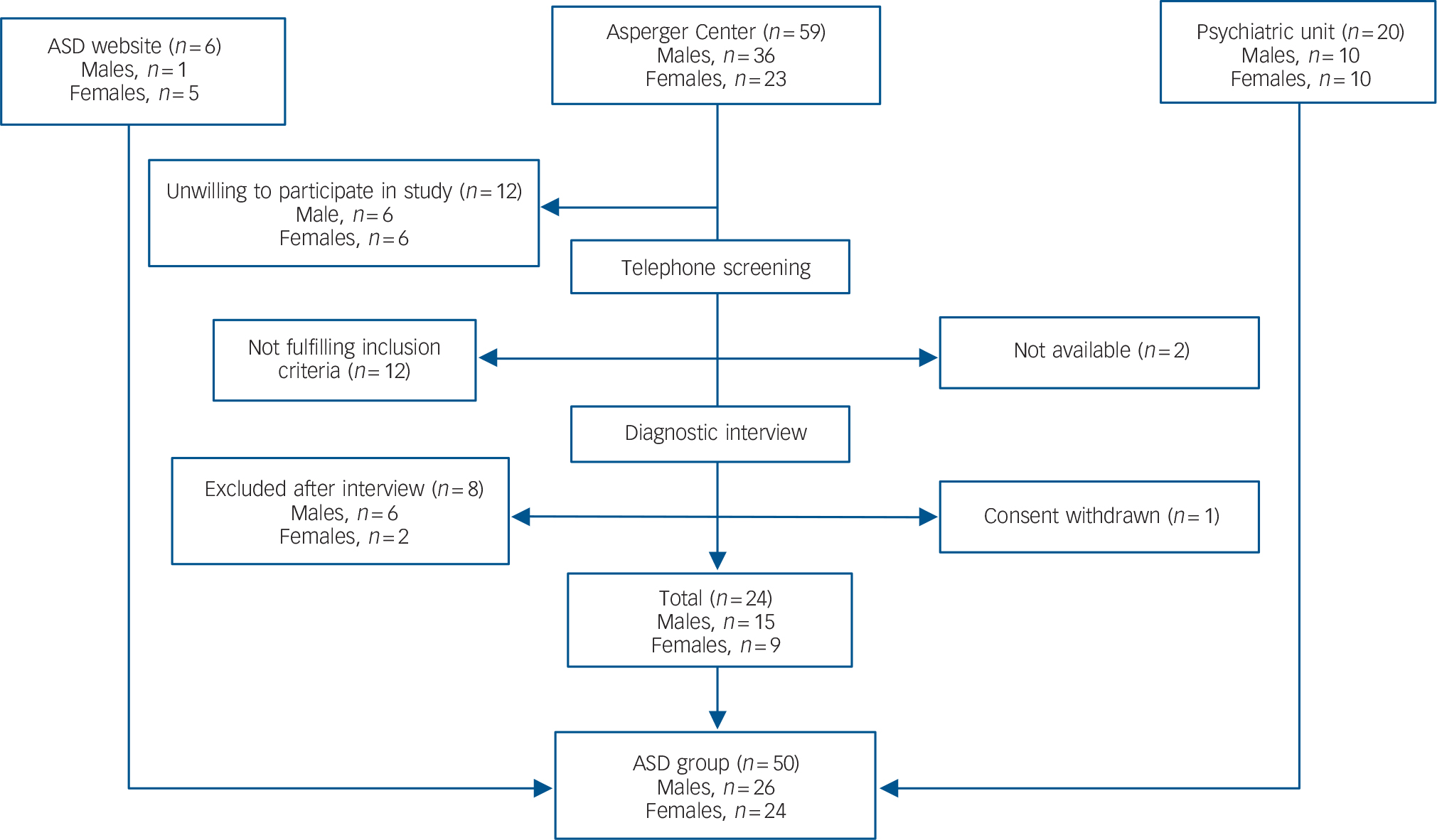
The study took place at the psychiatric clinic, St Göran Hospital, between November 2006 and October 2010. A total of 50 adults (24 women, 26 men) diagnosed with ASD and 53 healthy controls (25 women, 28 men) were included in this study (Table 1).
Participants
Inclusion criteria common to both groups were Swedish/White descent and age between 20 and 47 years. Exclusion criteria were any neurological or genetic syndrome, psychosis, any disease or medication affecting androgen status, any congenital syndrome, diagnosed malformations, intellectual disability or having attended special education in primary or secondary school. Additional exclusion criteria in the control group were ASD, ASD in a first-degree family member and (in order to match the ASD group) large or multiple tattoos or piercings. No participants reported use of sexual hormones other than the oral contraceptive combined pill, used by 4 women with ASD and 11 controls, and gestagens used additionally in 3 women in each group.
Autism spectrum disorder group
Only individuals that had previously been diagnosed with ASD were considered for this study. The standard diagnostic procedure in Sweden is rigorous and extensive. It includes developmental history obtained through structured parental interview, psychological tests and psychiatric assessments.
Individuals with ASD were recruited through: the out-patient tertiary psychiatric unit for adult attention-deficit hyperactivity disorder/autism-spectrum disorder at Northern Stockholm Psychiatry (n = 20), a community-based facility for adults with ASD in Stockholm; the Asperger Center (n = 24); and through a Swedish website for ASD (n = 6) (Fig. 1).
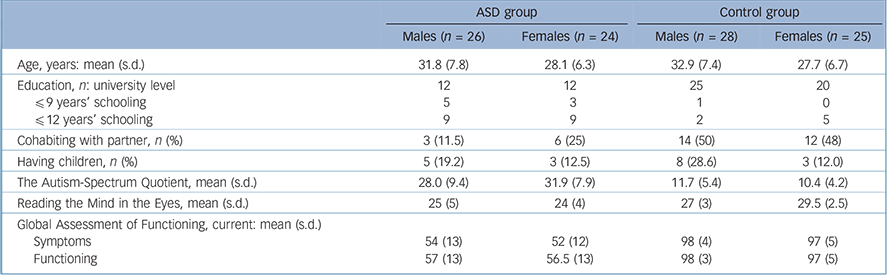
TABLE 1 Characterisation data for the final samples of the autism spectrum (ASD) group and the control group, separated by gender
| ASD group | Control group | |||
|---|---|---|---|---|
| Males (n = 26) | Females (n = 24) | Males (n = 28) | Females (n = 25) | |
| Age, years: mean (s.d.) | 31.8 (7.8) | 28.1 (6.3) | 32.9 (7.4) | 27.7 (6.7) |
| Education, n: university level | 12 | 12 | 25 | 20 |
| ≤9 years’ schooling | 5 | 3 | 1 | 0 |
| ≤12 years’ schooling | 9 | 9 | 2 | 5 |
| Cohabiting with partner, n (%) | 3 (11.5) | 6 (25) | 14 (50) | 12 (48) |
| Having children, n (%) | 5 (19.2) | 3 (12.5) | 8 (28.6) | 3 (12.0) |
| The Autism-Spectrum Quotient, mean (s.d.) | 28.0 (9.4) | 31.9 (7.9) | 11.7 (5.4) | 10.4 (4.2) |
| Reading the Mind in the Eyes, mean (s.d.) | 25 (5) | 24 (4) | 27 (3) | 29.5 (2.5) |
| Global Assessment of Functioning, current: mean (s.d.) | ||||
| Symptoms | 54 (13) | 52 (12) | 98 (4) | 97 (5) |
| Functioning | 57 (13) | 56.5 (13) | 98 (3) | 97 (5) |
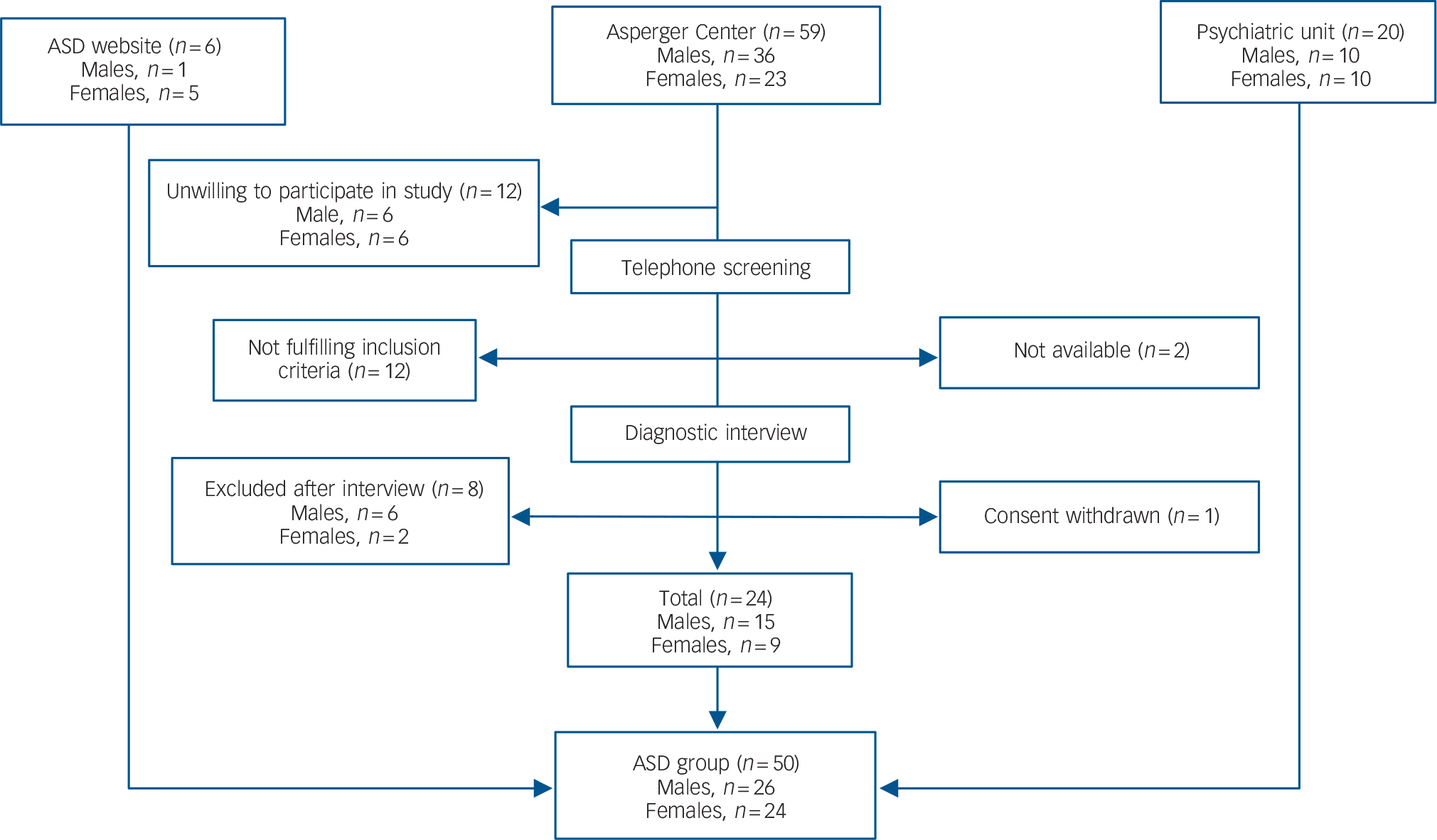
Fig. 1 Recruitment of the autism spectrum disorder (ASD) group.
Control group
Individuals were recruited from a non-profit keep-fit organisation (n = 23), Stockholm university (n = 8), university accommodation in Stockholm (n = 5), private companies (n = 4), dentists and vaccination centres (n = 5), employment agencies (n = 2) and through recommendations from friends (n = 7). We screened the healthy controls with the goal of recruiting a comparison group that would have similar gender and age distribution as the ASD group.
Procedures
Rating scales and anthropometry
Autism-spectrum disorder was confirmed by clinical assessment, supported by the Autism Diagnostic Observation Schedule (ADOS), Reference Lord, Rutter, Goode, Heemsbergen, Jordan and Mawhood9 review of previous patient records, the Autism-Spectrum Quotient Reference Baron-Cohen, Wheelwright, Skinner, Martin and Clubley10 and Reading the Mind in the Eyes. Reference Baron-Cohen, Wheelwright, Hill, Raste and Plumb11 Co-existing psychiatric disorders were assessed with the Mini International Neuropsychiatric Interview (Swedish version 5.0.0.), Reference Sheehan, Lecrubier, Harnett-Sheehan, Amorim, Janavs and Weiller12 and functional level was determined with Global Assessment of Functioning (GAF) 13 ranging from 0 to 100 for symptoms and functioning separately. A self-reported list of drugs and health remedies was administered.
A tape measure was used for determining the circumferences of head, wrists, ankles, chest, waist and hip. The waist was measured halfway between the lower rib and the iliac crest, and the hip over the widest part of the gluteal region. In women, the bosom was measured without removing the bra, and the size of the bra cup was noted. The length of the ears and 2nd to 4th ratios (2D:4D) were measured with Vernier callipers; digits were measured from the middle of the proximal crease to the fingertip. Each participant was weighed whereas height was reported by the participants. Three examiners from the research team performed all measurements (interrater reliability 0.96–1.0 (Cronbach's α), when independently assessing eight participants).
Assessment of gender coherence and age
All participants were photographed in a standardised manner, standing against a white wall in an examination room, which was used throughout the study. Digital photographs included the front and profile of both the face and full body. All participants wore underpants (and bras in females) and a shower cap to hide the hair. Obvious makeup and jewellery were removed before photographing. All photographs were digitally manipulated to show underwear of neutral shape and colour. Participants' voices were recorded with a dictation machine while reading a standardised 2-minute story.
A selected assessor group including four men and four women, ages 18–47 years, and from divergent socioeconomic backgrounds, individually assessed the digital photographs and voice samples presented on a computer. They were carefully informed to estimate whether the participants had more or less gender-typical features, but not to judge beauty. Femininity in the women and masculinity in the men were assessed, face and body unconnected, applying a 5-point Likert scale: very gender-typical; gender-typical; average; weak gender coherence; very weak gender coherence. Coded photographs and voice samples of the control and ASD groups, men and women separately, were shown in random order. First, front and profile photographs of each participant's face were shown, and the assessors were to estimate the age and gender coherence of the faces. Next, assessors estimated gender coherence from the body photographs, showing the shoulders and downwards. Finally, the assessors evaluated the voices, coded with a random number, on the computer according to the above-described 5-point Likert scale.
Interrater reliability among the assessors was calculated using Cronbach's α and was shown to be satisfactory: face gender coherence α = 0.88, body gender coherence α = 0.86 and voice gender coherence α = 0.86 (average inter-item correlations between 0.46 and 0.49). These α values did not increase when any evaluator's results were neglected. The intra-rater reliability was likewise satisfactory when a sample of the photographs was re-evaluated by six assessors a month later.
Laboratory tests
Blood samples for serum testosterone, sex hormone-binding globulin (SHBG) and dehydroepiandrosterone sulphate (DHEAS) were collected between 09.00 h and 13.20 h. The distribution of test times was roughly the same in all groups, and controlling for the time of sampling did not markedly modify the outcome.
Analysis was performed by chemiluminescence immunoassays with commercial kits obtained from Beckman Coulter, Fullerton, California, USA (‘Dxi’, serum testosterone and SHBG) and from Roche GmbH, Mannheim, Germany (‘Modular’ DHEAS). Detection limits and total assay coefficients of variation were for serum testosterone 0.35 nmol/l and 6.7%, for SHBG 0.2 nmol/l and 5.3% and for DHEAS 0.003 mol/l and 2.7% respectively. Bioactive testosterone (sum of free + albumin-bound serum testosterone) was calculated from serum testosterone, SHBG and a fixed albumin level of 40 g/l by Vermeulens equation. All analyses were carried out at the Department of Clinical Chemistry, Karolinska University Hospital.
Statistical analysis
Sociodemographic and other relevant characteristics are reported as percentages or means (standard deviation). Data distributions were analysed graphically, and no variables showed multimodal distribution. Comparisons between groups were made using Student's t-test for normally distributed data, the Mann-Whitney U-test for non-normal data and χ Reference Baron-Cohen2 distribution for categorical variables.
Bivariate correlations were performed to examine the relationships between the androgens, body mass index (BMI) and the body feature measures. Owing to skewness of androgen distributions, Spearman's rank correlation test was used for these data in addition to Pearson's r. Linear adjustments were made to compensate for strong loading of BMI on the variables ankle measure and testosterone. Data points for the presumably testosterone-dependent variables, included in Fig. 2, were converted to z-scores, based on the mean and standard deviation of the whole sample.
Cronbach reliability tests were conducted to determine the inter-reliability of assessments and measures of body features and for the intra-reliability of each assessor.
Missing data, assumed to be random, were present for 5 participants with ASD (3 men, 2 women) and 1 male control. A two-tailed P value of <0.05 was considered significant. We did not correct P values for multiple comparisons, and the statistical significance of the results must be interpreted with this taken into consideration. A k-means cluster analysis, based on body, face and voice gender coherence, was used to categorise participants into groups of varying gender typicality.
Ethical considerations
All participants provided their written informed consent to the study and received a £95 reimbursement. The regional ethics committee in Stockholm approved the study protocol. The study design was discussed in depth and approved by the local empowerment board within the Swedish Autism Society.
Results
Information on age, education, partnership, children and symptom rating is provided in Table 1; as expected, the groups differed on all symptom-related variables.
Gender and age coherence
The assessors regarded facial features as less feminine in the female ASD group than in female controls. Body constitution and voice were, however, assessed as equally feminine in the two groups.
In the male ASD group, both body constitution and voice quality were assessed as significantly less masculine than in male controls, while the assessment of facial features revealed no difference between the groups.
Androgynous signs, plausibly related to testosterone effect in participants with ASD relative to controls (with corrections for differences in BMI) are summarised in Fig. 2.
The accuracy of assessor-estimated age did not differ between groups.
Anthropometry
Women
The female ASD group had significantly higher BMI and waist-hip ratio than female controls. The difference with respect to waist-hip ratio disappeared when correcting for BMI. With or without adjustment for BMI, the bra cup size was equal in the two female groups. Although women with ASD displayed larger head and ankle circumference than female controls, there was no difference between the two groups with respect to length of ears, wrist circumference or 2D:4D ratio.
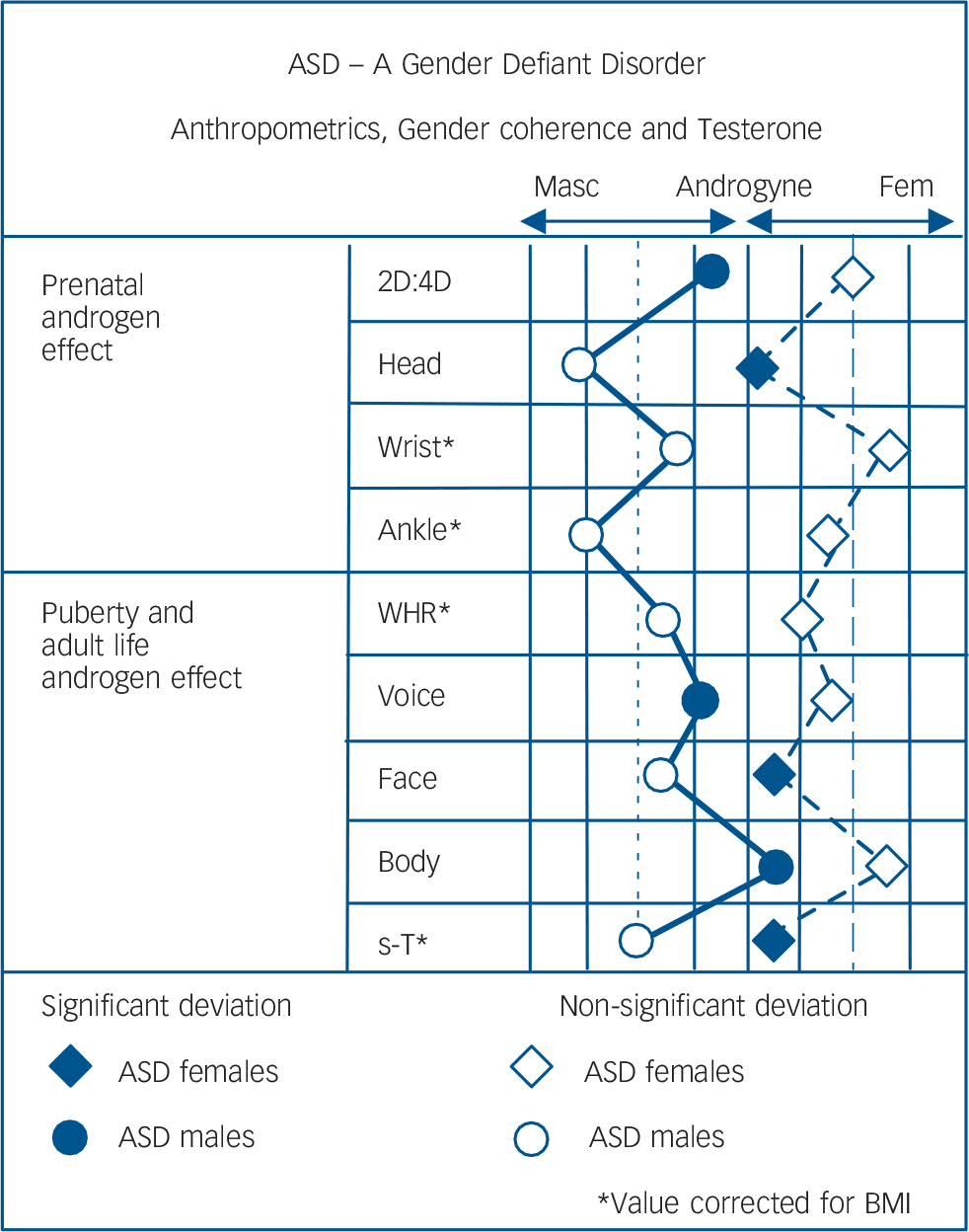
Fig. 2 Anthropometrics, gender coherence and testosterone.
Head, Wrist, Ankle refer to circumference; WHR, waist: hip ratio; Voice, Face, Body refer to gender coherence ratings; s-T, serum testosterone. Left dotted line represents the results of the male control group and right dotted line the female control group. Each solid black line represents a half standard deviation from the mean of the neurotypical control group.
Men
There was no difference between men with ASD and male controls with respect to BMI or waist-hip ratio. Men with ASD displayed higher 2D:4D ratio on the right hand and a non-significant trend for larger head circumference compared with male controls, but the two groups did not differ with respect to ankle or wrist circumferences or length of ears (Table 2).
Hormone levels
Women
The female ASD group had significantly higher total and bioactive testosterone levels compared with the female control group, a difference that remained significant after exclusion of participants on oral contraceptives and/or after correction for BMI. In two women in the ASD group, more than 4 weeks (30 and 47 days respectively) had passed since last menstruation. Their hormone levels did not differ from the rest of the women with ASD.
Men
There was no difference between the ASD group and control group with respect to serum levels of testosterone or bioactive testosterone, and this lack of difference remained also after correction for time of blood sampling. Also, a positive correlation between testosterone and SHBG was found in both groups.
DHEAS and SHBG
In each gender, neither levels of DHEAS nor SHBG differed between the two groups. In both control groups, DHEAS decreased with age (which is to be expected); this decrease was absent in both females and males with ASD (Table 3). However, in a general linear regression with DHEAS as the dependent variable, this age × group interaction was not significant.
Correlations between autistic traits and gender coherence
In the total sample, the Autism-Spectrum Quotient correlated negatively with gender coherence with respect to face and voice, but not with respect to body constitution (ρ = –0.33; P = 0.0006; ρ = –0.24; P = 0.016; ρ = –0.17; P = 0.8 respectively).
In the collapsed group of females, the Autism-Spectrum Quotient correlated negatively with facial coherence (P = 0.004), but not with body characteristics or voice (Table 4). After splitting for diagnosis, none of these correlations was significant. We found no relationship between gender coherence in facial features, body constitution or voice in females with or without ASD, and no significant correlations between serum testosterone and gender coherence or 2D:4D (data not shown).
In the collapsed male groups, a strong negative correlation was found between the Autism-Spectrum Quotient and body gender coherence (r = –0.46; P = 0.0007) but not for gender coherence of face or voice (Table 4). In control males, gender coherence in body (but not face or voice) correlated negatively with the Autism-Spectrum Quotient (ρ = –0.60; P = 0.0008), whereas the Autism-Spectrum Quotient did not correlate with any gender coherence parameters in men with ASD (possibly due to a plateau effect). In the male ASD group, gender coherence in facial features was strongly correlated with gender coherence in voice (r = 0.62; P = 0.001), which was not the case in the male control group (r = 0.23; P = 0.23), where face gender coherence instead correlated strongly with 2D:4D (r = 0.45 P = 0.018). No significant correlations between serum levels of testosterone and gender coherence or 2D:4D were found in the collapsed male groups.
Cluster analysis
In the k-means clustering based on the three gender coherence items (voice, face and body), two to six clusters were exploratively formed for each gender; however, no clusters of extreme groups (e.g. supermasculinised males) emerged. When the two clusters solution was used, significant differences between those two clusters were found in several additional variables relevant for gender coherence. Since these had not been included in the cluster formation, the validity of this clustering was supported. In this model, a significant majority of the male ASD group were categorised into the less masculine cluster, and likewise most of the female ASD group into the less feminine cluster (χ2 = 12.6 and 11.0 respectively; d.f. = 1, P<0.001), as shown in Table 5.
Discussion
The present findings provide some support for the clinical observations that prompted this study, i.e. that women with ASD often display less feminine characteristics than women without ASD, and that men with ASD often display less masculine characteristics than men without ASD. More autistic traits were thus related to effeminate body characteristics in men and less feminine facial features in women. In addition, a strong relationship was found between autistic traits, according to the Austism-Spectrum Quotient scores, and less gender coherence of face and voice in the total sample of participants, irrespective of gender and diagnosis. Also, the vast majority of participants
TABLE 2 Anthropometric measures and gender coherence in the autism spectrum disorder (ASD) and control groups
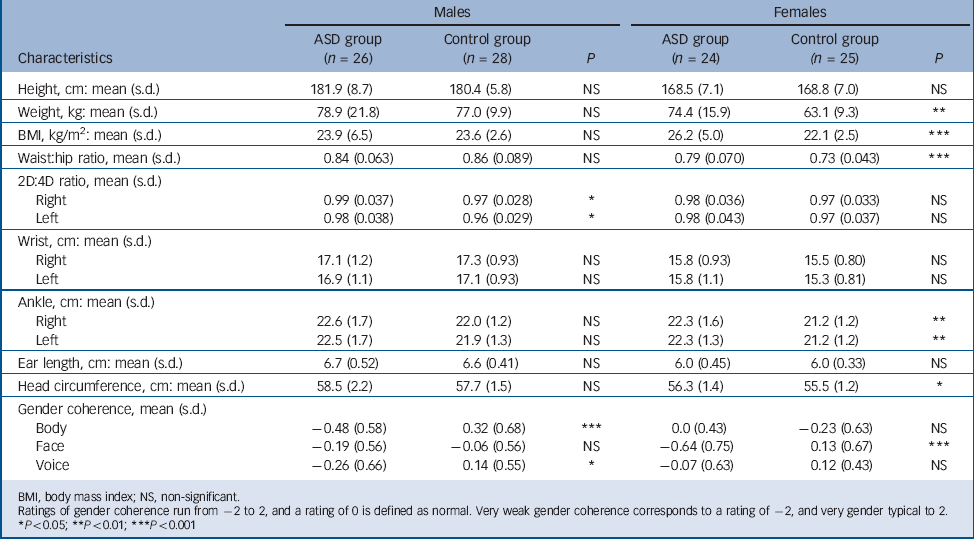
| Males | Females | |||||
|---|---|---|---|---|---|---|
| Characteristics | ASD group (n = 26) | Control group (n = 28) | P | ASD group (n = 24) | Control group (n = 25) | P |
| Height, cm: mean (s.d.) | 181.9 (8.7) | 180.4 (5.8) | NS | 168.5 (7.1) | 168.8 (7.0) | NS |
| Weight, kg: mean (s.d.) | 78.9 (21.8) | 77.0 (9.9) | NS | 74.4 (15.9) | 63.1 (9.3) | ** |
| BMI, kg/m2: mean (s.d.) | 23.9 (6.5) | 23.6 (2.6) | NS | 26.2 (5.0) | 22.1 (2.5) | *** |
| Waist:hip ratio, mean (s.d.) | 0.84 (0.063) | 0.86 (0.089) | NS | 0.79 (0.070) | 0.73 (0.043) | *** |
| 2D:4D ratio, mean (s.d.) | ||||||
| Right | 0.99 (0.037) | 0.97 (0.028) | * | 0.98 (0.036) | 0.97 (0.033) | NS |
| Left | 0.98 (0.038) | 0.96 (0.029) | * | 0.98 (0.043) | 0.97 (0.037) | NS |
| Wrist, cm: mean (s.d.) | ||||||
| Right | 17.1 (1.2) | 17.3 (0.93) | NS | 15.8 (0.93) | 15.5 (0.80) | NS |
| Left | 16.9 (1.1) | 17.1 (0.93) | NS | 15.8 (1.1) | 15.3 (0.81) | NS |
| Ankle, cm: mean (s.d.) | ||||||
| Right | 22.6 (1.7) | 22.0 (1.2) | NS | 22.3 (1.6) | 21.2 (1.2) | ** |
| Left | 22.5 (1.7) | 21.9 (1.3) | NS | 22.3 (1.3) | 21.2 (1.2) | ** |
| Ear length, cm: mean (s.d.) | 6.7 (0.52) | 6.6 (0.41) | NS | 6.0 (0.45) | 6.0 (0.33) | NS |
| Head circumference, cm: mean (s.d.) | 58.5 (2.2) | 57.7 (1.5) | NS | 56.3 (1.4) | 55.5 (1.2) | * |
| Gender coherence, mean (s.d.) | ||||||
| Body | –0.48 (0.58) | 0.32 (0.68) | *** | 0.0 (0.43) | –0.23 (0.63) | NS |
| Face | –0.19 (0.56) | –0.06 (0.56) | NS | –0.64 (0.75) | 0.13 (0.67) | *** |
| Voice | –0.26 (0.66) | 0.14 (0.55) | * | –0.07 (0.63) | 0.12 (0.43) | NS |
BMI, body mass index; NS, non-significant.
Ratings of gender coherence run from –2 to 2, and a rating of 0 is defined as normal. Very weak gender coherence corresponds to a rating of –2, and very gender typical to 2.
* P < 0.05
** P < 0.01
*** P < 0.001
TABLE 3 Age, BMI and androgen data for individuals with autism spectrum disorder (ASD) and for age-matched controls, females separated for use of combined oral contraceptives
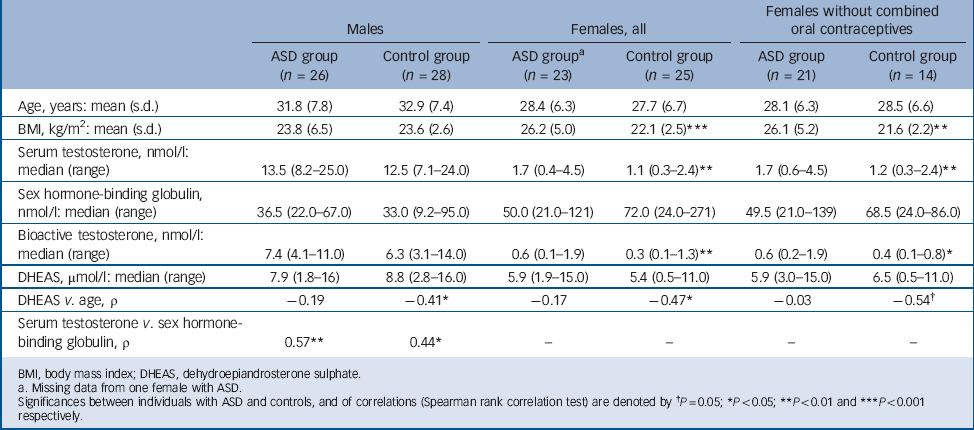
| Males | Females, all | Females without combined oral contraceptives | ||||
|---|---|---|---|---|---|---|
| ASD group (n = 26) | Control group (n = 28) | ASD group a (n = 23) | Control group (n = 25) | ASD group (n = 21) | Control group (n = 14) | |
| Age, years: mean (s.d.) | 31.8 (7.8) | 32.9 (7.4) | 28.4 (6.3) | 27.7 (6.7) | 28.1 (6.3) | 28.5 (6.6) |
| BMI, kg/m2: mean (s.d.) | 23.8 (6.5) | 23.6 (2.6) | 26.2 (5.0) | 22.1 (2.5) *** | 26.1 (5.2) | 21.6 (2.2) ** |
| Serum testosterone, nmol/l: median (range) | 13.5 (8.2–25.0) | 12.5 (7.1–24.0) | 1.7 (0.4–4.5) | 1.1 (0.3–2.4) ** | 1.7 (0.6–4.5) | 1.2 (0.3–2.4) ** |
| Sex hormone-binding globulin, nmol/l: median (range) | 36.5 (22.0–67.0) | 33.0 (9.2–95.0) | 50.0 (21.0–121) | 72.0 (24.0–271) | 49.5 (21.0–139) | 68.5 (24.0–86.0) |
| Bioactive testosterone, nmol/l: median (range) | 7.4 (4.1–11.0) | 6.3 (3.1–14.0) | 0.6 (0.1–1.9) | 0.3 (0.1–1.3) ** | 0.6 (0.2–1.9) | 0.4 (0.1–0.8) * |
| DHEAS, μmol/l: median (range) | 7.9 (1.8–16) | 8.8 (2.8–16.0) | 5.9 (1.9–15.0) | 5.4 (0.5–11.0) | 5.9 (3.0–15.0) | 6.5 (0.5–11.0) |
| DHEAS v. age, ρ | –0.19 | –0.41 * | –0.17 | –0.47 * | –0.03 | –0.54 † |
| Serum testosterone v. sex hormone-binding globulin, ρ | 0.57 ** | 0.44 * | – | – | – | – |
BMI, body mass index; DHEAS, dehydroepiandrosterone sulphate.
a Missing data from one female with ASD.
Significances between individuals with ASD and controls, and of correlations (Spearman rank correlation test) are denoted by respectively.
† P = 0.05
* P < 0.05
** P < 0.01
*** P < 0.001
TABLE 4 Correlations between gender coherence, 2D:4D and autistic traits in 54 males, above the diagonal, and 49 females, under the diagonal, with or without autism spectrum disorder
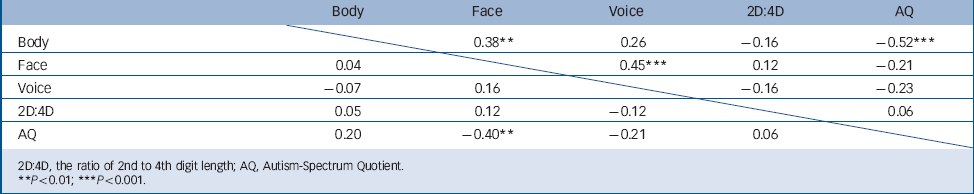
| Body | Face | Voice | 2D:4D | AQ | |
|---|---|---|---|---|---|
| Body | 0.38 ** | 0.26 | –0.16 | –0.52 *** | |
| Face | 0.04 | 0.45 *** | 0.12 | –0.21 | |
| Voice | –0.07 | 0.16 | –0.16 | –0.23 | |
| 2D:4D | 0.05 | 0.12 | –0.12 | 0.06 | |
| AQ | 0.20 | –0.40 ** | –0.21 | 0.06 | |
2D:4D, the ratio of 2nd to 4th digit length; AQ, Autism-Spectrum Quotient.
** P < 0.01
*** P < 0.001.
TABLE 5 Clusters of more (+) or less (–) gender coherent individuals within each gender, autism spectrum disorder (ASD) and control groups collapsed a
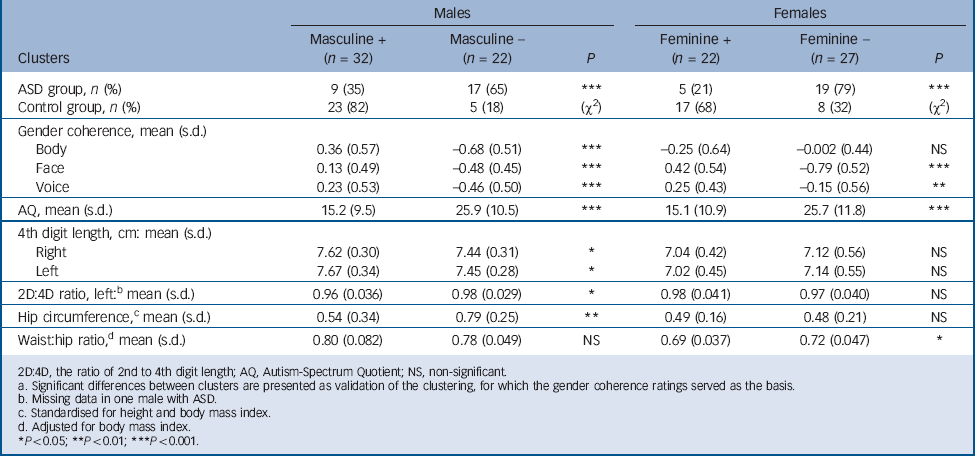
| Males | Females | |||||
|---|---|---|---|---|---|---|
| Clusters | Masculine + (n = 32) | Masculine – (n = 22) | P | Feminine + (n = 22) | Feminine – (n = 27) | P |
| ASD group, n (%) | 9 (35) | 17 (65) | *** | 5 (21) | 19 (79) | *** |
| Control group, n (%) | 23 (82) | 5 (18) | (χ2) | 17 (68) | 8 (32) | (χ2) |
| Gender coherence, mean (s.d.) | ||||||
| Body | 0.36 (0.57) | –0.68 (0.51) | *** | –0.25 (0.64) | –0.002 (0.44) | NS |
| Face | 0.13 (0.49) | –0.48 (0.45) | *** | 0.42 (0.54) | –0.79 (0.52) | *** |
| Voice | 0.23 (0.53) | –0.46 (0.50) | *** | 0.25 (0.43) | –0.15 (0.56) | ** |
| AQ, mean (s.d.) | 15.2 (9.5) | 25.9 (10.5) | *** | 15.1 (10.9) | 25.7 (11.8) | *** |
| 4th digit length, cm: mean (s.d.) | ||||||
| Right | 7.62 (0.30) | 7.44 (0.31) | * | 7.04 (0.42) | 7.12 (0.56) | NS |
| Left | 7.67 (0.34) | 7.45 (0.28) | * | 7.02 (0.45) | 7.14 (0.55) | NS |
| 2D:4D ratio, left: b mean (s.d.) | 0.96 (0.036) | 0.98 (0.029) | * | 0.98 (0.041) | 0.97 (0.040) | NS |
| Hip circumference, c mean (s.d.) | 0.54 (0.34) | 0.79 (0.25) | ** | 0.49 (0.16) | 0.48 (0.21) | NS |
| Waist:hip ratio, d mean (s.d.) | 0.80 (0.082) | 0.78 (0.049) | NS | 0.69 (0.037) | 0.72 (0.047) | * |
2D:4D, the ratio of 2nd to 4th digit length; AQ, Autism-Spectrum Quotient; NS, non-significant.
a Significant differences between clusters are presented as validation of the clustering, for which the gender coherence ratings served as the basis.
b Missing data in one male with ASD.
c Standardised for height and body mass index.
d Adjusted for body mass index.
* P < 0.05
** P < 0.01
*** P < 0.001.
with ASD of both genders were categorised into the less gender coherent clusters.
In contrast, no support was obtained for the observation that individuals with ASD often appear younger than age-matched controls; however, tentatively, this negative finding could be explained by the fact that the studied participants were relatively young, and that such a difference is more likely to be evident later in life.
Prenatal androgen influence
Sexual dimorphism is to a great extent (but not entirely) due to the influence of sex steroids exerting permanent so-called organisational effects prenatally (and later), and transient so-called activating effects in the adult organism. Reference Hines14 In this study we have explored some of the physical aspects supposedly influenced by testosterone in fetal life and throughout childhood and adulthood. Our results are compatible with the view that the androgen influence in ASD is enhanced in women but reduced in men. Moreover, in a study of children with ASD and gender identity disorder, almost all were male-to-female boys, but according to the early androgen impact hypothesis for ASD, the opposite should be expected. Reference de Vries, Noens, Cohen-Kettenis, van Berckelaer-Onnes and Doreleijers15 We thus modify the theory of Baron-Cohen, that autism should be regarded as the result of excessive masculinisation of the brain, by suggesting that it may rather be associated with androgynous features in both genders.
Our male participants with ASD were assessed as speaking with a higher pitch than the controls, which suggests a reduced impact of testosterone on the vocal tract. Reference Evans, Neave, Wakelin and Hamilton16 Moreover, gender coherence in facial features and voice pitch were strongly correlated in the male ASD group, suggesting that within this group these parameters are influenced by the same determinant, which is likely to be testosterone. No corresponding correlation was found in male controls. However, we did not find any relationship between voice pitch and serum testosterone, presumably because of the fact that vocal masculinisation appears to be completed during puberty. Also, testosterone levels are sensitive to a number of external factors, such as parenthood, mental stress, physical and sexual activity, season and age. Reference Zitzmann and Nieschlag17 Serum levels of testosterone, determined by the testicular and adrenal production, are probably inadequate as a measure of androgenisation. Reference Zitzmann and Nieschlag17 Nevertheless, several studies have indeed shown a relationship between higher levels of circulating testosterone and lower voice pitch. Reference Dabbs and Mallinger18
Evolutionary aspects
Physical signs of hormonal influence plays an evolutionary role; for example, low voice pitch in males is regarded as more attractive by females and has been shown to predict reproductive success in a population of hunter-gatherers, Reference Apicella, Feinberg and Marlowe19 and lower pitch is related to broader shoulders and chests in males. Reference Evans, Neave and Wakelin20 Thus, nose width, jaw shape, height, body configuration and voice pitch are parameters determined by the action of testosterone that are suggested to signal ‘hormonal health’ to potential female mates, in line with the ‘good genes’ model of sexual selection. Reference Evans, Neave and Wakelin20 It is likely that neuronal circuits regulating social recognition, social imitation, non-verbal communication and social reciprocity (i.e. functions that are all impaired in ASD) are also of importance for mating behaviour, and hence under strong influence from sex steroids. In line with this, abnormalities of sexual differentiation may influence both physical characteristics and social functioning, which could explain the apparent association between impaired sexual differentiation and autism symptomatology in this study.
Along the same vein, the brain neurotransmitters vasopressin and oxytocin, which are under the influence of sex steroids and display sexual dimorphism, have recently been attributed importance both for various sexually dimorphic functions related to reproductive behaviour Reference Insel, Winslow, Wang, Young and Hulihan21 and for behaviours related to social interaction. Reference Insel22 Interestingly, according to a growing body of literature, both vasopressin and oxytocin are implicated in the biology of ASD. Reference Carter23,Reference Sala, Braida, Lentini, Busnelli, Bulgheroni and Capurro24
Hormone levels and anthropometrics
A previous study found elevated testosterone levels in ASD of both genders, however females dispayed a much larger serum testosterone difference than males. Reference Geier and Geier7 Our study could only replicate this finding in women. The reasons for the higher androgen levels in women with ASD relative to controls, and to what extent this aberration is associated with the difference of gender characteristics between individuals with ASD and controls, remain to be disclosed. Being overweight increases serum androgen level, Reference Sutton-Tyrrell, Zhao, Santoro, Lasley, Sowers and Johnston25 but the relatively high BMI in the female ASD group in the present study is unlikely to explain the difference in serum testosterone levels, since this difference remained when women with BMI >25 were excluded from the analysis. Another plausible reason for high levels of serum testosterone is polycystic ovary syndrome or irregular menses, which unfortunately could not be ruled out in this study. Our finding of increased testosterone levels in women with ASD is inconsistent with a recent report showing no such difference between individuals with ASD or controls of either gender. Reference Ruta, Ingudomnukul, Taylor, Chakrabarti and Baron-Cohen26 On the other hand, these authors found markedly elevated levels of the androgen precursor androstendione in both genders, which also was reported by Geier & Geier. Reference Geier and Geier7
Total testosterone and SHBG were positively correlated in both male groups. This is well in line with previous data on other male populations and may be explained by SHBG exerting a stimulatory influence on testosterone levels.
Participants with ASD and controls did not differ with respect to DHEAS; findings that are consistent with those of Ruta et al Reference Ruta, Ingudomnukul, Taylor, Chakrabarti and Baron-Cohen26 and Tordjman et al in males with autism, Reference Tordjman, Anderson, McBride, Hertzig, Snow and Hall27 but in contrast to studies showing either trends towards higher DHEAS in boys with ASD Reference Mills, Hediger, Molloy, Chrousos, Manning-Courtney and Yu28 or lower levels compared with a non-gender-matched control group. Reference Strous, Golubchik, Maayan, Mozes, Tuati-Werner and Weizman29 Dehydroepiandrosterone sulfate normally decreases with age; Reference Maninger, Wolkowitz, Reus, Epel and Mellon30 however, this decrease was less marked in our ASD group, which suggests an abnormal hypothalamic-pituitary-adrenal axis. Whether the clinically observed ‘young look’ in middle-aged people with ASD is associated with a reduced decline in DHEAS or not is worth exploring in future studies.
The length ratio between the 2nd and 4th digit is related to prenatal androgenisation. Reference Rahman31 Accordingly, men have shorter index fingers in relation to the 4th digit compared with women. Moreover, abnormal 2D:4D ratios have been observed in women with congenital adrenal hyperplasia, Reference Okten, Kalyoncu and Yaris32 a disorder which results in an increased influence of androgen during fetal life and is associated with a more masculine cognitive profile. Sexual preferences may also be associated with 2D:4D ratio as homo-sexual men and women alike show significantly lower 2D:4D ratios in comparison to heterosexual men and women. Reference Rahman31 Furthermore, lesbians with a more ‘masculine’ appearance have a lower 2D:4D ratio relative to lesbians with a more feminine appearance, suggesting a larger androgen influence in the more masculine group. Reference Brown, Finn, Cooke and Breedlove33 Thus, the finding of low 2D:4D ratios in children with ASD Reference Manning, Baron-Cohen, Wheelwright and Sanders34,Reference De Bruin, De Nijs, Verheij, Verhagen and Ferdinand35 may further support the ‘extreme male brain’ hypothesis of ASD. However, in contrast to these previous reports, participants with ASD in our study did not have a lower 2D:4D ratio. On the contrary, men with ASD had a higher ratio, whereas that of the female ASD group was similar to that of the female control group. When interpreting this difference in outcome, it should be noted that the previous studies were conducted in children, and that the 2D:4D ratio is suggested to normalise with age. Reference De Bruin, De Nijs, Verheij, Verhagen and Ferdinand35 Almost all individuals with ASD have normal physical features but may display various minor anomalies such as an increased head size. Reference Herbert36 In our female ASD group this remained into adulthood.
Limitations
The representativeness of participants with ASD and controls is a key issue for the interpretation of our results. Needless to say, the physical appearance or the voice did not influence the inclusion of participants. However, a study on sexual hormones and anthropometry, including being photographed in underwear, will not attract a random sample of individuals. In an age-equivalent clinical sample consisting of 84 patients with ASD, cohabiting with a partner was equally frequent as in the present sample. Reference Rydén and Bejerot37 However, parenthood and university studies were twice as common in our study. This suggests that our participants with ASD were relatively resourceful and possibly may be regarded as more ‘neurotypical’ compared with individuals with ASD in general. Ideally, a control group should consist of a random sample of the population, which was not the case here. However, we have no reason to believe that our controls were more gender-typical than the general population. The data obtained may in fact underestimate rather than overstate the postulated differences in the parameters studied.
The participants were enrolled over a length of time, and individuals with ASD were in most cases recruited prior to the controls; moreover, the clinical assessments were not performed masked, as opposed to ratings made by the assessor group. In addition, we were not successful in matching the women for BMI. The relatively low BMI of the control group is plausibly related to the requirement of being photographed in underwear, or recruitments from a keep-fit organisation. This could result in less feminine body features in the female controls and, conversely, more masculine in the male controls.
A gender defiant disorder
Taken together, our results suggest that women with ASD have elevated serum testosterone levels and that, in several aspects, they display more masculine traits than women without ASD, and men with ASD display more feminine characteristics than men without ASD. Rather than being a disorder characterised by masculinisation in both genders, ASD thus seems to be a gender defiant disorder. Our results strongly suggest that gender incoherence in individuals with ASD is to be expected and should be regarded as one reflection of the wide autism phenotype. The prevalence of ASD appears to be rising and so does gender identity disorder. The comorbidity between these two disorders is striking. Reference de Vries, Noens, Cohen-Kettenis, van Berckelaer-Onnes and Doreleijers15 Endocrine disruptors, that is chemical substances that interfere with the reproductive endocrine system and abound in the current environment, may hypothetically play a role in this development. Reference Bejerot, Humble and Gardner38 Future research may gain from studying endocrine disruptors in fetal development, in relation to ASD and other increasingly prevalent diagnostic entities.
Funding
This study was supported by the St Göran Foundation, The Swedish Society of Medicine and the Thuring Foundation.
Acknowledgements
Technical support was provided by Christina Arlinde and Ann Lindgren. We thank all participants who agreed to sign up for the study.









eLetters
No eLetters have been published for this article.